Plasma Electrolytic Oxidation on Magnesium AZ31 with Sepiolite as Inhibitor Carrier for Improved Corrosion Protection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Loading of Sepiolite with Inhibitors
- Cerium(III) ions
- Phosphate ester
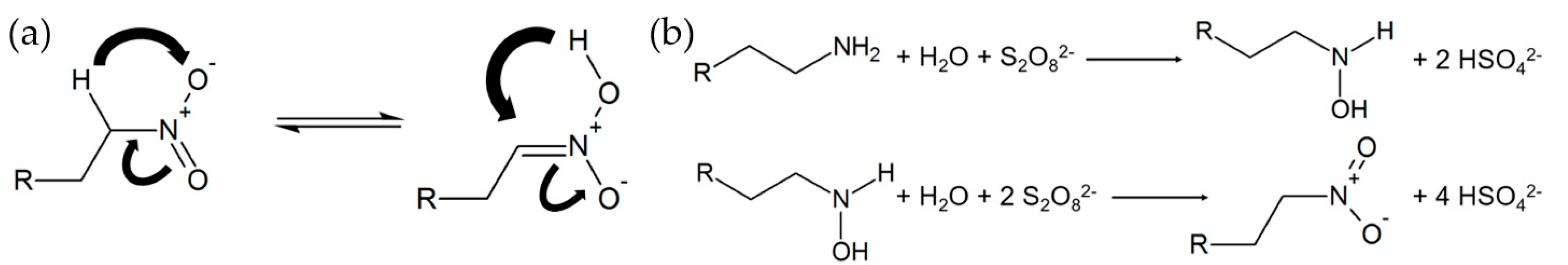
- Oxidized 1-dodecylamine (1-nitrododecane)
2.2. Suspension Stability
2.3. Leaching Tests
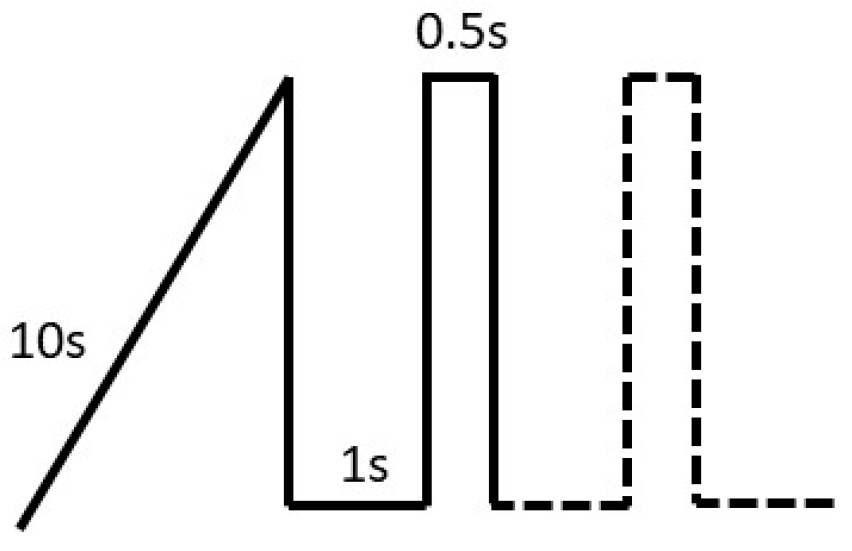
2.4. Plasma Electrolytic Oxidation
2.5. Electrochemical Impedance Spectroscopy
2.6. Linear Sweep Voltammetry (LSV)
2.7. Scanning Electron Microscopy (SEM)
3. Results and Discussion
3.1. Suspension Stability
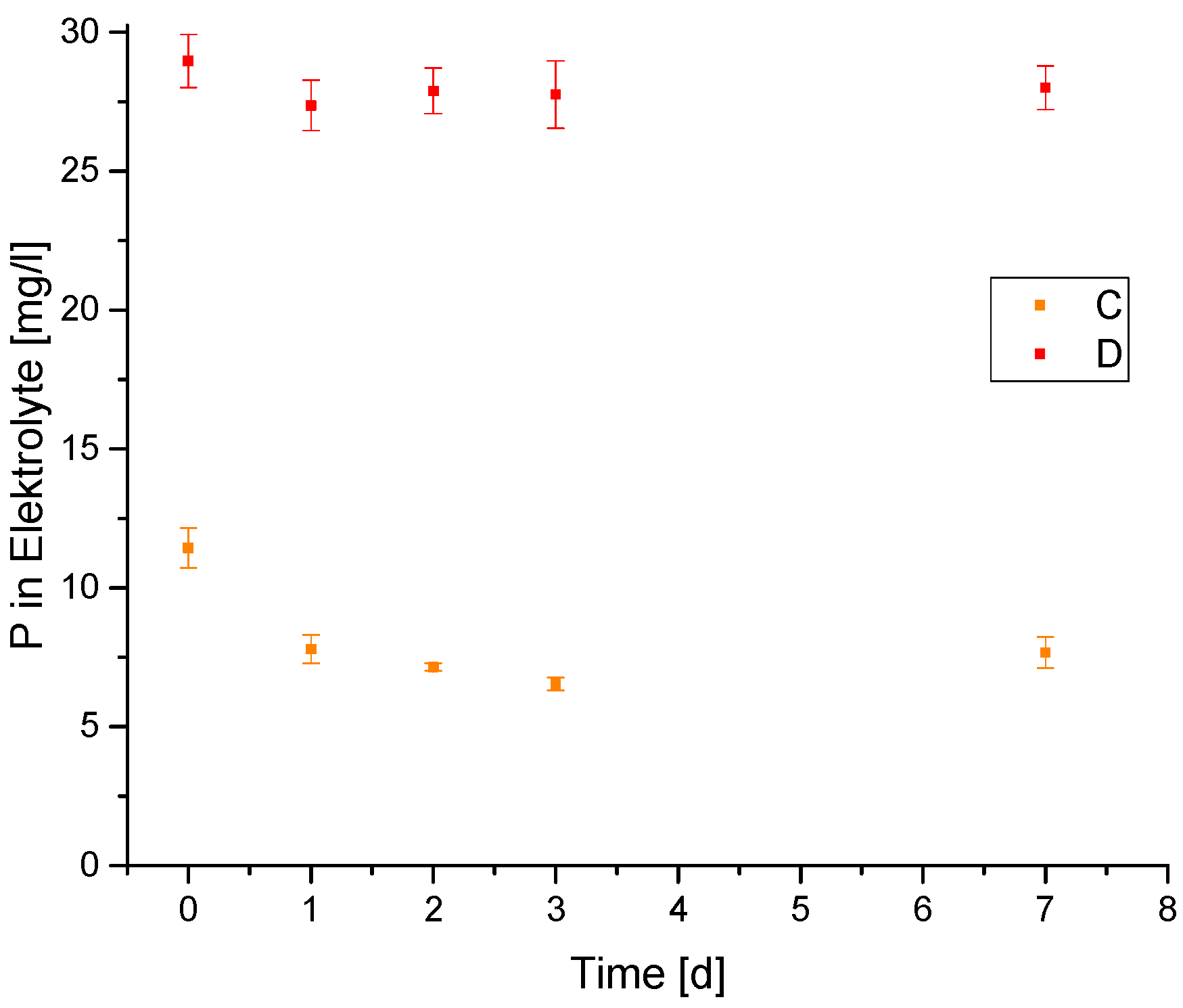
3.2. Leaching Tests
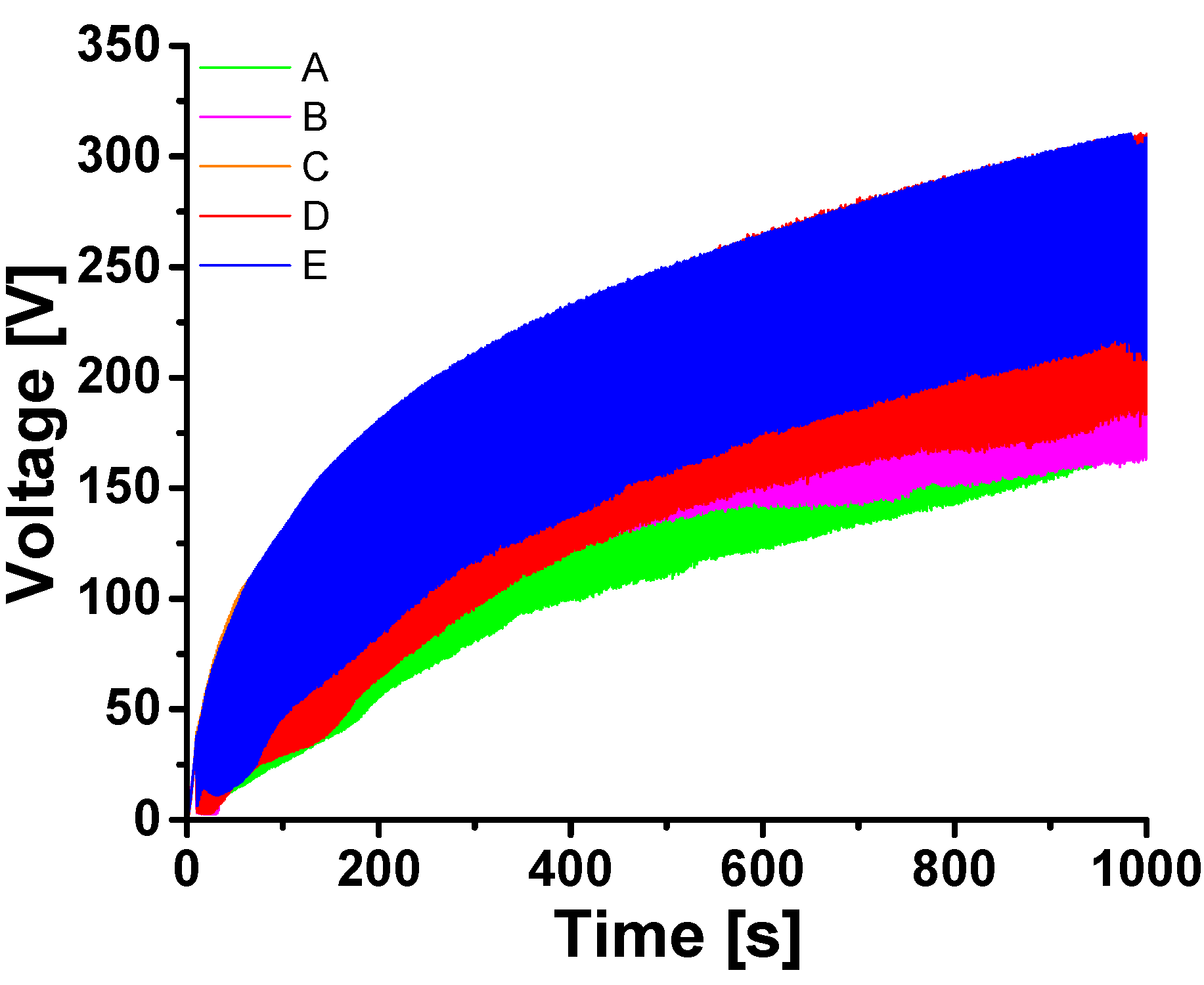
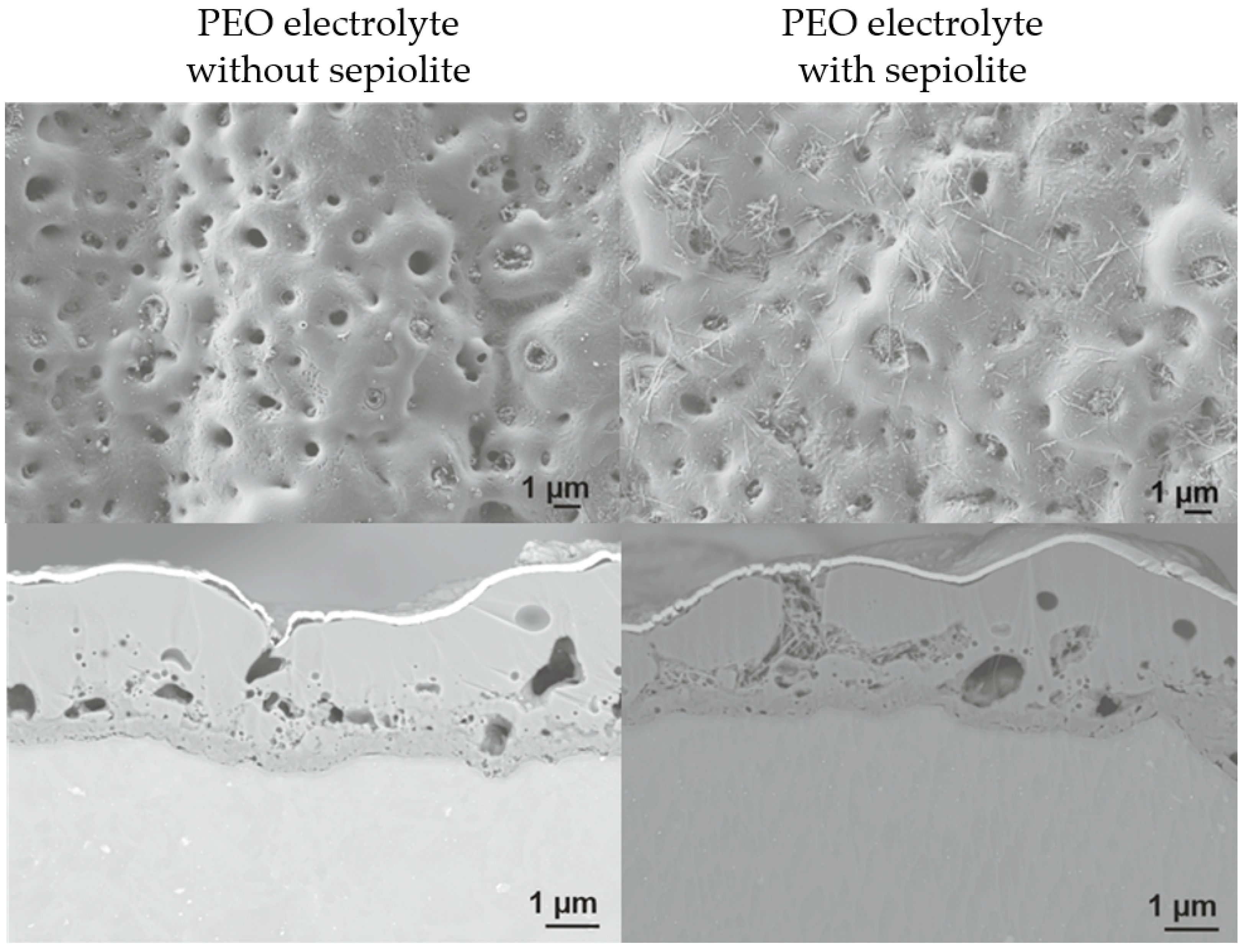
3.3. Plasma Electrolytic Oxidation
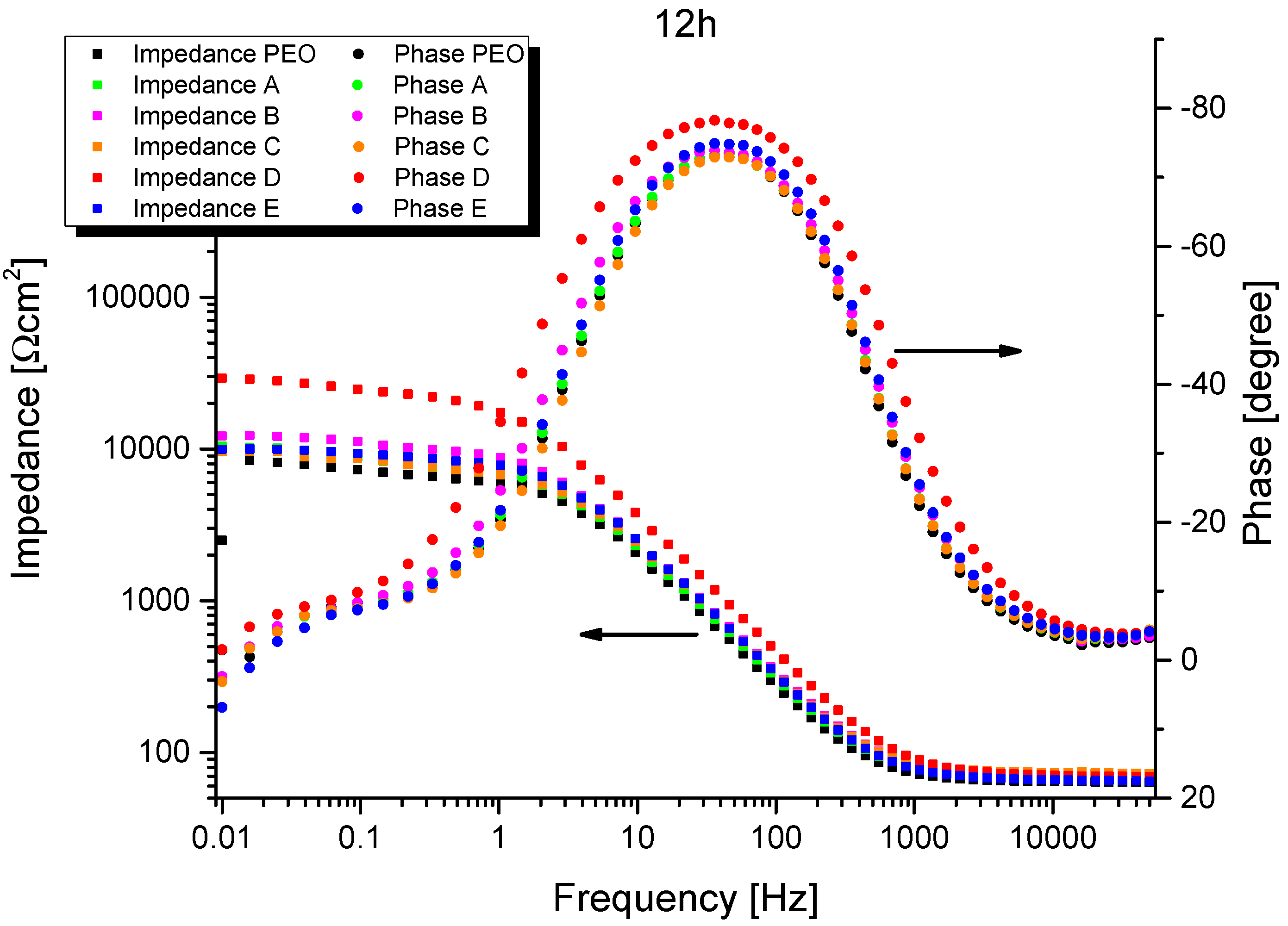
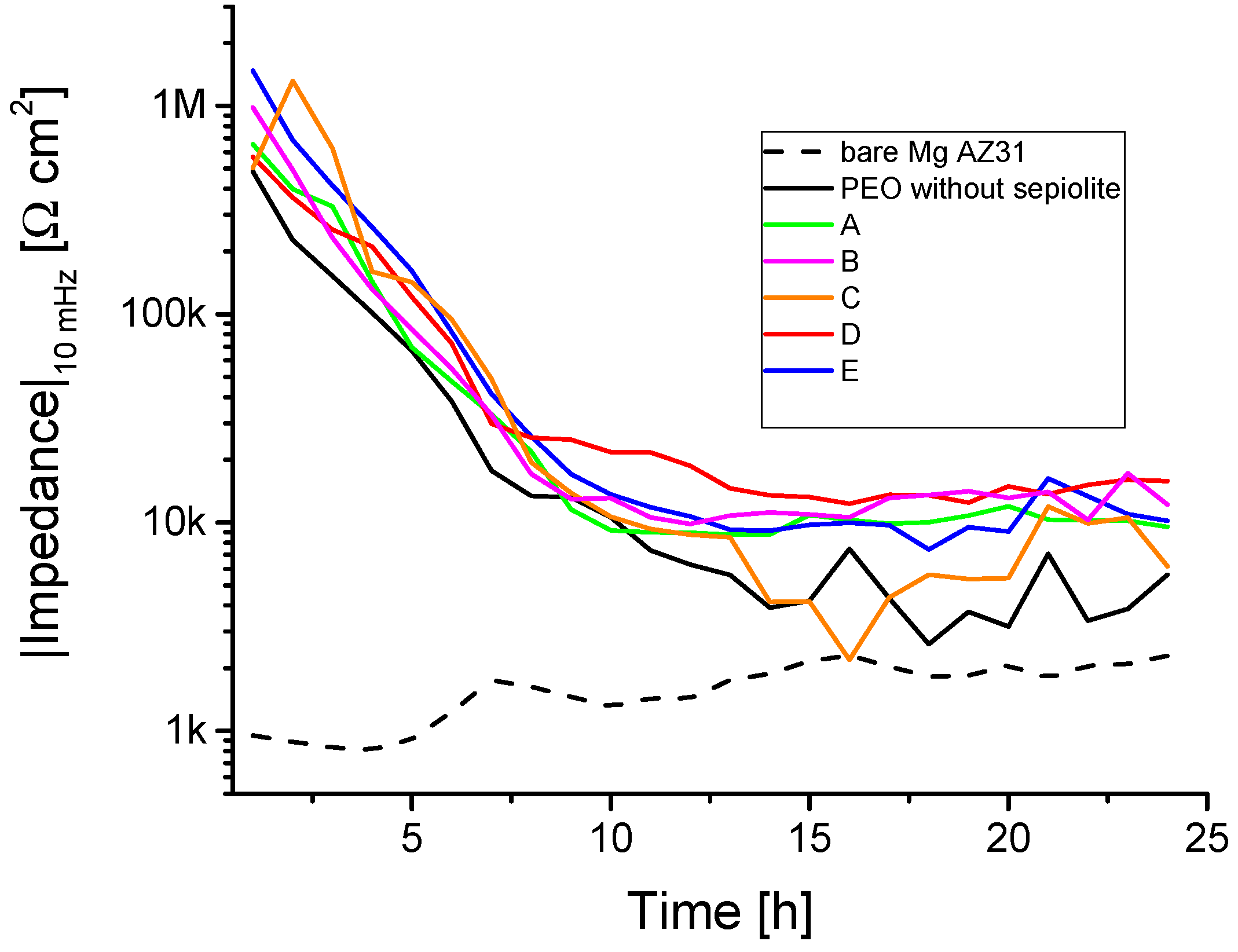
3.4. Electrochemical Impedance Spectroscopy (EIS)
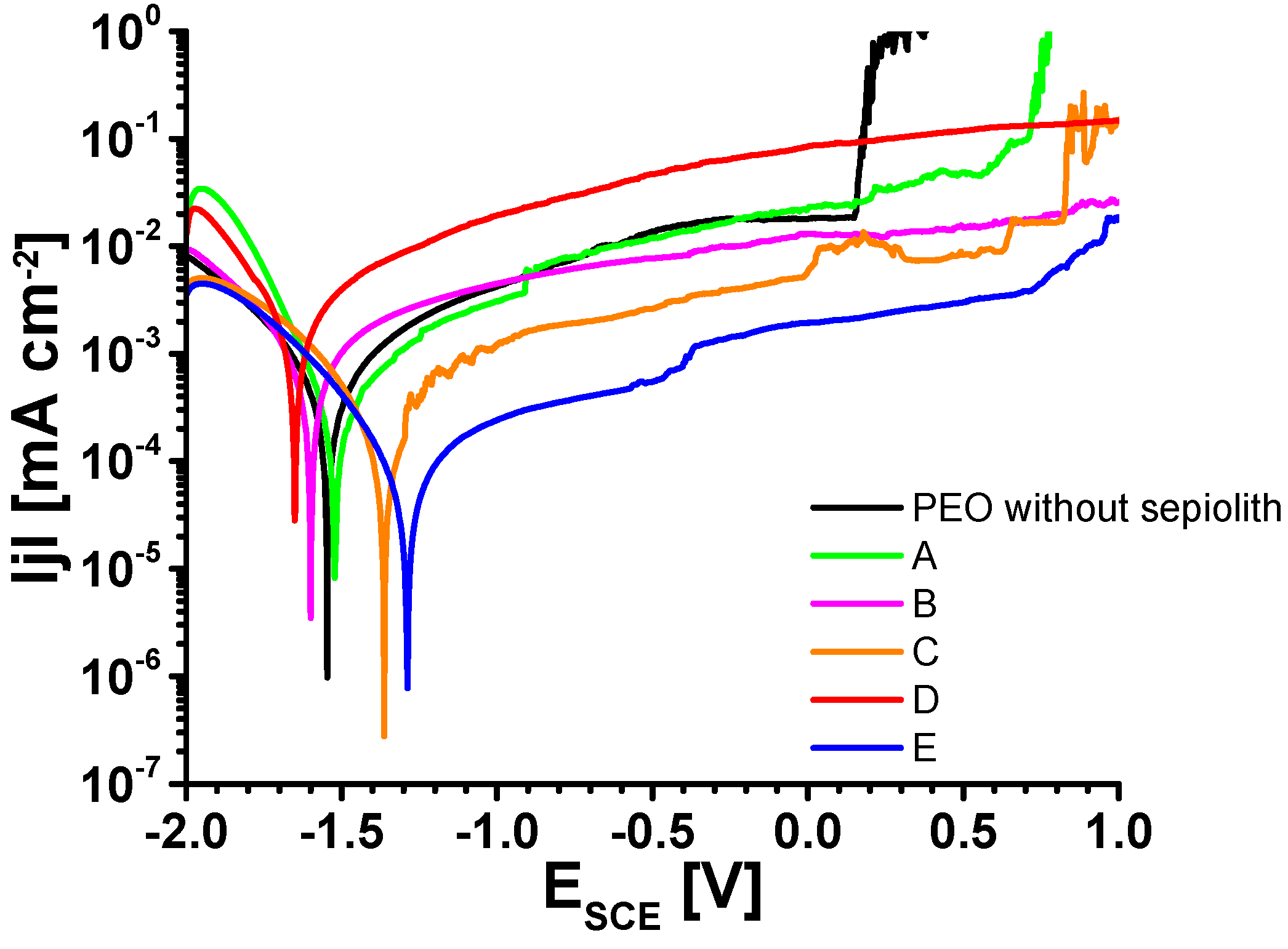
3.5. Linear Sweep Voltammetry (LSV)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Friedrich, H.E. Magnesium Technology: Metallurgy, Design Data, Applications; Springer: Berlin/Heidelberg, Germany, 2006; ISBN 3-540-20599-3. [Google Scholar]
- Rumble, J.R.; Bruno, T.J.; Doa, M.J. CRC Handbook of Chemistry and Physics: A Ready-Reference Book of Chemical and Physical Data, 101st ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: London, UK; New York, NY, USA, 2020; ISBN 0367417243. [Google Scholar]
- Song, G.-L. Corrosion of Magnesium Alloys; ebrary, Inc.: Oxford, UK; Woodhead Pub: Philadelphia, PA, USA, 2011; ISBN 9781845697082. [Google Scholar]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions; NACE International: Houston, TX, USA, 1974. [Google Scholar]
- Koppers, M.; Weber, K.; Dehnke, V.; Fuhrmann, J. Einfluss der chemischen Umgebung auf Morphologie und Struktur von Magnesiumhydroxid auf Magnesiumoberflächen. Mat.-Wiss. U. Werkstofftech. 2001, 32, 88–96. [Google Scholar] [CrossRef]
- Nordlien, J.H.; Nisancioglu, K.; Ono, S.; Masuko, N. Morphology and Structure of Water-Formed Oxides on Ternary MgAl Alloys. J. Electrochem. Soc. 1997, 144, 461–466. [Google Scholar] [CrossRef]
- Nordlien, J.H.; Ono, S.; Masuko, N.; Nisancioglu, K. Morphology and Structure of Oxide Films Formed on Magnesium by Exposure to Air and Water. J. Electrochem. Soc. 1995, 142, 3320–3322. [Google Scholar] [CrossRef]
- Arrabal, R.; Mohedano, M.; Matykina, E. Electrochemical Surface Treatments for Mg Alloys. In Encyclopedia of Materials: Metals and Alloys; Elsevier: Amsterdam, The Netherlands, 2022; pp. 87–112. ISBN 9780128197332. [Google Scholar]
- Barati Darband, G.; Aliofkhazraei, M.; Hamghalam, P.; Valizade, N. Plasma electrolytic oxidation of magnesium and its alloys: Mechanism, properties and applications. J. Magnes. Alloys 2017, 5, 74–132. [Google Scholar] [CrossRef]
- Sikdar, S.; Menezes, P.V.; Maccione, R.; Jacob, T.; Menezes, P.L. Plasma Electrolytic Oxidation (PEO) Process-Processing, Properties, and Applications. Nanomaterials 2021, 11, 1375. [Google Scholar] [CrossRef]
- Wierzbicka, E.; Vaghefinazari, B.; Mohedano, M.; Visser, P.; Posner, R.; Blawert, C.; Zheludkevich, M.; Lamaka, S.; Matykina, E.; Arrabal, R. Chromate-Free Corrosion Protection Strategies for Magnesium Alloys—A Review: Part II—PEO and Anodizing. Materials 2022, 15, 8515. [Google Scholar] [CrossRef]
- Sampatirao, H.; Radhakrishnapillai, S.; Dondapati, S.; Parfenov, E.; Nagumothu, R. Developments in plasma electrolytic oxidation (PEO) coatings for biodegradable magnesium alloys. Mater. Today Proc. 2021, 46, 1407–1415. [Google Scholar] [CrossRef]
- Sankara Narayanan, T.; Park, I.S.; Lee, M.H. Strategies to improve the corrosion resistance of microarc oxidation (MAO) coated magnesium alloys for degradable implants: Prospects and challenges. Prog. Mater. Sci. 2014, 60, 1–71. [Google Scholar] [CrossRef]
- Hussein, R.O.; Nie, X.; Northwood, D.O. An investigation of ceramic coating growth mechanisms in plasma electrolytic oxidation (PEO) processing. Electrochim. Acta 2013, 112, 111–119. [Google Scholar] [CrossRef]
- Kaseem, M.; Fatimah, S.; Nashrah, N.; Ko, Y.G. Recent progress in surface modification of metals coated by plasma electrolytic oxidation: Principle, structure, and performance. Prog. Mater. Sci. 2021, 117, 100735. [Google Scholar] [CrossRef]
- Adsul, S.H.; Soma Raju, K.; Sarada, B.V.; Sonawane, S.H.; Subasri, R. Evaluation of self-healing properties of inhibitor loaded nanoclay-based anticorrosive coatings on magnesium alloy AZ91D. J. Magnes. Alloys 2018, 6, 299–308. [Google Scholar] [CrossRef]
- Vaghefinazari, B.; Wang, C.; Mercier, D.; Mei, D.; Seyeux, A.; Marcus, P.; Blawert, C.; Lamaka, S.V.; Zheludkevich, M.L. Adverse effect of 2,5PDC corrosion inhibitor on PEO coated magnesium. Corros. Sci. 2021, 192, 109830. [Google Scholar] [CrossRef]
- Wang, S.Y.; Li, Q.; Zhong, X.K.; Li, L.Q.; Chen, F.N.; Luo, F.; Dai, Y.; Gao, H.; Liu, F.; Zhang, H.X. Effects of NO −3 in NaCl solution on corrosion protection of AZ91D magnesium alloy coated with silane films. Trans. IMF 2012, 90, 78–85. [Google Scholar] [CrossRef]
- Ouyang, Y.; Li, L.-X.; Xie, Z.-H.; Tang, L.; Wang, F.; Zhong, C.-J. A self-healing coating based on facile pH-responsive nanocontainers for corrosion protection of magnesium alloy. J. Magnes. Alloys 2022, 10, 836–849. [Google Scholar] [CrossRef]
- Zhang, G.; Wu, L.; Tang, A.; Ma, Y.; Song, G.-L.; Zheng, D.; Jiang, B.; Atrens, A.; Pan, F. Active corrosion protection by a smart coating based on a MgAl-layered double hydroxide on a cerium-modified plasma electrolytic oxidation coating on Mg alloy AZ31. Corros. Sci. 2018, 139, 370–382. [Google Scholar] [CrossRef]
- Zhang, G.; Wu, L.; Tang, A.; Ding, X.; Jiang, B.; Atrens, A.; Pan, F. Smart epoxy coating containing zeolites loaded with Ce on a plasma electrolytic oxidation coating on Mg alloy AZ31 for active corrosion protection. Prog. Org. Coat. 2019, 132, 144–147. [Google Scholar] [CrossRef]
- Zhang, G.; Jiang, E.; Wu, L.; Ma, W.; Yang, H.; Tang, A.; Pan, F. Corrosion protection properties of different inhibitors containing PEO/LDHs composite coating on magnesium alloy AZ31. Sci. Rep. 2021, 11, 2774. [Google Scholar] [CrossRef]
- Yang, J.; Blawert, C.; Lamaka, S.V.; Snihirova, D.; Lu, X.; Di, S.; Zheludkevich, M.L. Corrosion protection properties of inhibitor containing hybrid PEO-epoxy coating on magnesium. Corros. Sci. 2018, 140, 99–110. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Mashtalyar, D.V.; Gnedenkov, S.V. Localized corrosion of the Mg alloys with inhibitor-containing coatings: SVET and SIET studies. Corros. Sci. 2016, 102, 269–278. [Google Scholar] [CrossRef]
- Dong, Q.; Ba, Z.; Jia, Y.; Chen, Y.; Lv, X.; Zhang, X.; Wang, Z. Effect of solution concentration on sealing treatment of Mg-Al hydrotalcite film on AZ91D Mg alloy. J. Magnes. Alloys 2017, 5, 320–325. [Google Scholar] [CrossRef]
- Fattah-alhosseini, A.; Chaharmahali, R.; Babaei, K. Effect of particles addition to solution of plasma electrolytic oxidation (PEO) on the properties of PEO coatings formed on magnesium and its alloys: A review. J. Magnes. Alloys 2020, 8, 799–818. [Google Scholar] [CrossRef]
- Toorani, M.; Aliofkhazraei, M.; Sabour Rouhaghdam, A. Microstructural, protective, inhibitory and semiconducting properties of PEO coatings containing CeO2 nanoparticles formed on AZ31 Mg alloy. Surf. Coat. Technol. 2018, 352, 561–580. [Google Scholar] [CrossRef]
- Chaharmahali, R.; Fattah-alhosseini, A.; Esfahani, H. Increasing the in-vitro corrosion resistance of AZ31B-Mg alloy via coating with hydroxyapatite using plasma electrolytic oxidation. J. Asian Ceram. Soc. 2020, 8, 39–49. [Google Scholar] [CrossRef]
- Lu, X.; Mohedano, M.; Blawert, C.; Matykina, E.; Arrabal, R.; Kainer, K.U.; Zheludkevich, M.L. Plasma electrolytic oxidation coatings with particle additions—A review. Surf. Coat. Technol. 2016, 307, 1165–1182. [Google Scholar] [CrossRef]
- Mashtalyar, D.V.; Imshinetskiy, I.M.; Nadaraia, K.V.; Gnedenkov, A.S.; Suchkov, S.N.; Opra, D.P.; Pustovalov, E.V.; Yu Ustinov, A.; Sinebryukhov, S.L.; Gnedenkov, S.V. Effect of TiO2 nanoparticles on the photocatalytic properties of PEO coatings on Mg alloy. J. Magnes. Alloys 2023, 11, 735–752. [Google Scholar] [CrossRef]
- Zehra, T.; Patil, S.A.; Shrestha, N.K.; Fattah-alhosseini, A.; Kaseem, M. Anionic assisted incorporation of WO3 nanoparticles for enhanced electrochemical properties of AZ31 Mg alloy coated via plasma electrolytic oxidation. J. Alloys Compd. 2022, 916, 165445. [Google Scholar] [CrossRef]
- Mingo, B.; Guo, Y.; Němcová, A.; Gholinia, A.; Mohedano, M.; Sun, M.; Matthews, A.; Yerokhin, A. Incorporation of halloysite nanotubes into forsterite surface layer during plasma electrolytic oxidation of AM50 Mg alloy. Electrochim. Acta 2019, 299, 772–788. [Google Scholar] [CrossRef]
- Mingo, B.; Guo, Y.; Leiva-Garcia, R.; Connolly, B.J.; Matthews, A.; Yerokhin, A. Smart Functionalization of Ceramic-Coated AZ31 Magnesium Alloy. ACS Appl. Mater. Interfaces 2020, 12, 30833–30846. [Google Scholar] [CrossRef]
- Sun, M.; Yerokhin, A.; Bychkova, M.; Shtansky, D.V.; Levashov, E.A.; Matthews, A. Self-healing plasma electrolytic oxidation coatings doped with benzotriazole loaded halloysite nanotubes on AM50 magnesium alloy. Corros. Sci. 2016, 111, 753–769. [Google Scholar] [CrossRef]
- Schneider, M.; Kremmer, K.; Tabatabai, D.; Fürbeth, W. Nano-sized zeolite particles as inhibitor carrier in plasma electrolytic oxide layers on AZ31. Mater. Corros. 2018, 69, 971–977. [Google Scholar] [CrossRef]
- Guo, X.-P.; Song, G.-L.; Hu, J.-Y.; Huang, D.-B. Corrosion inhibition of magnesium (Mg) Alloys. In Corrosion Prevention of Magnesium Alloys; Song, G.-L., Ed.; WP Woodhead Publisching: Oxford, UK; Cambridge, UK; Philadelphia, PA, USA, 2013; pp. 61–84. ISBN 9780857094377. [Google Scholar]
- McCafferty, E. Introduction to Corrosion Science; Springer: New York, NY, USA, 2010. [Google Scholar] [CrossRef]
- Palanisamy, G. Corrosion Inhibitors. In Corrosion Inhibitors; Singh, A., Ed.; IntechOpen: 2019; ISBN 978-1-78984-714-7.
- Lamaka, S.V.; Vaghefinazari, B.; Mei, D.; Petrauskas, R.P.; Höche, D.; Zheludkevich, M.L. Comprehensive screening of Mg corrosion inhibitors. Corros. Sci. 2017, 128, 224–240. [Google Scholar] [CrossRef]
- Galvão, T.L.P. CORDATA. Available online: https://datacor.shinyapps.io/cordata/ (accessed on 15 November 2022).
- Song, H.; Xu, Z.; Benabou, L.; Yin, Z.; Guan, H.; Yan, H.; Chao, L.; Hu, Z.; Wang, X. Sodium dodecyl sulfate (SDS) as an effective corrosion inhibitor for Mg-8Li-3Al alloy in aqueous NaCl: A combined experimental and theoretical investigation. J. Magnes. Alloys 2021, 11, 287–300. [Google Scholar] [CrossRef]
- Li, Y.; Lu, X.; Wu, K.; Yang, L.; Zhang, T.; Wang, F. Exploration the inhibition mechanism of sodium dodecyl sulfate on Mg alloy. Corros. Sci. 2020, 168, 108559. [Google Scholar] [CrossRef]
- Calado, L.M.; Taryba, M.G.; Morozov, Y.; Carmezim, M.J.; Montemor, M.F. Cerium phosphate-based inhibitor for smart corrosion protection of WE43 magnesium alloy. Electrochim. Acta 2021, 365, 137368. [Google Scholar] [CrossRef]
- Hu, J.; Huang, D.; Zhang, G.; Song, G.-L.; Guo, X. Research on the inhibition mechanism of tetraphenylporphyrin on AZ91D magnesium alloy. Corros. Sci. 2012, 63, 367–378. [Google Scholar] [CrossRef]
- Rudnick, L.R. Synthetics, Mineral oils, and Bio-Based Lubricants: Chemistry and Technology, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2020; ISBN 9781138068216. [Google Scholar]
- Poppe, L.J.; Paskevich, V.F.; Hathaway, J.C.; Blackwood, D.S. A Laboratory Manual for X-ray Powder Diffraction. Open-File Report 01-041. Available online: https://pubs.usgs.gov/of/2001/of01-041/ (accessed on 15 November 2022).
- Álvarez, A.; Santarén, J.; Esteban-Cubillo, A.; Aparicio, P. Current Industrial Applications of Palygorskite and Sepiolite. In Developments in Palygorskite-Sepiolite Research; Elsevier: Amsterdam, The Netherlands, 2011; pp. 281–298. ISBN 9780444536075. [Google Scholar]
- Harvey, C.C.; Lagaly, G. Industrial Applications. In Handbook of Clay Science; Elsevier: Amsterdam, The Netherlands, 2013; pp. 451–490. ISBN 9780080993645. [Google Scholar]
- Sabah, E.; Majdan, M. Removal of phosphorus from vegetable oil by acid-activated sepiolite. J. Food Eng. 2009, 91, 423–427. [Google Scholar] [CrossRef]
- Murray, H.H.; Pozo, M.; Galán, E. An Introduction to Palygorskite and Sepiolite Deposits—Location, Geology and Uses. In Developments in Palygorskite-Sepiolite Research; Elsevier: Amsterdam, The Netherlands, 2011; pp. 85–99. ISBN 9780444536075. [Google Scholar]
- Robert, J.D.; Caserio, M.C. Basic Principles of Organic Chemistry. Available online: https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Book%3A_Basic_Principles_of_Organic_Chemistry_(Roberts_and_Caserio)/23%3A_Organonitrogen_Compounds_I_-_Amines/23.11%3A_Oxidation_of_Amines (accessed on 15 November 2022).
- Schneider, M.; Kremmer, K.; Höhn, S. Corrosion protection of thickness reduced plasma electrolytic layers on AZ31. Mater. Corros. 2016, 67, 921–928. [Google Scholar] [CrossRef]
- Schneider, M.; Kremmer, K.; Fürbeth, W.; Lederer, S.; Sottor, R. Sepiolite reinforced PEO layer on AZ 31. Surf. Coat. Technol. 2023, 459, 129369. [Google Scholar] [CrossRef]
- Hsu, C.H.; Mansfeld, F. Technical Note: Concerning the Conversion of the Constant Phase Element Parameter Y 0 into a Capacitance. CORROSION 2001, 57, 747–748. [Google Scholar] [CrossRef]




| Adsorption Inhibitors | Film-Forming Inhibitors | ||
|---|---|---|---|
| Passivating Inhibitors | Precipitation Inhibitors | ||
| Oxidizing Inhibitors | Non-Oxidizing Inhibitors | ||
| sodium dodecyl- sulfate [41,42] sodium dodecylbenzene-sulfonate [36,39] hexamethylene-tetramine [36] bis(2-ethylhexyl)-phosphate [43] | nitrate [18,36,39] chromate [36,39] hydrogen peroxide [36] permanganate [36] | phosphate [39] fluoride [36,39] 5,10,15,20-tetraphenyl- prophyrin [36,44] 8-hydroxychinolin [23,39] sodium aminopropyl- triethoxysilicate [36] | |
| Nomenclature | Loaded with… | Loading Capacity [mg/g] |
|---|---|---|
| A | Unloaded/natural | - |
| B | Ce3+ | 6.2 |
| C | tris (2-ethylhexyl) phosphate | 200 |
| D | tris (2-ethylhexyl) phosphate +Apricon OPE | 100 + 100 |
| E | oxidized 1-dodecylamine | 200 |
| Al | Zn | Mn | Si | Cu | Ca | Fe | Ni | Others | Mg |
|---|---|---|---|---|---|---|---|---|---|
| 2.5–3.5 | 0.7–1.3 | 0.2 | Max. 0.05 | Max. 0.05 | Max. 0.04 | Max. 0.005 | Max. 0.005 | 0.3 max total | balanced |
| Sepiolite Type | OCP vs. SCE [V] | R0 [Ω cm2] | R1 [kΩ cm2] | C1 [µF/cm2] | R2 [kΩ cm2] | C2 [µF/cm2] | Overall Fit Error [%] |
|---|---|---|---|---|---|---|---|
| After 1 h of exposure | |||||||
| PEO without sepiolite | −1.454 ± 0.088 | 68 ± 3 | 5.9 ± 2.9 | 0.12 ± 0.8 | 790 ± 760 | 0.61 ± 0.26 | 8.8 ± 3.1 |
| A | −1.444 ± 0.079 | 69 ± 4 | 2.7 ± 2.3 | 0.081 ± 0.045 | 1060 ± 1080 | 0.88 ± 0.28 | 12.9 ± 2.2 |
| B | −1.460 ± 0.183 | 74 ± 2 | 2.0 ± 0.2 | 0.071 ± 0.010 | 1500 ± 300 | 0.94 ± 0.15 | 13.5 ± 1.2 |
| C | −1.493 ± 0.027 | 62 ± 2 | 3.2 ± 2.1 | 0.073 ± 0.060 | 490 ± 230 | 0.56 ± 0.22 | 11.8 ± 3.2 |
| D | −1.532 ± 0.029 | 67 ± 4 | 40.4 ± 11.2 | 0.11 ± 0.01 | 660 ± 290 | 0.25 ± 0.12 | 6.8 ± 4.1 |
| E | −1.452 ± 0.049 | 60 ± 2 | 10.6 ± 2.7 | 0.062 ± 0.015 | 2300 ± 1100 | 0.47 ± 0.11 | 10.5 ± 1.1 |
| After 12 h of exposure | |||||||
| PEO without sepiolite | −1.499 ± 0.014 | 66 ± 2 | - | - | 6.5 ± 1.6 | 3.5 ± 0.6 | 9.4 ± 0.9 |
| A | −1.505 ± 0.002 | 66 ± 2 | - | - | 7.3 ± 0.9 | 3.2 ± 0.2 | 9.4 ± 0.6 |
| B | −1.511 ± 0.009 | 70 ± 2 | - | - | 8.4 ± 3.0 | 3.4 ± 0.5 | 9.2 ± 2.0 |
| C | −1.508 ± 0.010 | 71 ± 3 | - | - | 6.3 ± 2.0 | 3.4 ± 0.6 | 9.2 ± 1.2 |
| D | −1.517 ± 0.002 | 70 ± 1 | - | - | 16.1 ± 6.0 | 2.6 ± 0.3 | 9.3 ± 0.4 |
| E | −1.501 ± 0.003 | 67 ± 3 | - | - | 9.3 ± 0.9 | 3.1 ± 0.3 | 7.5 ± 0.1 |
| PEO | A | B | C | D | E | |
|---|---|---|---|---|---|---|
| Ecorr/ [V vs. SCE] | −1.44 ± 0.12 | −1.50 ± 0.10 | 1.49 ± 0.09 | −1.40 ± 0.04 | −1.65 ± 0.06 | −1.42 ± 0.15 |
| /jcorr/ [µA cm−2] | 0.12 ± 0.11 | 0.98 ± 0.90 | 0.25 ± 0.15 | 0.14 ± 0.02 | 1.00 ± 0.68 | 0.36 ± n.a. |
| Ebreak down [V vs. SCE] | −0.28 ± 0.43 | −0.65 ± 0.53 | 0.57 ± 0.32 | 0.04 ± 0.75 | −0.92 ± 0.10 | −0.06 ± 1.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sottor, R.; Gruen, R.; Kremmer, K.; Lederer, S.; Schneider, M.; Fuerbeth, W. Plasma Electrolytic Oxidation on Magnesium AZ31 with Sepiolite as Inhibitor Carrier for Improved Corrosion Protection. Corros. Mater. Degrad. 2023, 4, 488-502. https://doi.org/10.3390/cmd4030025
Sottor R, Gruen R, Kremmer K, Lederer S, Schneider M, Fuerbeth W. Plasma Electrolytic Oxidation on Magnesium AZ31 with Sepiolite as Inhibitor Carrier for Improved Corrosion Protection. Corrosion and Materials Degradation. 2023; 4(3):488-502. https://doi.org/10.3390/cmd4030025
Chicago/Turabian StyleSottor, Robert, Ricarda Gruen, Kerstin Kremmer, Stephan Lederer, Michael Schneider, and Wolfram Fuerbeth. 2023. "Plasma Electrolytic Oxidation on Magnesium AZ31 with Sepiolite as Inhibitor Carrier for Improved Corrosion Protection" Corrosion and Materials Degradation 4, no. 3: 488-502. https://doi.org/10.3390/cmd4030025






