Protection of Testis against Lipopolysaccharide-Induced Toxicity: Mildronate-Induced L-Carnitine Depletion as a Modulator of Gut Microbiome Composition and Gastrointestinal Inflammation
Abstract
:1. Introduction
2. Results
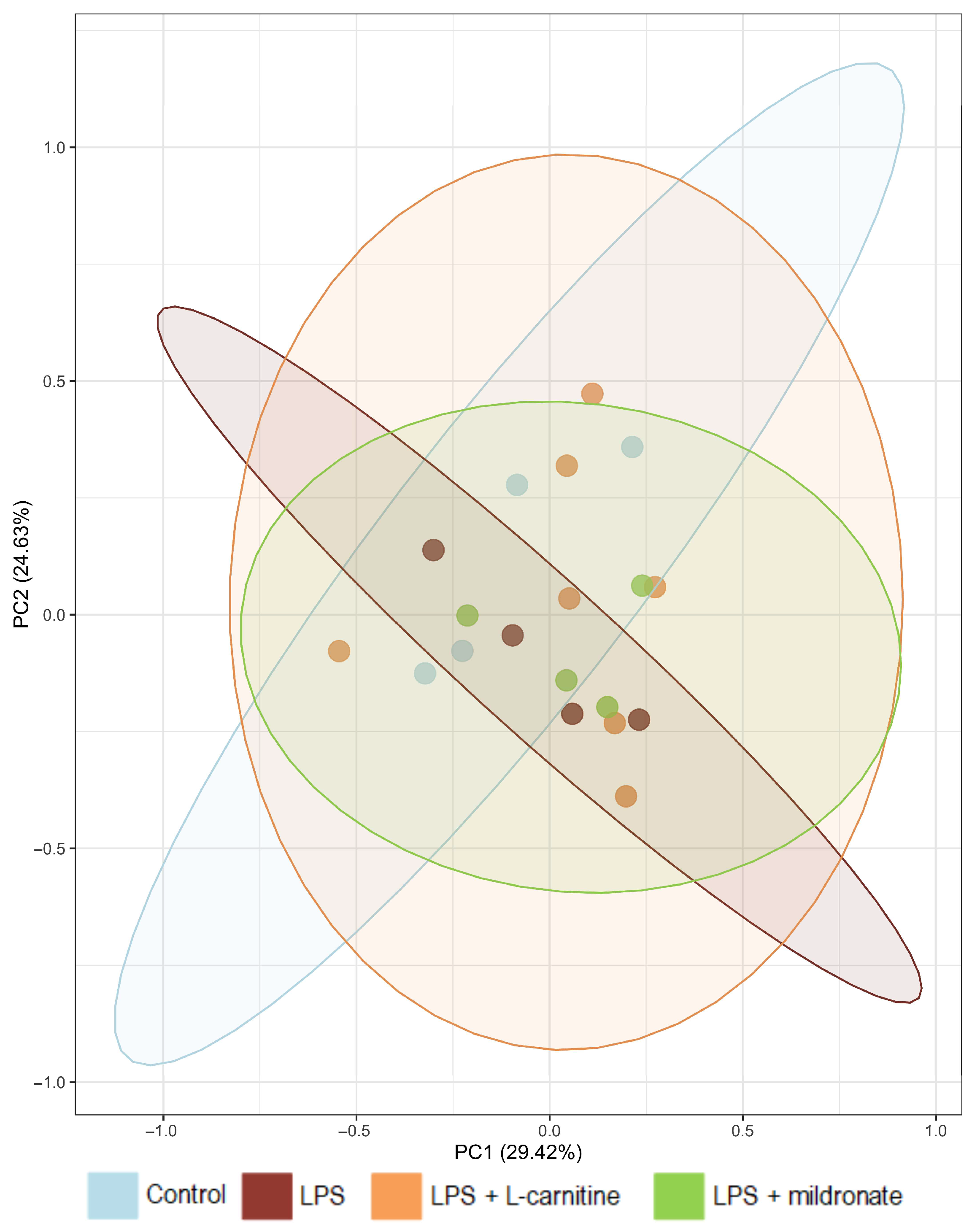
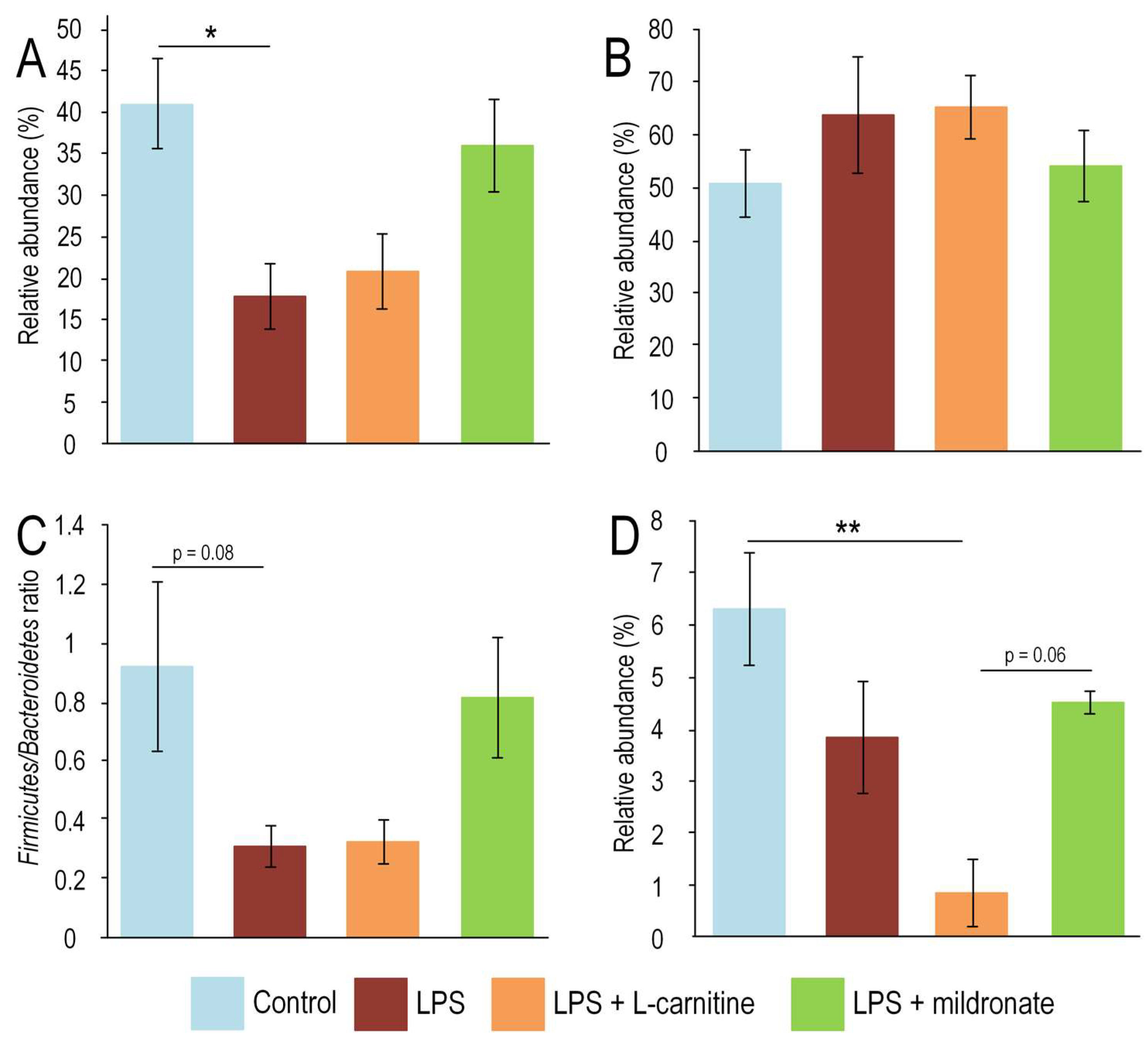
2.1. Bacterial Composition of the Gut Microbiome
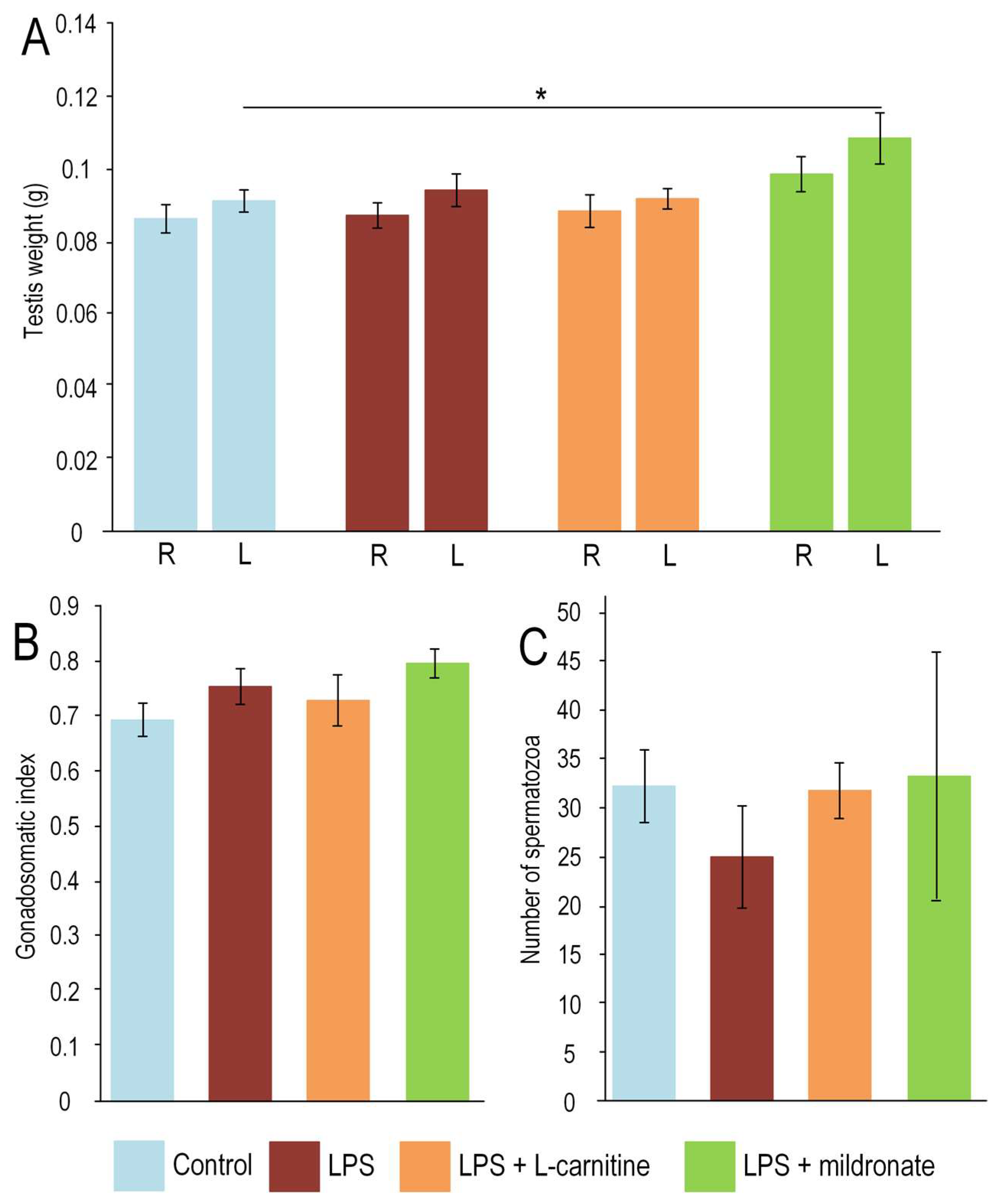
2.2. Effect of LPS Injection and Modulators of L-Carnitine Metabolism on Testis Weight and Sperm Quality
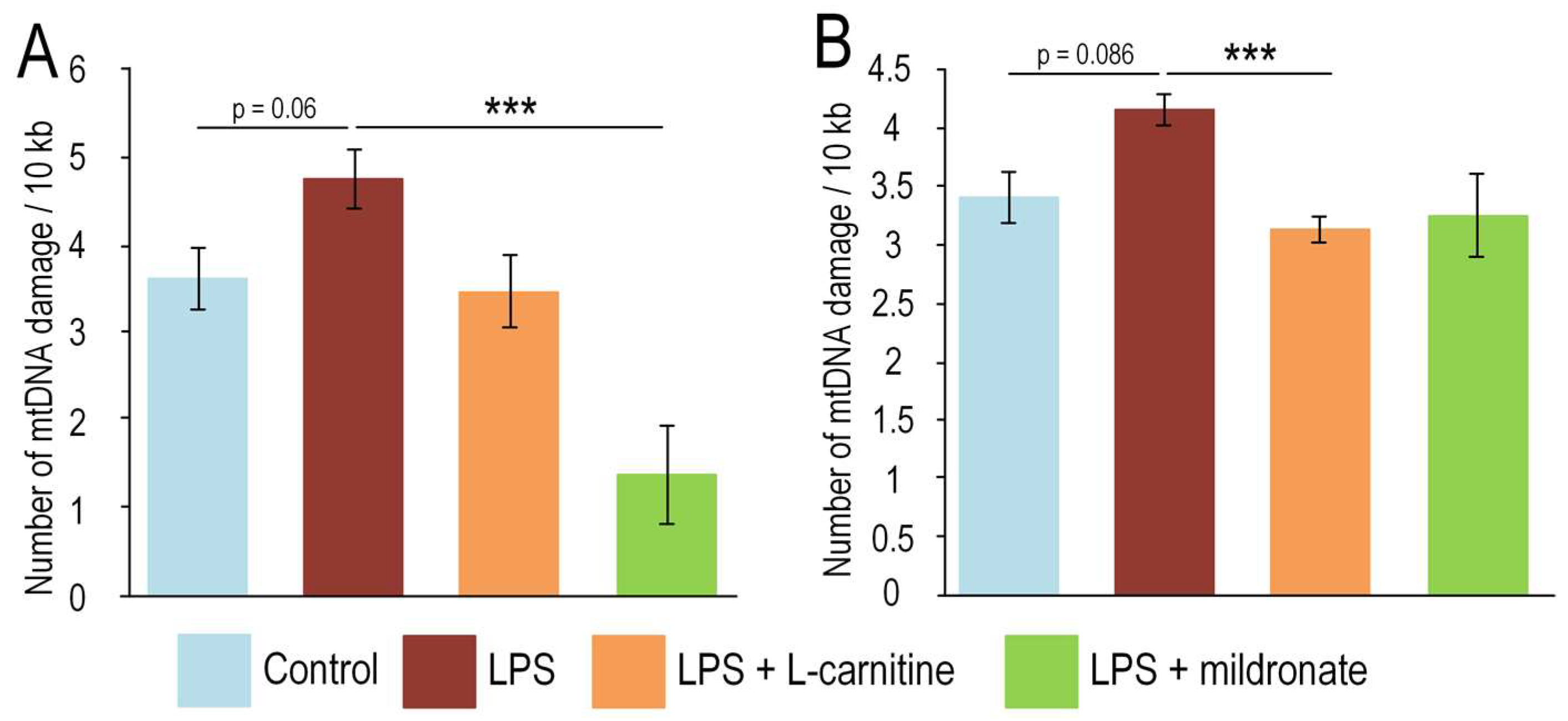
2.3. Impact of the LPS, L-Carnitine and Mildronate on the mtDNA Integrity in the Gut and Testis
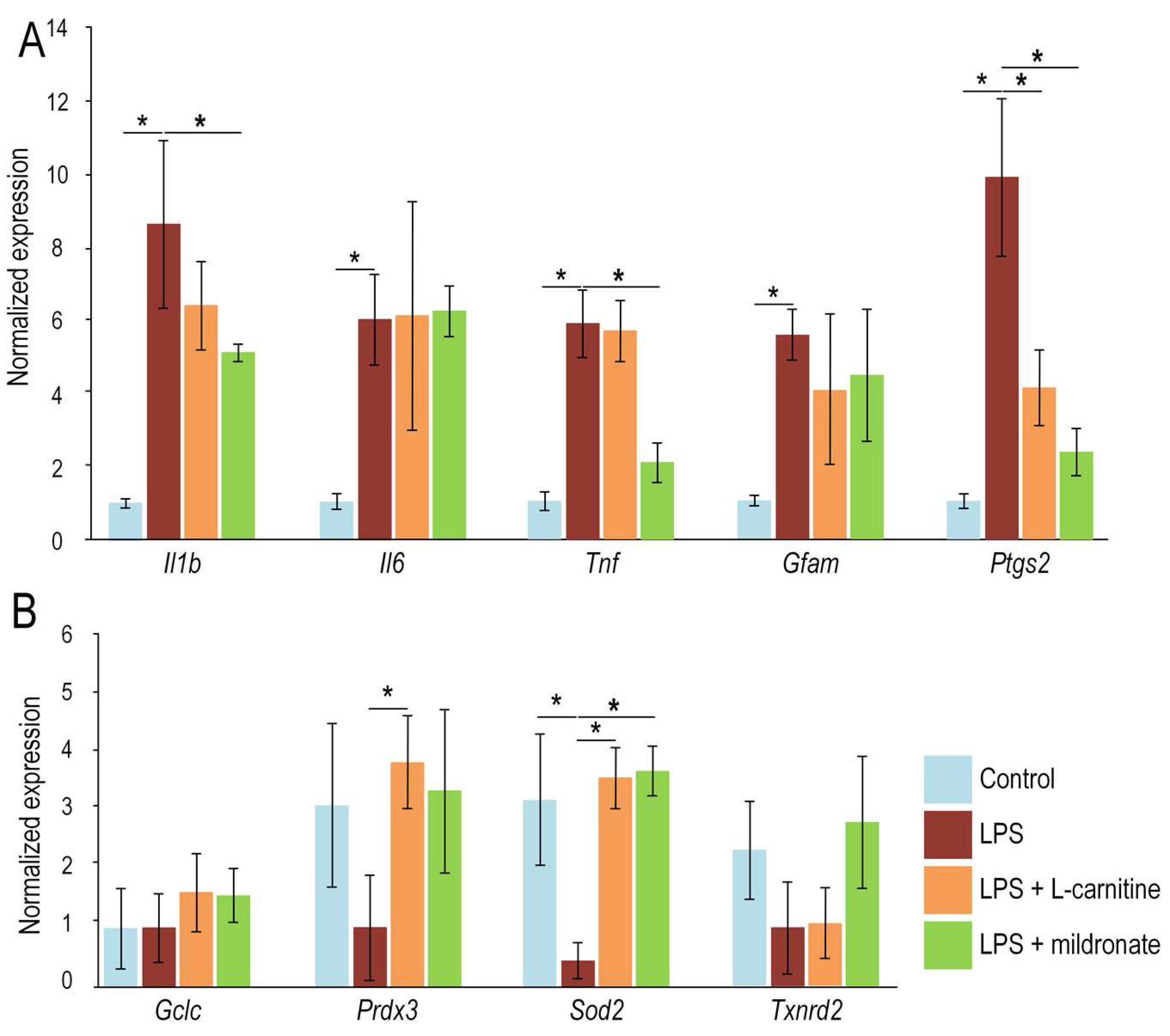
2.4. Effect of LPS, L-Carnitine and Mildronate on the Expression of Inflammatory Markers in the Gut
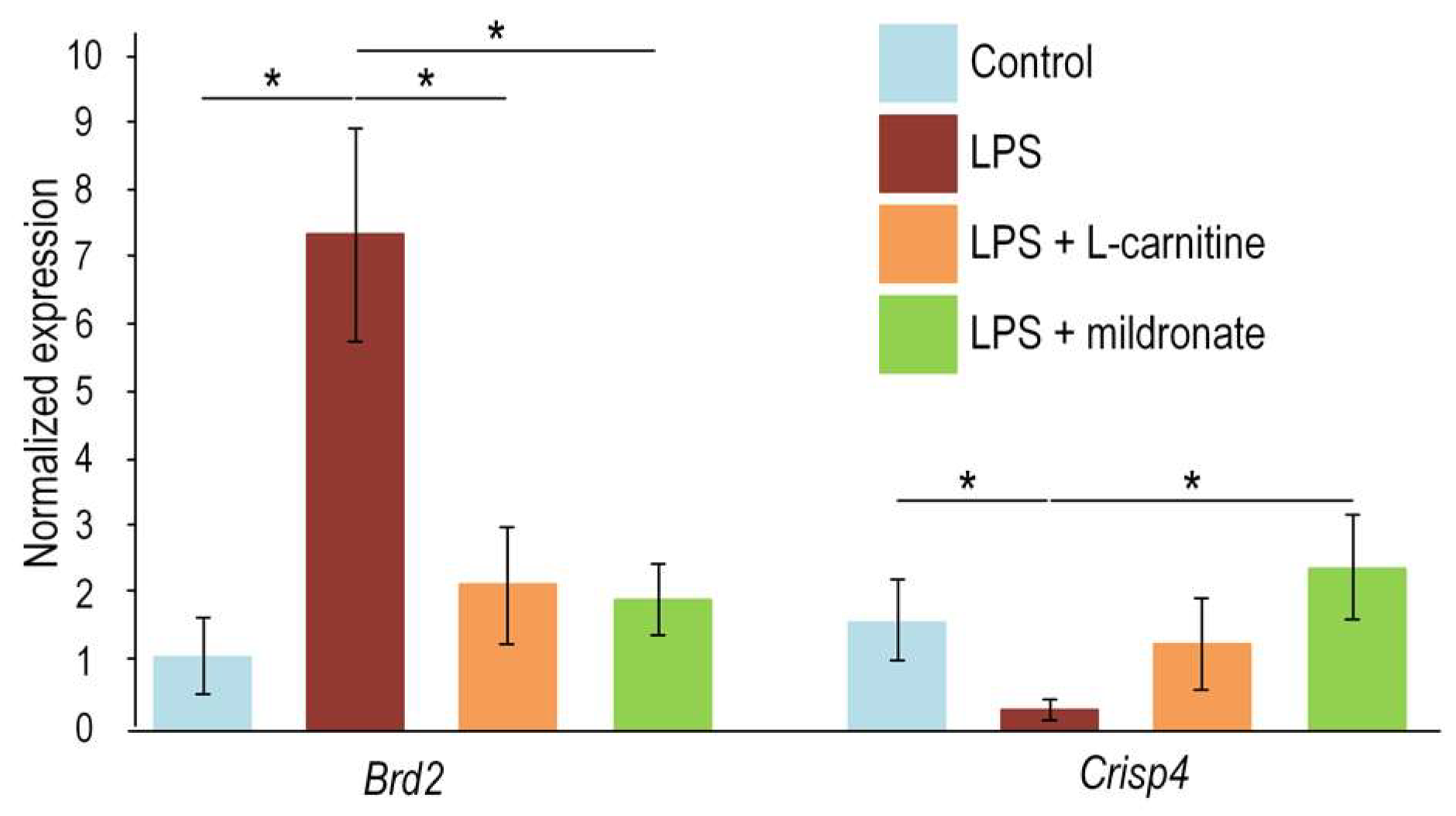
2.5. Effect of LPS Injection and Modulators of L-Carnitine Metabolism on the Gene Expression in the Testis
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experiment Design
4.3. Testis Analysis
4.4. Measurement of Number of mtDNA Damage
4.5. Gene Expression Measurement
4.6. Analysis of Bacterial Composition of Gut Microbiome
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Azenabor, A.; Ekun, A.O.; Akinloye, O. Impact of Inflammation on Male Reproductive Tract. J. Reprod. Infertil. 2015, 16, 123–129. [Google Scholar] [PubMed]
- D’Argenio, V.; Salvatore, F. The role of the gut microbiome in the healthy adult status. Clin. Chim. Acta 2015, 451, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Kell, D.B.; Pretorius, E. The Role of Lipopolysaccharide-Induced Cell Signalling in Chronic Inflammation. Chronic Stress 2022, 6, 24705470221076390. [Google Scholar] [CrossRef] [PubMed]
- Claudino Dos Santos, J.C.; Oliveira, L.F.; Noleto, F.M.; Gusmão, C.T.P.; Brito, G.A.C.; Viana, G.S.B. Gut-microbiome-brain axis: The crosstalk between the vagus nerve, alpha-synuclein and the brain in Parkinson’s disease. Neural Regen. Res. 2023, 18, 2611–2614. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xie, Z. Exploring the role of gut microbiome in male reproduction. Andrology 2022, 10, 441–450. [Google Scholar] [CrossRef]
- Mardanshahi, T.; Rezaei, N.; Zare, Z.; Malekzadeh Shafaroudi, M.; Mohammadi, H. Effects of L-Carnitine on the sperm parameters disorders, apoptosis of spermatogenic cells and testis histopathology in diabetic Rats. Int. J. Reprod. Biomed. 2018, 17, 325–336. [Google Scholar] [CrossRef]
- Aliabadi, E.; Soleimani Mehranjani, M.; Borzoei, Z.; Talaei-Khozani, T.; Mirkhani, H.; Tabesh, H. Effects of L-carnitine and L-acetyl-carnitine on testicular sperm motility and chromatin quality. Iran J. Reprod. Med. 2012, 10, 77–82. [Google Scholar]
- Abd-Allah, A.R.; Helal, G.K.; Al-Yahya, A.A.; Aleisa, A.M.; Al-Rejaie, S.S.; Al-Bakheet, S.A. Pro-inflammatory and oxidative stress pathways which compromise sperm motility and survival may be altered by L-carnitine. Oxid. Med. Cell. Longev. 2009, 2, 73–81. [Google Scholar] [CrossRef]
- Sjakste, N.; Gutcaits, A.; Kalvinsh, I. Mildronate: An antiischemic drug for neurological indications. CNS Drug Rev. 2005, 11, 151–168. [Google Scholar] [CrossRef]
- Heggermont, W.A.; Papageorgiou, A.P.; Heymans, S.; van Bilsen, M. Metabolic support for the heart: Complementary therapy for heart failure? Eur. J. Heart Fail. 2016, 18, 1420–1429. [Google Scholar] [CrossRef]
- Isajevs, S.; Isajeva, D.; Beitnere, U.; Jansone, B.; Kalvinsh, I.; Klusa, V. Mildronate as a regulator of protein expression in a rat model of Parkinson’s disease. Medicina 2011, 47, 79. [Google Scholar] [CrossRef] [PubMed]
- Beitnere, U.; van Groen, T.; Kumar, A.; Jansone, B.; Klusa, V.; Kadish, I. Mildronate improves cognition and reduces amyloid-β pathology in transgenic Alzheimer’s disease mice. J. Neurosci. Res. 2014, 92, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Bezuglov, E.; Talibov, O.; Butovskiy, M.; Khaitin, V.; Achkasov, E.; Waśkiewicz, Z.; Lazarev, A. The Inclusion in WADA Prohibited List Is Not Always Supported by Scientific Evidence: A Narrative Review. Asian J. Sports Med. 2021, 12, e110753. [Google Scholar] [CrossRef]
- Liepinsh, E.; Konrade, I.; Skapare, E.; Pugovics, O.; Grinberga, S.; Kuka, J.; Kalvinsh, I.; Dambrova, M. Mildronate treatment alters γ-butyrobetaine and l-carnitine concentrations in healthy volunteers. J. Pharm. Pharmacol. 2011, 63, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xu, J.; Zheng, J.; Zhang, X.; Shao, J.; Zhao, L.; Hao, J. Anti-Inflammatory and Antioxidant Effects of Acetyl-L-Carnitine on Atherosclerotic Rats. Med. Sci. Monit. 2020, 26, e920250-1. [Google Scholar] [CrossRef] [PubMed]
- Zhitkova, V.S.; Khramtsova, Y.S. The influence of excessive physical activity on spermatogenesis in rats against the background of the introduction of mildronate Youth and science in the North. In Proceedings of the III All-Russian (XVIII) Youth Scientific Conference, Rep. Komi, Syktyvkar, Russia, 12–16 March 2018. [Google Scholar]
- Dambrova, M.; Cirule, H.; Svalbe, B.; Zvejniece, L.; Pugovichs, O.; Zorenko, T.; Kalvinsh, I.; Liepinsh, E.; Belozertseva, I. Effect of inhibiting carnitine biosynthesis on male rat sexual performance. Physiol. Behav. 2008, 95, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Dambrova, M.; Skapare-Makarova, E.; Konrade, I.; Pugovics, O.; Grinberga, S.; Tirzite, D.; Petrovska, R.; Kalvins, I.; Liepins, E. Meldonium decreases the diet-increased plasma levels of trimethylamine N-oxide, a metabolite associated with atherosclerosis. J. Clin. Pharmacol. 2013, 53, 1095–1098. [Google Scholar] [CrossRef]
- Berlato, D.G.; Bairros, A.V. Meldonium: Pharmacological, toxicological, and analytical aspects. Toxicol. Res. Appl. 2020, 4, 2397847320915143. [Google Scholar] [CrossRef]
- Duttaroy, A.K. Role of Gut Microbiota and Their Metabolites on Atherosclerosis, Hypertension and Human Blood Platelet Function: A Review. Nutrients 2021, 13, 144. [Google Scholar] [CrossRef]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef]
- Gureev, A.P.; Shaforostova, E.A.; Vitkalova, I.Y.; Sadovnikova, I.S.; Kalinina, Y.I.; Cherednichenko, V.R.; Reznikova, K.A.; Valuyskikh, V.V.; Popov, V.N. Long-term mildronate treatment increased Proteobacteria level in gut microbiome, and caused behavioral deviations and transcriptome change in liver, heart and brain of healthy mice. Toxicol. Appl. Pharmacol. 2020, 398, 115031. [Google Scholar] [CrossRef] [PubMed]
- Semet, M.; Paci, M.; Saïas-Magnan, J.; Metzler-Guillemain, C.; Boissier, R.; Lejeune, H.; Perrin, J. The impact of drugs on male fertility: A review. Andrology 2017, 5, 640–663. [Google Scholar] [CrossRef] [PubMed]
- Ajayi, A.; Akhigbe, R.E. The physiology of male reproduction: Impact of drugs and their abuse on male fertility. Andrologia 2020, 52, e13672. [Google Scholar] [CrossRef] [PubMed]
- Gureev, A.P.; Shaforostova, E.A.; Starkov, A.A.; Popov, V.N. Simplified qPCR method for detecting excessive mtDNA damage induced by exogenous factors. Toxicology 2017, 382, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, G.; Brukman, N.G.; Weigel Muñoz, M.; Battistone, M.A.; Guazzone, V.A.; Ikawa, M.; Haruhiko, M.; Lustig, L.; Breton, S.; Cuasnicu, P.S. Impaired male fertility and abnormal epididymal epithelium differentiation in mice lacking CRISP1 and CRISP4. Sci. Rep. 2018, 8, 17531. [Google Scholar] [CrossRef] [PubMed]
- Belkina, A.C.; Nikolajczyk, B.S.; Denis, G.V. BET protein function is required for inflammation: Brd2 genetic disruption and BET inhibitor JQ1 impair mouse macrophage inflammatory responses. J. Immunol. 2013, 190, 3670–3678. [Google Scholar] [CrossRef] [PubMed]
- Mruk, D.D.; Silvestrini, B.; Mo, M.Y.; Cheng, C.Y. Antioxidant superoxide dismutase—A review: Its function, regulation in the testis, and role in male fertility. Contraception 2002, 65, 305–311. [Google Scholar] [CrossRef]
- Lu, S.C. Glutathione synthesis. Biochim. Biophys. Acta 2013, 1830, 3143–3153. [Google Scholar] [CrossRef]
- Holzerova, E.; Danhauser, K.; Haack, T.B.; Kremer, L.S.; Melcher, M.; Ingold, I.; Kobayashi, S.; Terrile, C.; Wolf, P.; Schaper, J.; et al. Human thioredoxin 2 deficiency impairs mitochondrial redox homeostasis and causes early-onset neurodegeneration. Brain 2016, 139, 346–354. [Google Scholar] [CrossRef]
- Chen, L.; Na, R.; Gu, M.; Salmon, A.B.; Liu, Y.; Liang, H.; Qi, W.; Van Remmen, H.; Richardson, A.; Ran, Q. Reduction of mitochondrial H2O2 by overexpressing peroxiredoxin 3 improves glucose tolerance in mice. Aging Cell 2008, 7, 866–878. [Google Scholar] [CrossRef]
- Li, R.; Xie, J.; Xu, W.; Zhang, L.; Lin, H.; Huang, W. LPS-induced PTGS2 manipulates the inflammatory response through trophoblast invasion in preeclampsia via NF-κB pathway. Reprod. Biol. 2022, 22, 100696. [Google Scholar] [CrossRef] [PubMed]
- Morgan, X.C.; Tickle, T.L.; Sokol, H.; Gevers, D.; Devaney, K.L.; Ward, D.V.; Reyes, J.A.; Shah, S.A.; LeLeiko, N.; Snapper, S.B.; et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012, 13, R79. [Google Scholar] [CrossRef]
- Kelly, T.N.; Bazzano, L.A.; Ajami, N.J.; He, H.; Zhao, J.; Petrosino, J.F.; Correa, A.; He, J. Gut Microbiome Associates with Lifetime Cardiovascular Disease Risk Profile Among Bogalusa Heart Study Participants. Circ. Res. 2016, 119, 956–964. [Google Scholar] [CrossRef]
- Oh, J.K.; Vasquez, R.; Kim, S.H.; Hwang, I.C.; Song, J.H.; Park, J.H.; Kim, I.H.; Kang, D.K. Multispecies probiotics alter fecal short-chain fatty acids and lactate levels in weaned pigs by modulating gut microbiota. J. Anim. Sci. Technol. 2021, 63, 1142–1158. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Vitetta, L. The Role of Butyrate in Attenuating Pathobiont-Induced Hyperinflammation. Immune Netw. 2020, 20, e15. [Google Scholar] [CrossRef] [PubMed]
- Cretoiu, D.; Ionescu, R.F.; Enache, R.M.; Cretoiu, S.M.; Voinea, S.C. Gut Microbiome, Functional Food, Atherosclerosis, and Vascular Calcifications-Is There a Missing Link? Microorganisms 2021, 9, 1913. [Google Scholar] [CrossRef]
- Coutinho-Wolino, K.S.; de F Cardozo, L.F.M.; de Oliveira Leal, V.; Mafra, D.; Stockler, M.B. Can diet modulate trimethylamine N-oxide (TMAO) production? What do we know so far? Eur. J. Nutr. 2021, 60, 3567–3584. [Google Scholar] [CrossRef]
- Roncal, C.; Martínez-Aguilar, E.; Orbe, J.; Ravassa, S.; Fernandez-Montero, A.; Saenz-Pipaon, G.; Ugarte, A.; Estella-Hermoso de Mendoza, A.; Rodriguez, J.A.; Fernández-Alonso, S.; et al. Trimethylamine-N-Oxide (TMAO) Predicts Cardiovascular Mortality in Peripheral Artery Disease. Sci. Rep. 2019, 9, 15580. [Google Scholar] [CrossRef]
- Ghonimy, A.; Zhang, D.M.; Farouk, M.H.; Wang, Q. The Impact of Carnitine on Dietary Fiber and Gut Bacteria Metabolism and Their Mutual Interaction in Monogastrics. Int. J. Mol. Sci. 2018, 19, 1008. [Google Scholar] [CrossRef]
- Sadovnikova, I.S.; Gureev, A.P.; Ignatyeva, D.A.; Gryaznova, M.V.; Chernyshova, E.V.; Krutskikh, E.P.; Novikova, A.G.; Popov, V.N. Nrf2/ARE Activators Improve Memory in Aged Mice via Maintaining of Mitochondrial Quality Control of Brain and the Modulation of Gut Microbiome. Pharmaceuticals 2021, 14, 607. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gureev, A.P.; Babenkova, P.I.; Nesterova, V.V.; Tsvetkova, A.D.; Gryaznova, M.V.; Shaforostova, E.A. Protection of Testis against Lipopolysaccharide-Induced Toxicity: Mildronate-Induced L-Carnitine Depletion as a Modulator of Gut Microbiome Composition and Gastrointestinal Inflammation. Gastrointest. Disord. 2023, 5, 536-548. https://doi.org/10.3390/gidisord5040044
Gureev AP, Babenkova PI, Nesterova VV, Tsvetkova AD, Gryaznova MV, Shaforostova EA. Protection of Testis against Lipopolysaccharide-Induced Toxicity: Mildronate-Induced L-Carnitine Depletion as a Modulator of Gut Microbiome Composition and Gastrointestinal Inflammation. Gastrointestinal Disorders. 2023; 5(4):536-548. https://doi.org/10.3390/gidisord5040044
Chicago/Turabian StyleGureev, Artem P., Polina I. Babenkova, Veronika V. Nesterova, Arina D. Tsvetkova, Mariya V. Gryaznova, and Ekaterina A. Shaforostova. 2023. "Protection of Testis against Lipopolysaccharide-Induced Toxicity: Mildronate-Induced L-Carnitine Depletion as a Modulator of Gut Microbiome Composition and Gastrointestinal Inflammation" Gastrointestinal Disorders 5, no. 4: 536-548. https://doi.org/10.3390/gidisord5040044





