Kinetic Modeling for the “One-Pot” Hydrogenolysis of Cellulose to Glycols over Ru@Fe3O4/Polymer Catalyst
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Catalyst Synthesis
2.3. Characterization
2.4. Catalyst TESTING Procedure and Product Analysis
3. Results
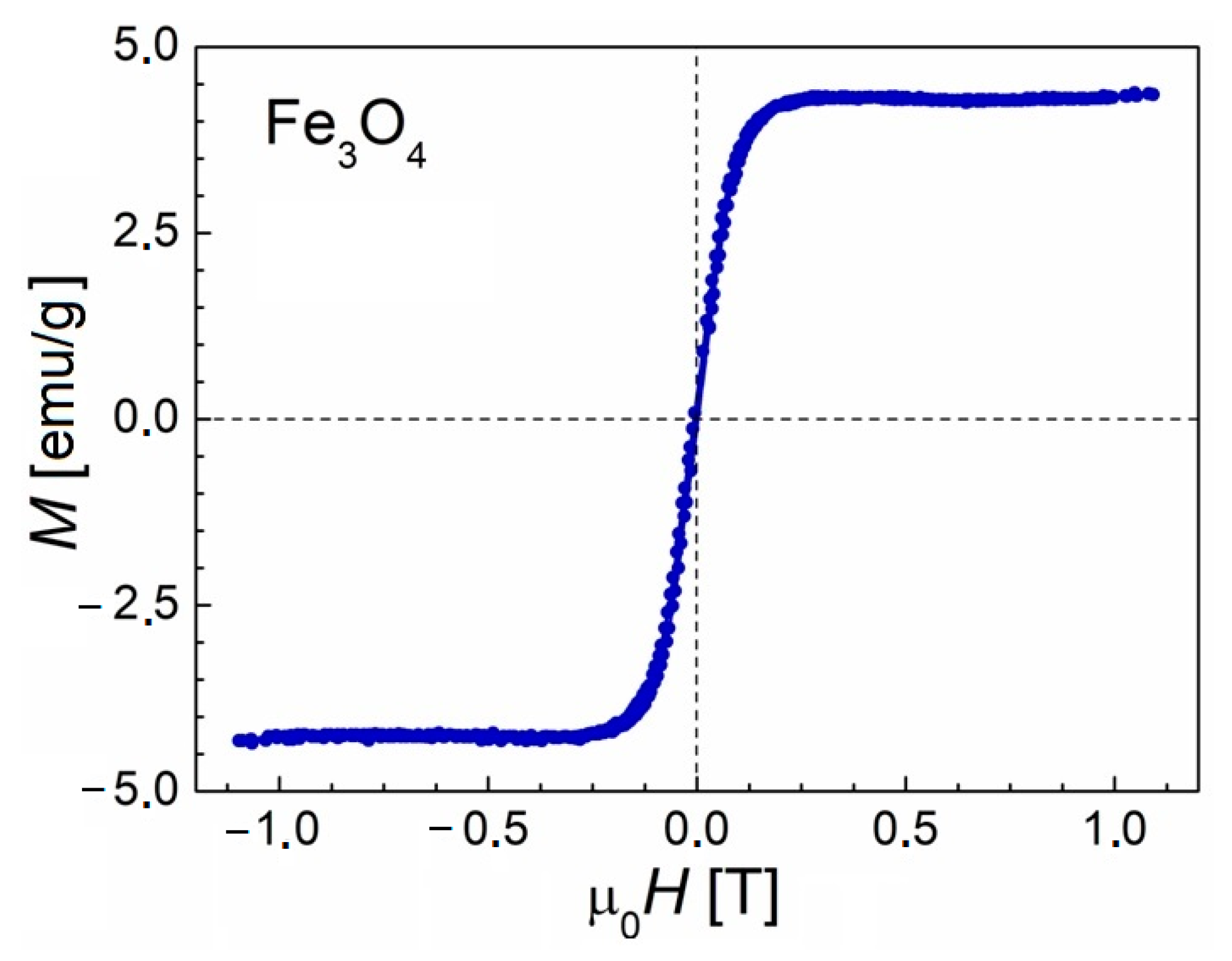
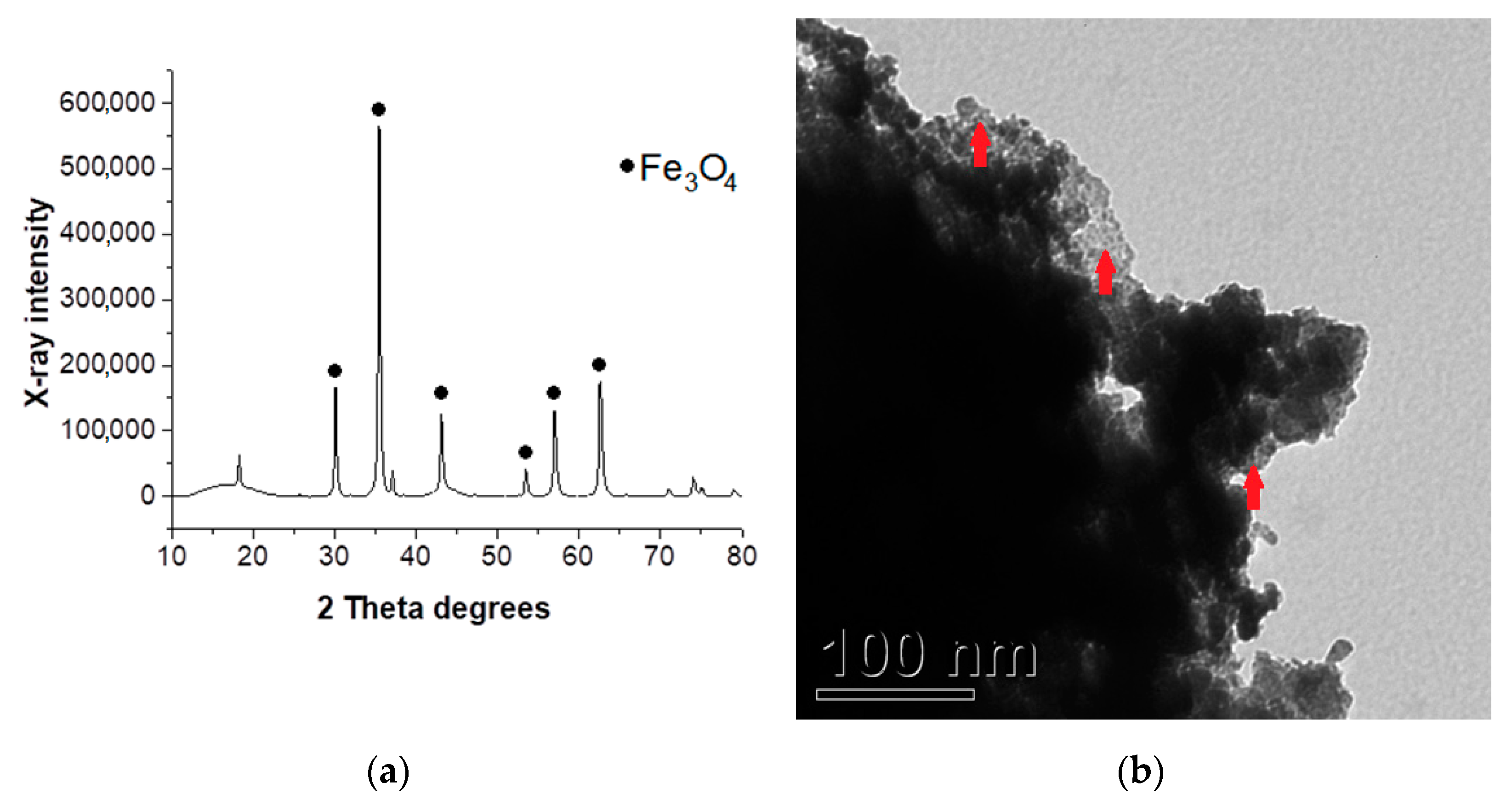
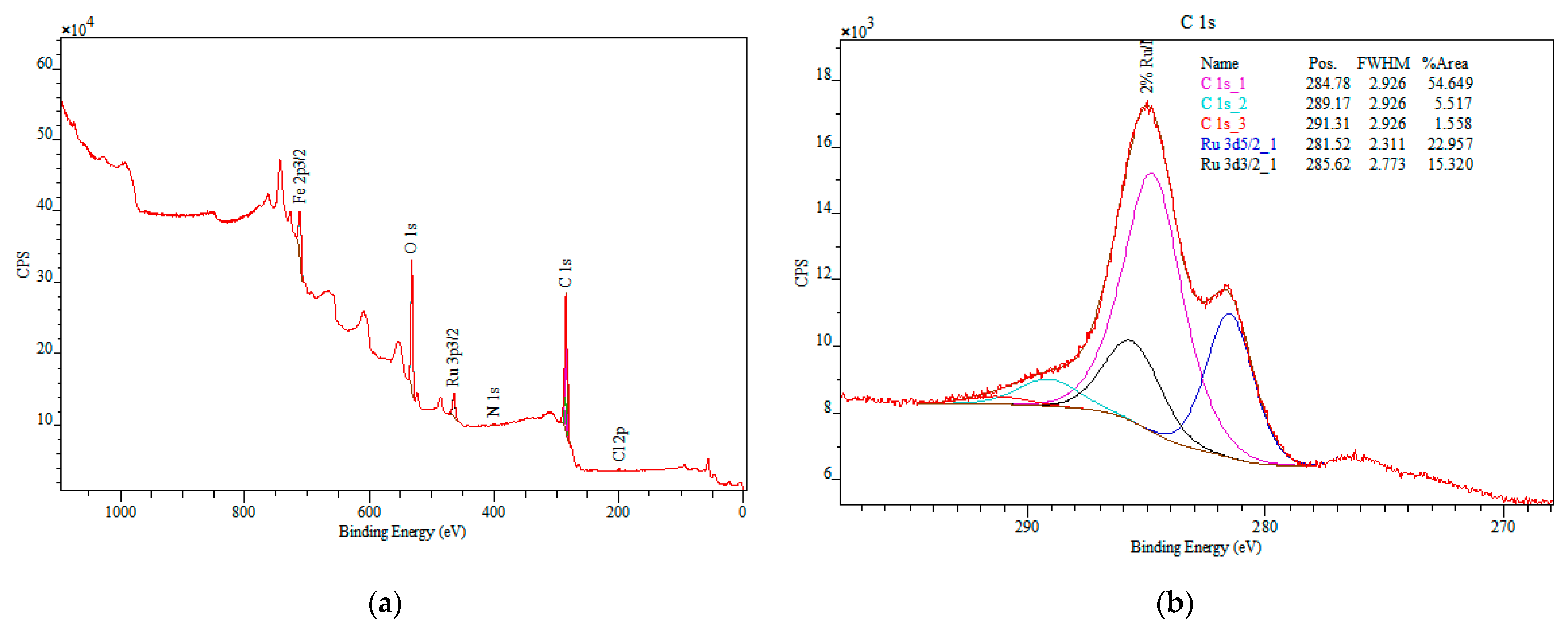
3.1. Catalyst Characterization
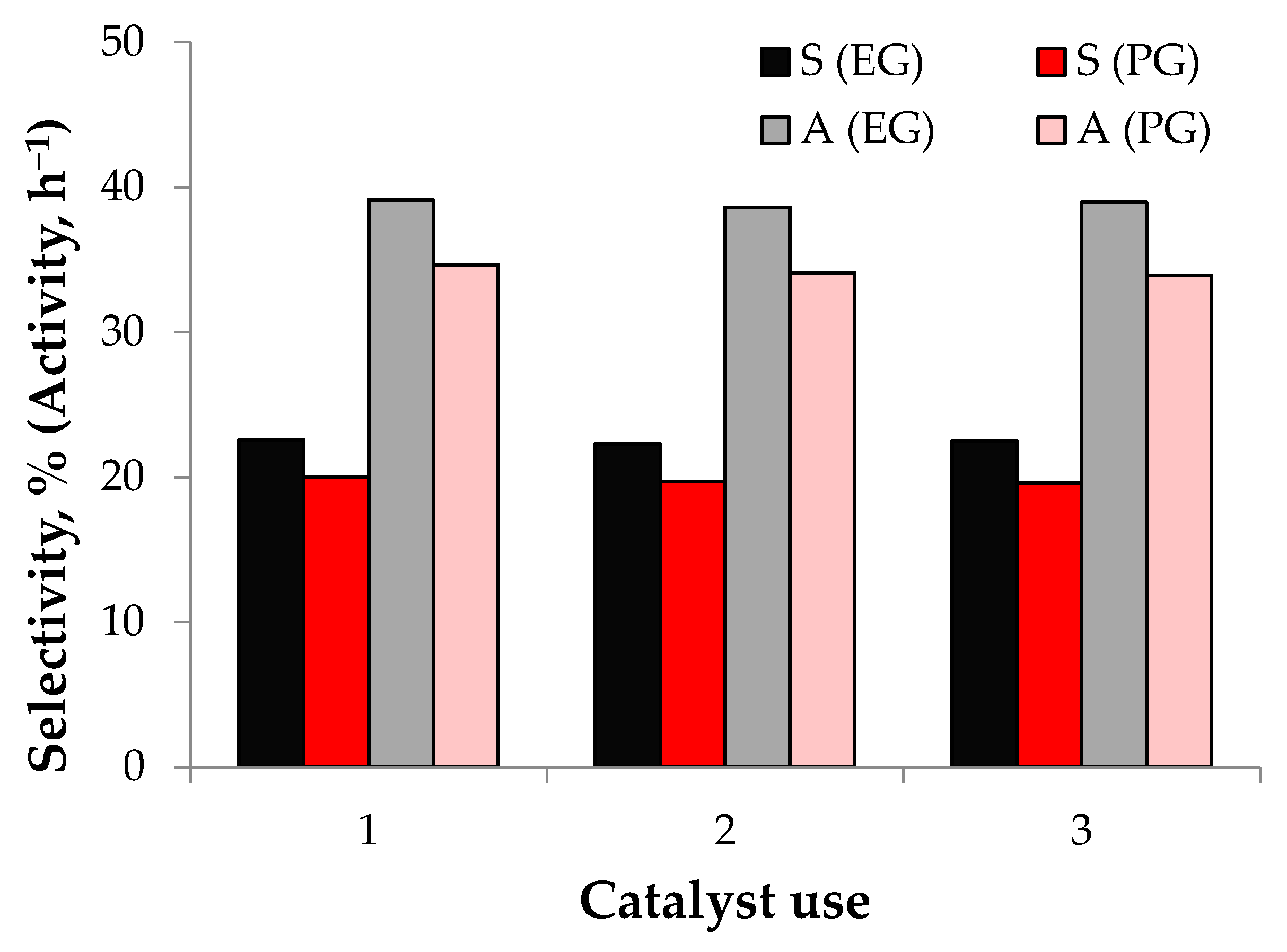
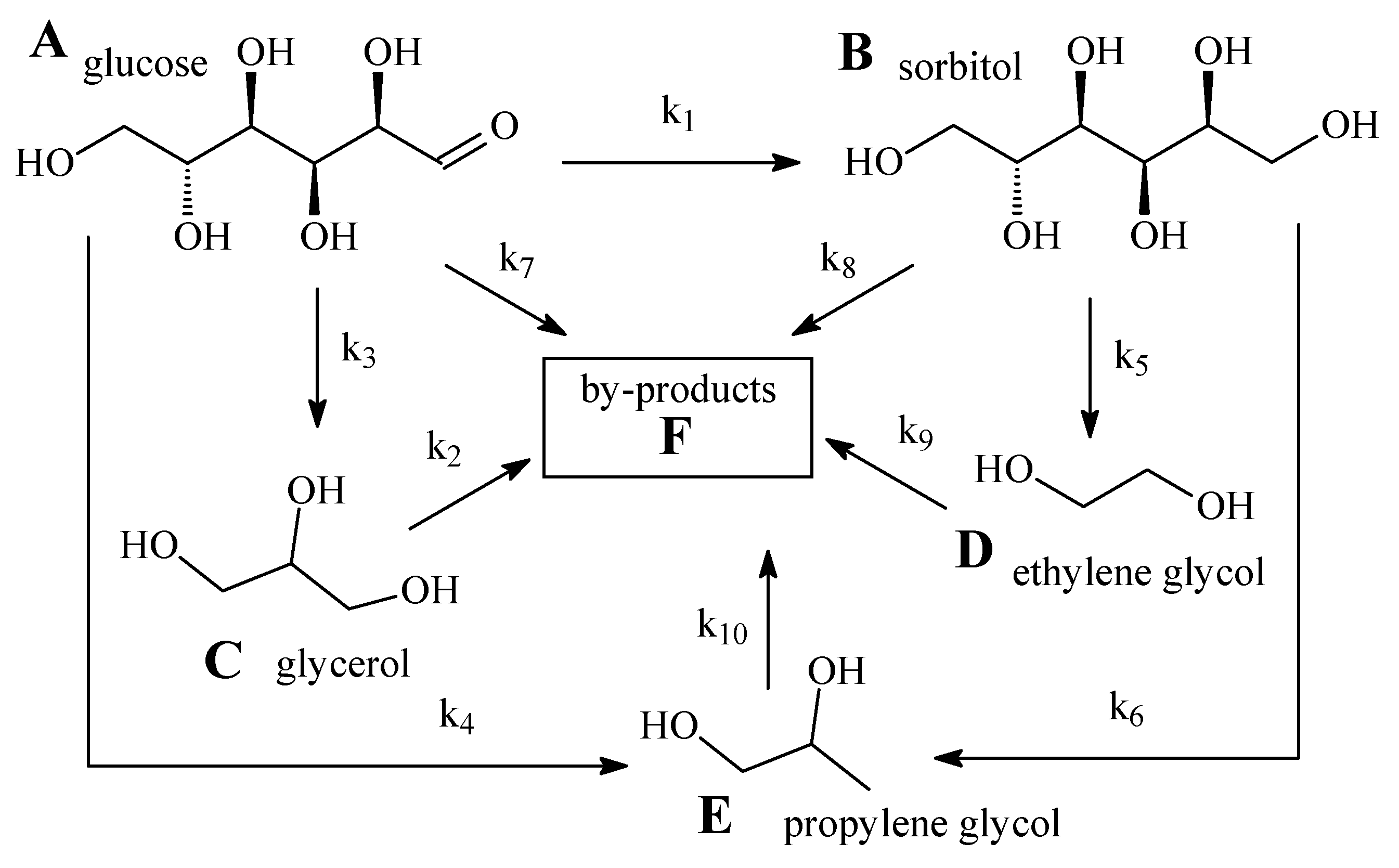
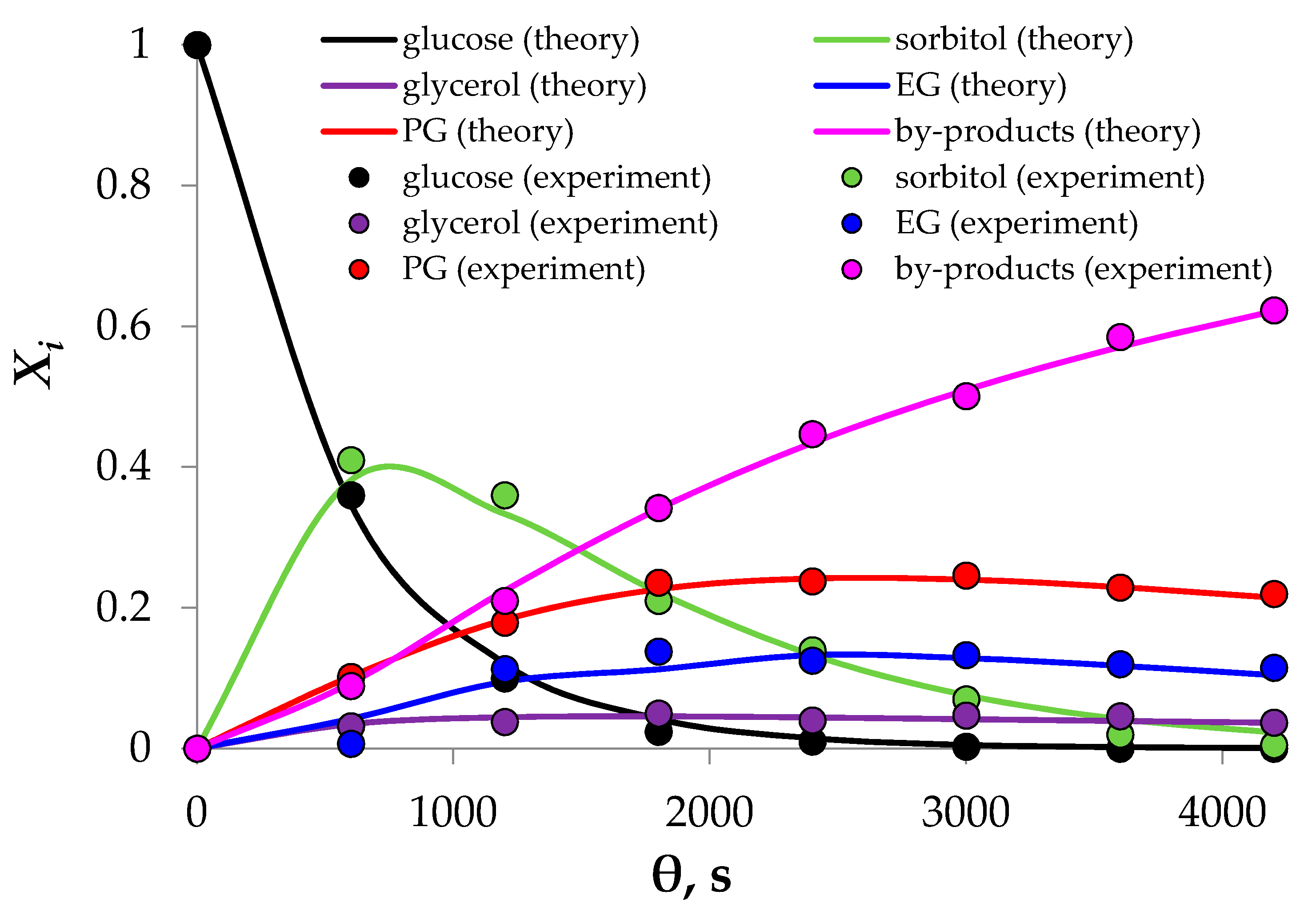
3.2. Cellulose Hydrogenolysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xin, H.; Hu, X.; Cai, C.; Wang, H.; Zhu, C.; Li, S.; Xiu, Z.; Zhang, X.; Liu, Q.; Ma, L. Catalytic Production of Oxygenated and Hydrocarbon Chemicals From Cellulose Hydrogenolysis in Aqueous Phase. Front. Chem. 2020, 8, 146. [Google Scholar] [CrossRef]
- Cai, C.; Wang, H.; Xin, H.; Zhu, C.; Zhang, Q.; Zhang, X.; Wang, C.; Liu, Q.; Ma, L. Hydrogenolysis of biomass-derived sorbitol over La-promoted Ni/ZrO2 catalysts. RSC Adv. 2020, 10, 3993. [Google Scholar] [CrossRef] [Green Version]
- Godina, L.I.; Kirilin, A.V.; Tokarev, A.V.; Simakova, I.L.; Murzin, D.Y. Sibunit-Supported Mono- and Bimetallic Catalysts Used in Aqueous-Phase Reforming of Xylitol. Ind. Eng. Chem. Res. 2018, 57, 2050–2067. [Google Scholar] [CrossRef]
- Mounguengui-Diallo, M.; Sadier, A.; Noly, E.; Da Silva Perez, D.; Pinel, C.; Perret, N.; Besson, M. C-O Bond Hydrogenolysis of Aqueous Mixtures of Sugar Polyols and Sugars over ReOx-Rh/ZrO2 Catalyst: Application to an Hemicelluloses Extracted Liquor. Catalysts 2019, 9, 740. [Google Scholar] [CrossRef] [Green Version]
- Yue, H.; Zhao, Y.; Ma, X.; Gong, J. Ethylene glycol: Properties, synthesis, and applications. Chem. Soc. Rev. 2012, 41, 4218–4244. [Google Scholar] [CrossRef]
- Zheng, M.; Pang, J.; Sun, R.; Wang, A.; Zhang, T. Selectivity Control for Cellulose to Diols: Dancing on Eggs. ACS Catal. 2017, 7, 1939–1954. [Google Scholar] [CrossRef]
- Gu, M.; Shen, Z.; Yang, L.; Dong, W.; Kong, L.; Zhang, W.; Peng, B.-Y.; Zhang, Y. Reaction Route Selection for Cellulose Hydrogenolysis into C2/C3 Glycols by ZnO-Modified Ni-W/β-zeolite Catalysts. Sci. Rep. 2019, 9, 11938. [Google Scholar] [CrossRef]
- te Molder, T.D.J.; Kersten, S.R.A.; Lange, J.P.; Ruiz, M.P. Ethylene Glycol from Lignocellulosic Biomass: Impact of Lignin on Catalytic Hydrogenolysis. Ind. Eng. Chem. Res. 2021, 60, 7043–7049. [Google Scholar] [CrossRef]
- Ribeiro, L.S.; Orfao, J.J.M.; Pereira, M.F.R. Insights into the effect of the catalytic functions on selective production of ethylene glycol from lignocellulosic biomass over carbon supported ruthenium and tungsten catalysts. Bioresour. Technol. 2018, 263, 402–409. [Google Scholar] [CrossRef]
- Seretis, A.; Diamantopoulou, P.; Thanou, I.; Tzevelekidis, P.; Fakas, C.; Lilas, P.; Papadogianakis, G. Recent Advances in Ruthenium-Catalyzed Hydrogenation Reactions of Renewable Biomass-Derived Levulinic Acid in Aqueous Media. Front. Chem. 2020, 8, 221. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Guan, J.; Li, B.; Wang, X.; Mu, X.; Liu, H. Conversion of biomass-derived sorbitol to glycols over carbon materials supported Ru-based catalysts. Sci. Rep. 2015, 5, 16451–16460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Liu, G.; Sui, Z.; Zhou, X.; Yuan, W. Hydrogenolysis of sorbitol to glycols over carbon nanofibers- supported ruthenium catalyst: The role of base promoter. Chin. J. Catal. 2014, 35, 692–702. [Google Scholar] [CrossRef]
- Pang, J.; Zhang, B.; Jiang, Y.; Zhao, Y.; Li, C.; Zheng, M.; Zhang, T. Complete conversion of lignocellulosic biomass to mixed organic acids and ethylene glycol via cascade steps. Green Chem. 2021, 23, 2427–2436. [Google Scholar] [CrossRef]
- Shifrina, Z.B.; Bronstein, L.M. Magnetically Recoverable Catalysts: Beyond Magnetic Separation. Front. Chem. 2018, 6, 298. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Z. Catalytic Conversion of Biomass into Chemicals and Fuels over Magnetic Catalysts. ACS Catal. 2016, 6, 326–338. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Qi, W.; Shi, J.; Zhang, J.; Xu, Y.; Pang, J. Preparation of magnetic biomass-based solid acid catalyst and effective catalytic conversion of cellulose into high yields of reducing sugar. BioRes 2015, 10, 6720–6729. [Google Scholar] [CrossRef]
- Espro, C.; Gumina, B.; Paone, E.; Mauriello, F. Upgrading Lignocellulosic Biomasses: Hydrogenolysis of Platform Derived Molecules Promoted by Heterogeneous Pd-Fe Catalysts. Catalysts 2017, 7, 78. [Google Scholar] [CrossRef]
- García-Sancho, C.; Luque, R. Editorial Catalysts: Special Issue on Heterogeneous Catalysis for Valorization of Lignocellulosic Biomass. Catalysts 2021, 11, 649. [Google Scholar] [CrossRef]
- Shifrina, Z.B.; Matveeva, V.G.; Bronstein, L.M. Role of Polymer Structures in Catalysis by Transition Metal and Metal Oxide Nanoparticle Composites. Chem. Rev. 2020, 120, 1350–1396. [Google Scholar] [CrossRef]
- Axet, M.R.; Philippot, K. Catalysis with Colloidal Ruthenium Nanoparticles. Chem. Rev. Am. Chem. Soc. 2020, 120, 1085–1145. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Tan, B. Hypercrosslinked porous polymer materials: Design, synthesis, and applications. Chem. Soc. Rev. 2017, 46, 3322. [Google Scholar] [CrossRef] [PubMed]
- Sidorov, S.N.; Volkov, I.V.; Davankov, V.A.; Tsyurupa, M.P.; Valetsky, P.M.; Bronstein, L.M.; Karlinsey, R.; Zwanziger, J.W.; Matveeva, V.G.; Sulman, E.M.; et al. Platinum-Containing Hyper-Cross-Linked Polystyrene as a Modifier-Free Selective Catalyst for L-Sorbose Oxidation. J. Am. Chem. Soc. 2001, 123, 10502–10510. [Google Scholar] [CrossRef]
- Bronstein, L.M.; Goerigk, G.; Kostylev, M.; Pink, M.; Khotina, I.A.; Valetsky, P.M.; Matveeva, V.G.; Sulman, E.M.; Sulman, M.G.; Bykov, A.V.; et al. Structure and Catalytic Properties of Pt-Modified Hyper-Cross-Linked Polystyrene Exhibiting Hierarchical Porosity. J. Phys. Chem. B 2004, 108, 18234–18242. [Google Scholar] [CrossRef] [Green Version]
- Nikoshvili, L.Z.; Bykov, A.V.; Khudyakova, T.E.; LaGrange, T.; Heroguel, F.; Luterbacher, J.S.; Matveeva, V.G.; Sulman, E.M.; Dyson, P.J.; Kiwi-Minsker, L. Promotion Effect of Alkali Metal Hydroxides on Polymer-Stabilized Pd Nanoparticles for Selective Hydrogenation of C-C Triple Bonds in Alkynols. Ind. Eng. Chem. Res. 2017, 56, 13219–13227. [Google Scholar] [CrossRef] [Green Version]
- Nemygina, N.A.; Nikoshvili, L.Z.; Tiamina, I.Y.; Bykov, A.V.; Smirnov, I.S.; LaGrange, T.; Kaszkur, Z.; Matveeva, V.G.; Sulman, E.M.; Kiwi-Minsker, L. Au Core-Pd Shell Bimetallic Nanoparticles Immobilized within Hyper-Cross-Linked Polystyrene for Mechanistic Study of Suzuki Cross-Coupling: Homogeneous or Heterogeneous Catalysis? Org. Process Res. Dev. 2018, 22, 1606–1613. [Google Scholar] [CrossRef]
- Matveeva, V.G.; Sulman, E.M.; Manaenkov, O.V.; Filatova, A.E.; Kislitza, O.V.; Sidorov, A.I.; Doluda, V.Y.; Sulman, M.G.; Rebrov, E.V. Hydrolytic Hydrogenation of Cellulose in Subcritical Water with the Use of the Ru-Containing Polymeric Catalysts. Catal. Today 2017, 280, 45–50. [Google Scholar] [CrossRef]
- Manaenkov, O.; Mann, J.; Kislitza, O.; Losovyj, Y.; Stein, B.; Morgan, D.; Pink, M.; Lependina, O.; Shifrina, Z.; Matveeva, V.; et al. Ru-containing Magnetically Recoverable Catalysts: A Sustainable Pathway from Cellulose to Ethylene and Propylene Glycols. ACS Appl. Mater. Interfaces 2016, 8, 21285–21293. [Google Scholar] [CrossRef] [Green Version]
- Schlaf, M.; Zhang, Z.C. Reaction Pathways and Mechanisms in Thermocatalytic Biomass Conversion I. In Mechanism and Kinetic Analysis of the Hydrogenolysis of Cellulose to Polyols; Chapter 9; Springer: Singapore, 2016; pp. 227–260. [Google Scholar] [CrossRef]
- Tajvidi, K.; Hausoul, P.J.C.; Palkovits, R. Hydrogenolysis of Cellulose over Cu-Based Catalysts—Analysis of the Reaction Network. ChemSusChem 2014, 7, 1311–1317. [Google Scholar] [CrossRef] [PubMed]
- Schlögl, R. Heterogeneous Catalysis. Angew. Chem. Int. Ed. 2015, 54, 3465–3520. [Google Scholar] [CrossRef] [Green Version]
- Barbato, M.; Bruno, C. Heterogeneous Catalysis: Theory, Models and Applications. Mol. Phys. Hypersonic Flows 1996, 139–160. [Google Scholar] [CrossRef]

| Sample | SBET, m2/g | SL, m2/g | St, m2/g | V, cm3/g |
|---|---|---|---|---|
| HPS | 1075 | 1191 | 265 (1); 807 (2); 1072 | 0.37 |
| Fe3O4/HPS | 450 | 480 | 160 (1); 289 (2); 449 | 0.13 |
| Ru@Fe3O4/HPS | 364 | 392 | 175 (1); 189 (2); 364 | 0.08 |
| Element | C 1s | O 1s | N 1s | Cl 2p | Ru 3p3/2 | Fe 2p3/2 |
|---|---|---|---|---|---|---|
| %at/%wt. | 66.8/45.5 | 25.8/23.5 | 0.2/0.2 | 0.4/0.8 | 3.3/18.9 | 3.5/11.1 |
| Catalyst | Selectivity, % | Specific Catalytic Activity Calculated as a Gram of EG or PG per Gram of Ru per Hour, h−1 | ||
|---|---|---|---|---|
| EG | PG | EG | PG | |
| Ru@Fe3O4/HPS | 22.6 | 20.0 | 39.12 | 34.62 |
| 5% Ru-Fe3O4/SiO2 | 19.1 | 20.9 | 25.29 | 27.72 |
| 3% Ru/HPS | 7.4 | 12.5 | 7.51 | 12.71 |
| Parameter, (mol/mol)n·s−1 | Value | Parameter, (mol/mol)n·s−1 | Value |
|---|---|---|---|
| k1 | 1.47 ± 0.07 × 10−3 | k6 | 3.41 ± 0.17 × 10−4 |
| k2 | 1.14 ± 0.06 × 10−4 | k7 | 4.55 ± 0.23 × 10−5 |
| k3 | 9.72 ± 0.49 × 10−5 | k8 | 4.35 ± 0.22 × 10−4 |
| k4 | 1.45 ± 0.07 × 10−4 | k9 | 2.81 ± 0.14 × 10−4 |
| k5 | 2.90 ± 0.14 × 10−4 | k10 | 1.61 ± 0.08 × 10−4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manaenkov, O.; Kosivtsov, Y.; Sapunov, V.; Kislitsa, O.; Sulman, M.; Bykov, A.; Sidorov, A.; Matveeva, V. Kinetic Modeling for the “One-Pot” Hydrogenolysis of Cellulose to Glycols over Ru@Fe3O4/Polymer Catalyst. Reactions 2022, 3, 1-11. https://doi.org/10.3390/reactions3010001
Manaenkov O, Kosivtsov Y, Sapunov V, Kislitsa O, Sulman M, Bykov A, Sidorov A, Matveeva V. Kinetic Modeling for the “One-Pot” Hydrogenolysis of Cellulose to Glycols over Ru@Fe3O4/Polymer Catalyst. Reactions. 2022; 3(1):1-11. https://doi.org/10.3390/reactions3010001
Chicago/Turabian StyleManaenkov, Oleg, Yuriy Kosivtsov, Valentin Sapunov, Olga Kislitsa, Mikhail Sulman, Alexey Bykov, Alexander Sidorov, and Valentina Matveeva. 2022. "Kinetic Modeling for the “One-Pot” Hydrogenolysis of Cellulose to Glycols over Ru@Fe3O4/Polymer Catalyst" Reactions 3, no. 1: 1-11. https://doi.org/10.3390/reactions3010001






