Biofuels from Pyrolysis of Third-Generation Biomass from Household and Garden Waste Composting Bin: Kinetics Analysis
Abstract
:1. Introduction


2. Experimental Section
2.1. Materials and Methods
2.2. Estimation of the Biomass Composition and Kinetic Studies
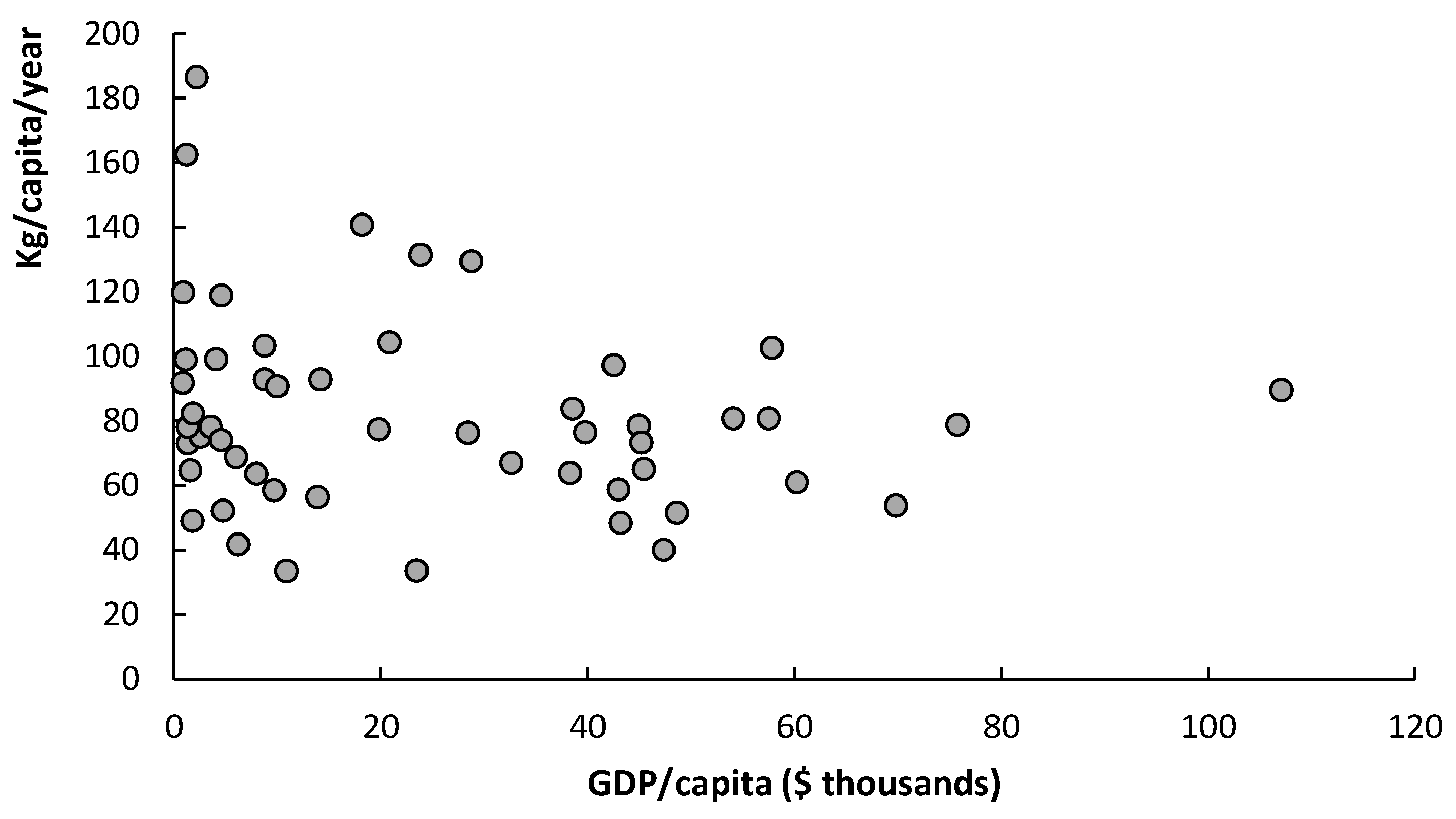
3. Results and Discussion
3.1. Biomass Characterization
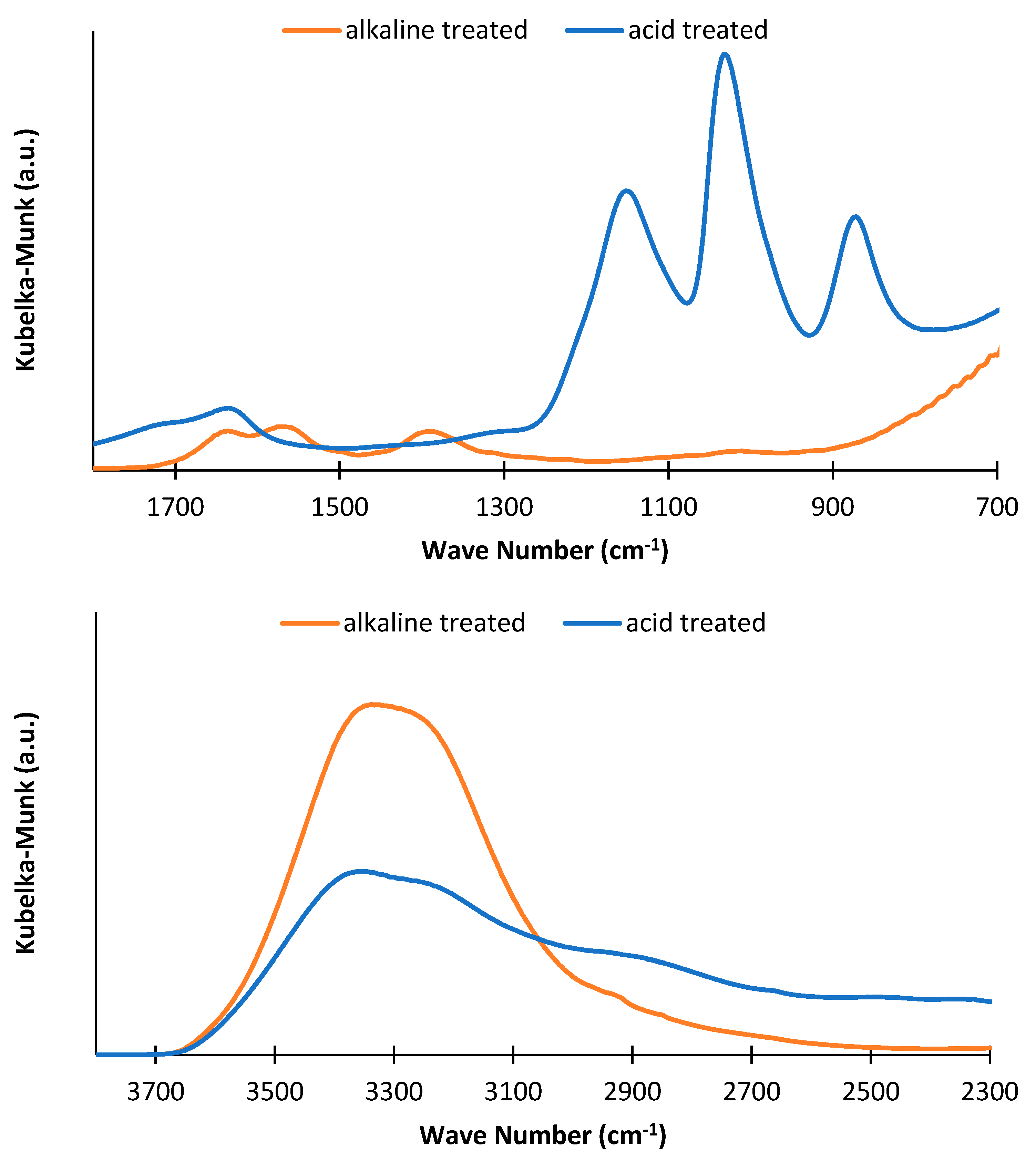
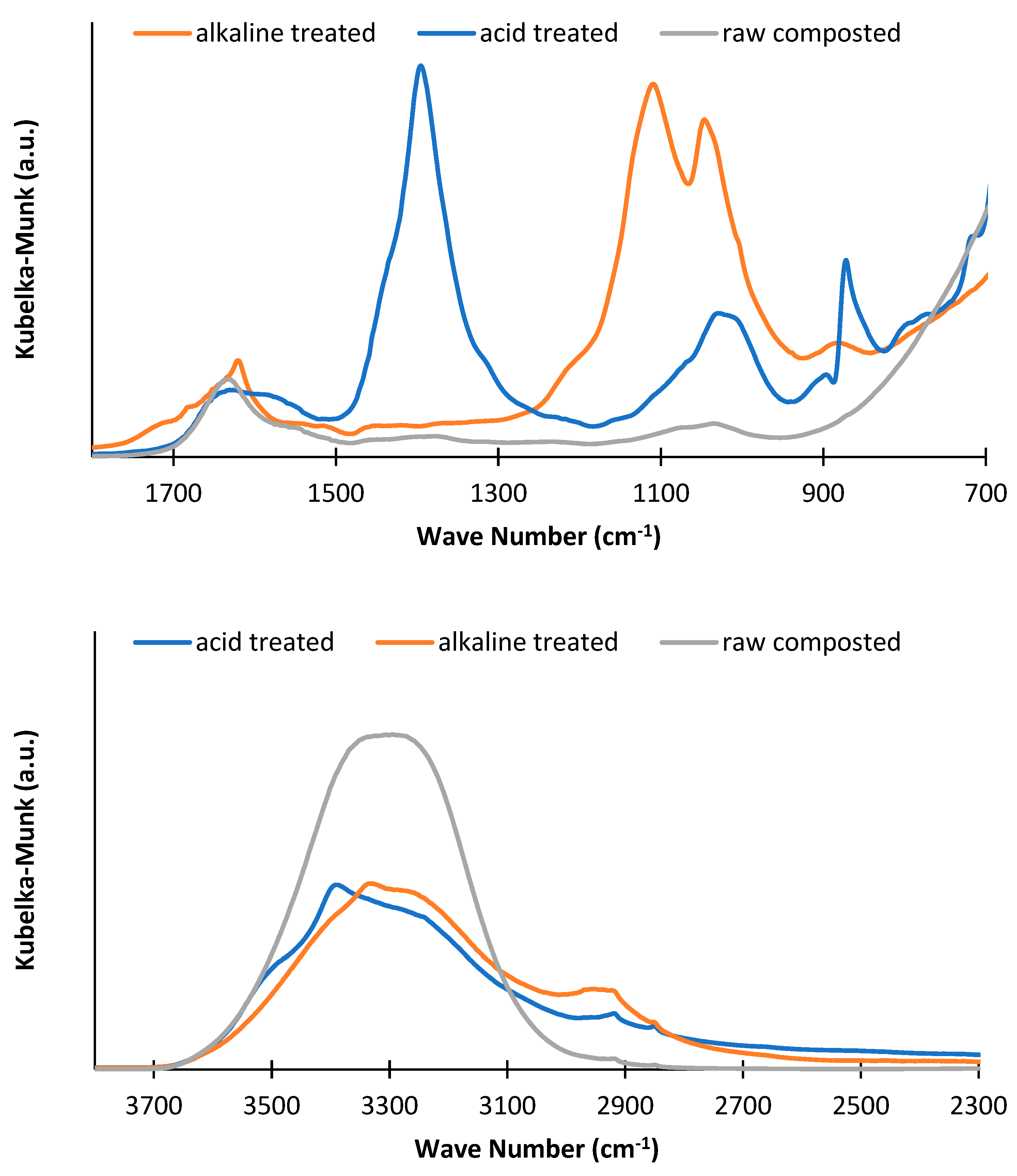
3.2. Infrared Spectroscopy
3.2.1. Liquid Fraction from Treatments
3.2.2. Solid Fractions after Treatments
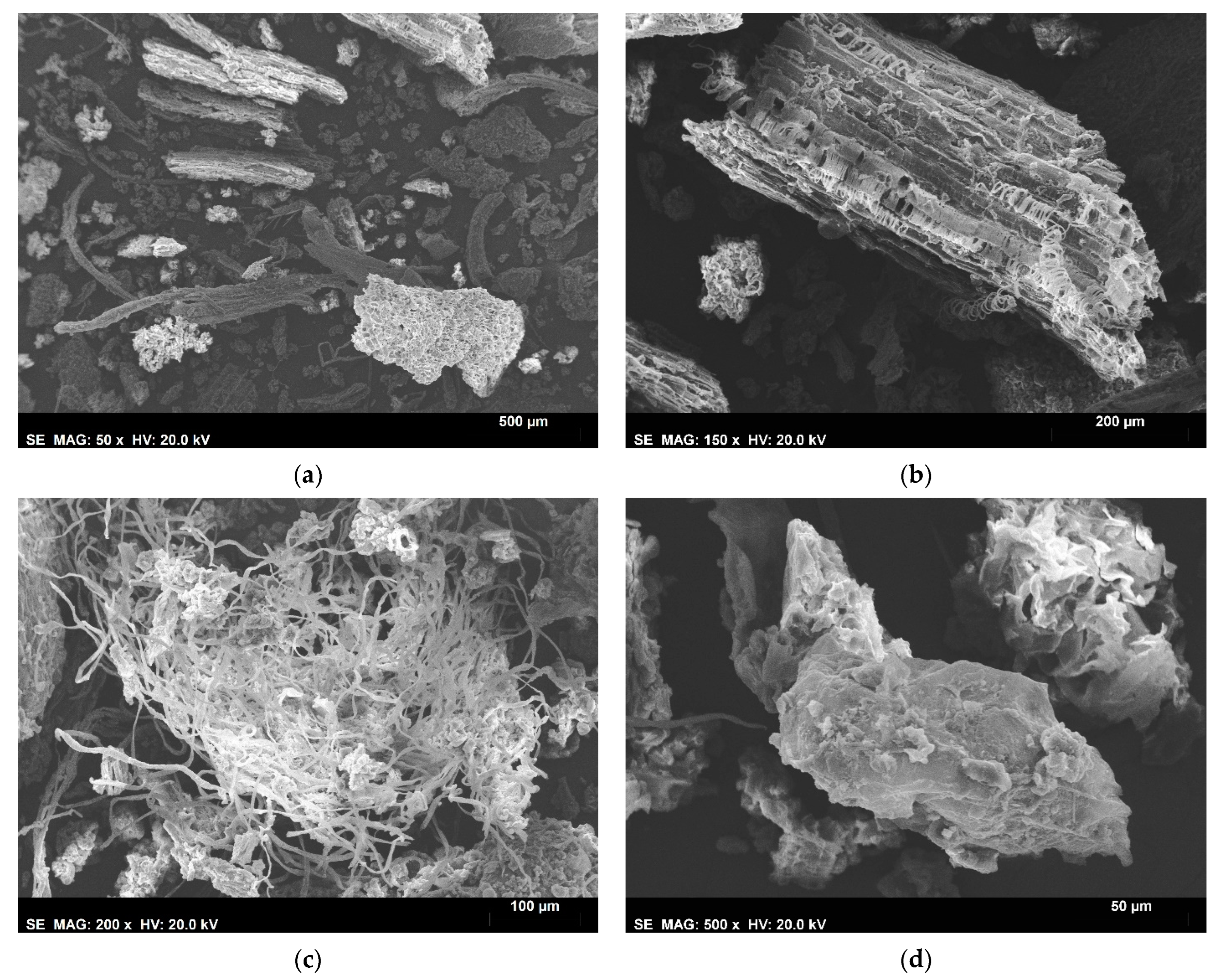
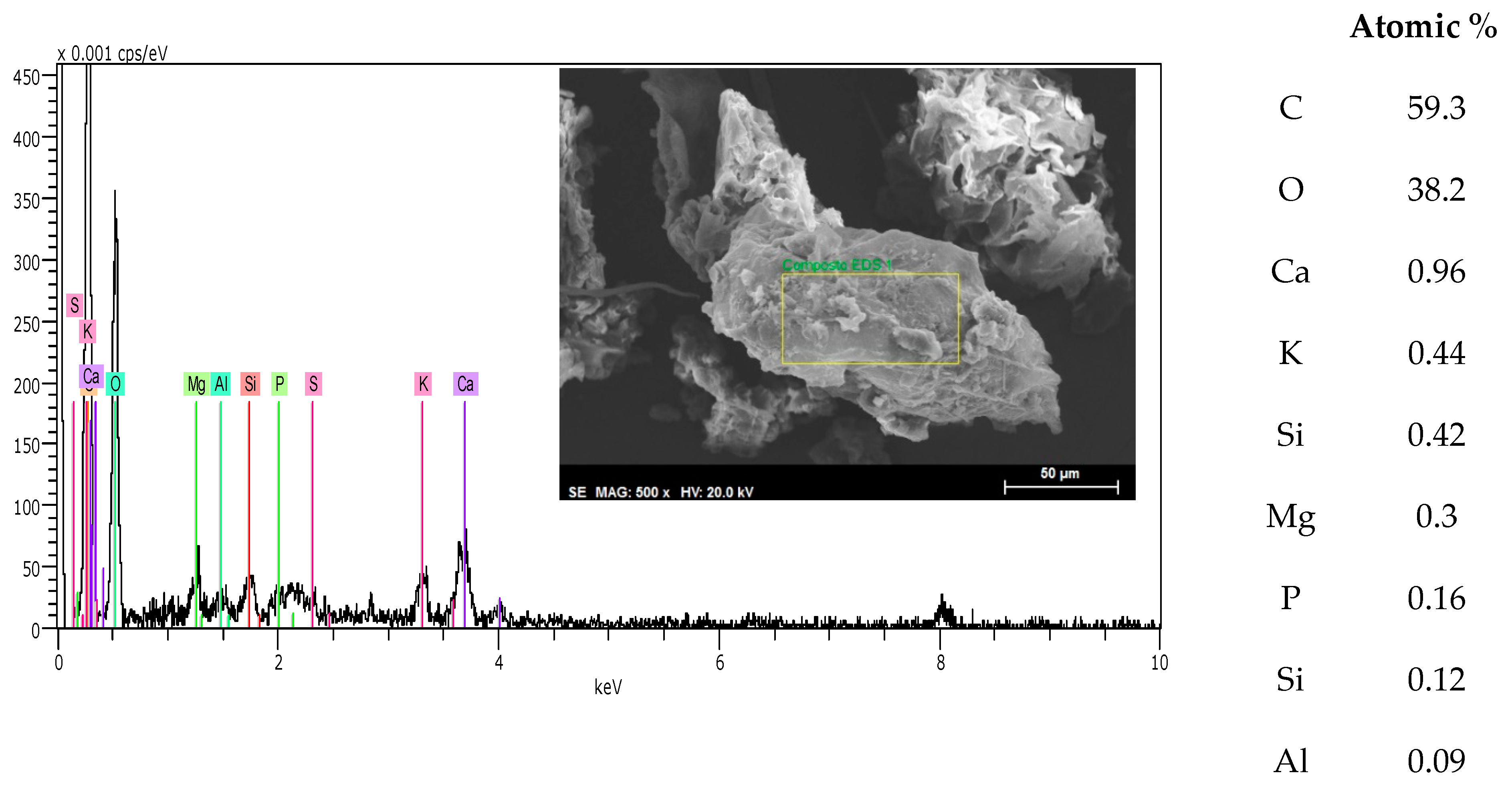
3.3. SEM—EDS
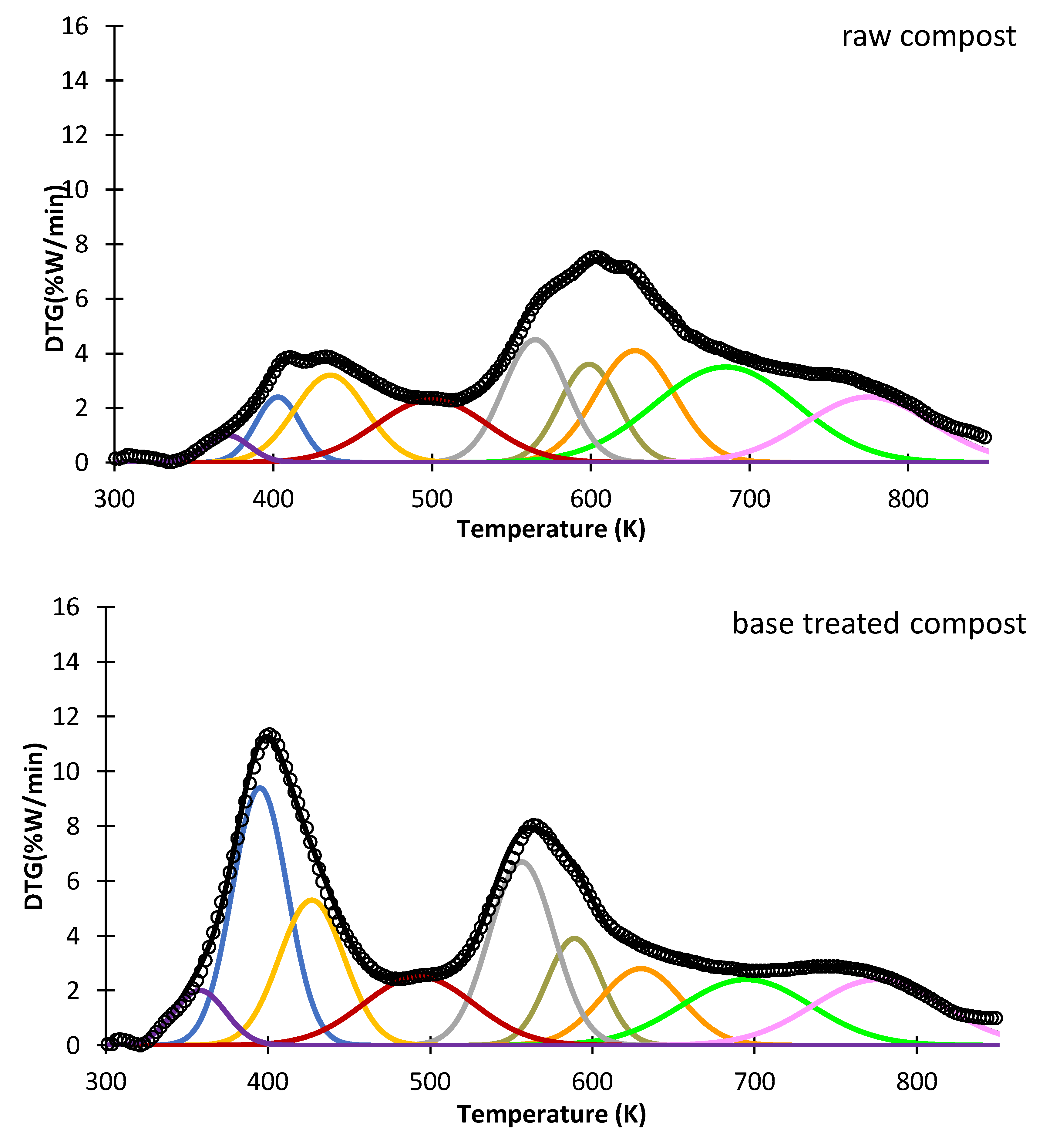
3.4. Thermogravimetry Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A | Pre-exponential factor of Arrhenius equation (s−1) |
| ATR | Attenuated total reflectance |
| DTG | Rate of thermal degradation (%wt./min) |
| Ea | Activation energy (kJ/mol) |
| EDS | Energy dispersive X-ray spectroscopy |
| FTIR | Fourier transform infrared spectroscopy |
| GDP | Gross domestic product |
| MSW | Municipal solid waste |
| R | Ideal gas constant (J/(mol.K) |
| SEM | Scanning electron microscopy |
| TG | Thermogravimetry |
| Tm | Temperature corresponding to the maximum rate of thermal degradation (K) |
| XRD | X-ray diffraction |
| β | Heating rate (K/min) |
References
- Lopez Barrera, E.; Hertel, T. Global food waste across the income spectrum: Implications for food prices, production and resource use. Food Policy 2021, 98, 101874. [Google Scholar] [CrossRef]
- Cakar, B.; Aydin, S.; Varank, G.; Ozcan, H.K. Assessment of environmental impact of FOOD waste in Turkey. J. Clean. Prod. 2020, 244, 118846. [Google Scholar] [CrossRef]
- Hiç, C.; Pradhan, P.; Rybski, D.; Kropp, J.P. Food Surplus and Its Climate Burdens. Environ. Sci. Technol. 2016, 50, 4269–4277. [Google Scholar] [CrossRef] [Green Version]
- UN Environment Program. Food Waste Index Report 2021. 2021. Available online: https://www.unep.org/resources/report/unep-food-waste-index-report-2021#:~:text=UNEP%20Food%20Waste%20Index%20Report%202021%2004%20March,are%20associated%20with%20food%20that%20is%20not%20consumed (accessed on 4 March 2021).
- Wu, D.; Wei, Z.; Mohamed, T.A.; Zheng, G.; Qu, F.; Wang, F.; Zhao, Y.; Songet, C. Lignocellulose biomass bioconversion during composting: Mechanism of action of lignocellulase, pretreatment methods and future perspectives. Chemosphere 2022, 286, 131635. [Google Scholar] [CrossRef] [PubMed]
- El Fels, L.; Zamama, M.; El Asli, A.; Hafidi, M. Assessment of biotransformation of organic matter during co-composting of sewage sludge-lignocelullosic waste by chemical, FTIR analyses, and phytotoxicity tests. Int. Biodeterior. Biodegrad. 2014, 87, 128–137. [Google Scholar] [CrossRef]
- Roulia, M. Humic Substances: A Novel Eco-Friendly Fertilizer. Agronomy 2022, 12, 754. [Google Scholar] [CrossRef]
- Kim, M.H.; Kim, J.W. Comparison through a LCA evaluation analysis of food waste disposal options from the perspective of global warming and resource recovery. Sci. Total Environ. 2010, 408, 3998–4006. [Google Scholar] [CrossRef]
- Available online: https://amplisource.com/sustainability/ (accessed on 16 May 2023).
- Sipra, A.T.; Gao, N.; Sarwar, H. Municipal solid waste (MSW) pyrolysis for bio-fuel production: A review of effects of MSW components and catalysts. Fuel Process. Technol. 2018, 175, 131–147. [Google Scholar] [CrossRef]
- Ma, J.; Kong, W.; Di, W.; Zhang, Z.; Wang, Z.; Feng, S.; Shen, B.; Mu, L. Synergistic effect of bulking agents and biodegradation on the pyrolysis of biodried products derived from municipal organic wastes: Product distribution and biochar physicochemical characteristics. Energy 2022, 248, 123512. [Google Scholar] [CrossRef]
- Barneto, A.G.; Carmona, J.A.; Jesús Díaz Blanco, M. Effect of the previous composting on volatiles production during biomass pyrolysis. J. Phys. Chem. A 2010, 114, 3756–3763. [Google Scholar] [CrossRef]
- Barneto, A.G.; Carmona, J.A.; Gálvez, A.; Conesa, J.A. Effects of the composting and the heating rate on biomass gasification. Energy Fuels 2009, 23, 951–957. [Google Scholar] [CrossRef]
- Trubetskaya, A. Reactivity Effects of Inorganic Content in Biomass Gasification: A Review. Energies 2022, 15, 3137. [Google Scholar] [CrossRef]
- Palma, A.; Doña-Grimaldi, V.M.; Ruiz-Montoya, M.; Giráldez, I.; García, J.C.; Loaiza, J.M.; López, F.; Díaz, M.J. MSW compost valorization by pyrolysis: Influence of composting process parameters. ACS Omega 2020, 5, 20810–20816. [Google Scholar] [CrossRef] [PubMed]
- Juchelková, D.; Corsaro, A.; Hlavsová, A.; Raclavská, H. Effect of composting on the production of syngas during pyrolysis of perennial grasses. Fuel 2015, 154, 380–390. [Google Scholar] [CrossRef]
- Xie, G.; Bruce, D.C.; Challacombe, J.F.; Chertkov, O.; Detter, J.C.; Gilna, P.; Han, C.S.; Lucas, S.; Misra, M.; Myers, G.L.; et al. Genome sequence of the cellulolytic gliding bacterium Cytophaga hutchinsonii. Appl. Environ. Microbiol. 2007, 73, 3536–3546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-González, D.; Ramirez, A.A.; Ghorbel, L.; Rouissi, T.; Brar, S.K.; Giroir-Fendler, A.; Godbout, S.; Palacios, J.; Silva, L.S.; Valverde, J.L. Valorization of compost from animal breeding via pyrolysis and combustion. In 2014 Montreal, Quebec Canada July 13–July 16, 2014; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2014; Volume 7, p. 1. [Google Scholar] [CrossRef]
- Griffiths, P.R. IR Spectroscopic Data Processing. In Encyclopedia of Spectroscopy and Spectrometry, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 428–436. [Google Scholar]
- E1755 Standard Test Method for Ash in Biomass. Available online: https://www.astm.org/e1755-01r20.html (accessed on 28 April 2023).
- Rijo, B.; Soares Dias, A.P.; Ramos, M.; Ameixa, M. Valorization of forest waste biomass by catalyzed pyrolysis. Energy 2022, 243, 122766. [Google Scholar] [CrossRef]
- Dias AP, S.; Rijo, B.; Ramos, M.; Casquilho, M.; Rodrigues, A.; Viana, H.; Rosa, F. Pyrolysis of burnt maritime pine biomass from forest fires. Biomass Bioenergy 2022, 163, 106535. [Google Scholar] [CrossRef]
- Song, B.; Lin, R.; Lam, C.H.; Wu, H.; Tsui, T.H.; Yu, Y. Recent advances and challenges of inter-disciplinary biomass valorization by integrating hydrothermal and biological techniques. Renew. Sustain. Energy Rev. 2021, 135, 110370. [Google Scholar] [CrossRef]
- Duan, D.; Ruan, R.; Wang, Y.; Liu, Y.; Dai, L.; Zhao, Y.; Zhou, Y.; Wu, Q. Microwave-assisted acid pretreatment of alkali lignin: Effect on characteristics and pyrolysis behavior. Bioresour. Technol. 2018, 251, 57–62. [Google Scholar] [CrossRef]
- Machado, W.; Franchini, J.C.; de Fátima Guimarães, M.; Filho, J.T. Spectroscopic characterization of humic and fulvic acids in soil aggregates, Brazil. Heliyon 2020, 6, e04078. [Google Scholar] [CrossRef]
- Md Salim, R.; Asik, J.; Sarjadi, M.S. Chemical functional groups of extractives, cellulose and lignin extracted from native Leucaena leucocephala bark. Wood Sci. Technol. 2021, 55, 295–313. [Google Scholar] [CrossRef]
- Grube, M.; Lin, J.G.; Lee, P.H.; Kokorevicha, S. Evaluation of sewage sludge-based compost by FT-IR spectroscopy. Geoderma 2006, 130, 324–333. [Google Scholar] [CrossRef]
- Madejová, J. FTIR techniques in clay mineral studies. Vib. Spectrosc. 2003, 31, 1–10. [Google Scholar] [CrossRef]
- Cai, G.B.; Chen, S.F.; Liu, L.; Jiang, J.; Yao, H.B.; Xu, A.W.; Yu, S.H. 1,3-Diamino-2-hydroxypropane-N,N,N′,N′-tetraacetic acid stabilized amorphous calcium carbonate: Nucleation, transformation and crystal growth. CrystEngComm 2009, 12, 234–241. [Google Scholar] [CrossRef]
- Lee, H.V.; Hamid, S.B.A.; Zain, S.K. Conversion of lignocellulosic biomass to nanocellulose: Structure and chemical process. Sci. World J. 2014, 2014, 631013. [Google Scholar] [CrossRef] [Green Version]
- Jiang, F.; Hsieh, Y.L. Rice Straw Nanocelluloses: Process-Linked Structures, Properties, and Self-Assembling into Ultra-Fine Fibers. ACS Symp. Ser. 2017, 1251, 133–150. [Google Scholar] [CrossRef]
- Czarnecka-Komorowska, D.; Tomasik, M.; Thakur, V.K.; Kostecka, E.; Rydzkowski, T.; Jursa-Kulesza, J.; Bryll, K.; Mysłowski, J.; Gawdzińska, K. Biocomposite composting based on the sugar-protein condensation theory. Ind. Crops Prod. 2022, 183, 114974. [Google Scholar] [CrossRef]
- Alptekin, F.M.; Celiktas, M.S. Review on Catalytic Biomass Gasification for Hydrogen Production as a Sustainable Energy Form and Social, Technological, Economic, Environmental, and Political Analysis of Catalysts. ACS Omega 2022, 7, 24918–24941. [Google Scholar] [CrossRef]
- De Carvalho, D.M.; de Queiroz, J.H.; Colodette, J.L. Assessment of alkaline pretreatment for the production of bioethanol from eucalyptus, sugarcane bagasse and sugarcane straw. Ind. Crops Prod. 2016, 94, 932–941. [Google Scholar] [CrossRef]
- Komilis, D.P.; Ham, R.K. The effect of lignin and sugars to the aerobic decomposition of solid wastes. Waste Manag. 2003, 23, 419–423. [Google Scholar] [CrossRef]
- Subhedar, P.B.; Gogate, P.R. Alkaline and ultrasound assisted alkaline pretreatment for intensification of delignification process from sustainable raw-material. Ultrason. Sonochem. 2014, 21, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Baruah, J.; Nath, B.K.; Sharma, R.; Kumar, S.; Deka, R.C.; Baruah, D.C.; Kalita, E. Recent trends in the pretreatment of lignocellulosic biomass for value-added products. Front. Energy Res. 2018, 6, 141. [Google Scholar] [CrossRef]
- Sahoo, D.; Ummalyma, S.B.; Okram, A.K.; Pandey, A.; RKS, M.S. Effect of dilute acid pretreatment of wild rice grass (Zizania latifolia) from Loktak Lake for enzymatic hydrolysis. Bioresour. Technol. 2018, 253, 252–255. [Google Scholar] [CrossRef]
- Li, K.; Wan, J.; Wang, X.; Wang, J.; Zhang, J. Comparison of dilute acid and alkali pretreatments in production of fermentable sugars from bamboo: Effect of Tween 80. Ind. Crops Prod. 2016, 83, 414–422. [Google Scholar] [CrossRef]
- Kurian, J.K.; Gariepy, Y.; Orsat, V.; Raghavan, G.S.V. Microwave-assisted lime treatment and recovery of lignin from hydrothermally treated sweet sorghum bagasse. Biofuels 2015, 6, 341–355. [Google Scholar] [CrossRef]




| C | H | N | S | Ash | O ★ | Atomic Ratio | ||
|---|---|---|---|---|---|---|---|---|
| H/C | O/C | |||||||
| (Wt. %) | ||||||||
| Composted Biomass | 35.4 ± 0.7 | 4.7 ± 0.2 | 2.7 ± 0.3 | 0.3 ± 0.4 | 37.6 | 19.3 | 1.6 | 0.4 |
| Compost | Weight Composition (%) | ||||||
|---|---|---|---|---|---|---|---|
| Moisture | Extractive Volatile | Component 1 | Component 2 | Component 3 | Lignin 1 | Lignin 2 | |
| Raw | 4.7 | 23.4 | 12.6 | 9.4 | 14.4 | 22.1 | 13.5 |
| Base-treated | 17.5 | 28.4 | 15.6 | 6.3 | 8.8 | 11.6 | 11.8 |
| Acid-treated | 20.0 | 43.2 | 9.7 | 6.5 | 5.4 | 10.6 | 4.7 |
| Raw Compost | Base-Treated Compost | Acid-Treated Compost | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Tm (K) | Ea (kJ mol) | k (s−1) | Tm (K) | Ea (kJ mol) | k (s−1) | Tm (K) | Ea (kJ mol) | k (s−1) | |
| Component 1 | 559 | 201 | 551 | 132 | 542 | 187 | |||
| Component 2 | 595 | 288 | 581 | 167 | 586 | 212 | |||
| Component 3 | 623 | 307 | 626 | 345 | 634 | 300 | |||
| Lignin 1 | 679 | 250 | 692 | 548 | 700 | 484 | |||
| Lignin 2 | 767 | 198 | 772 | 471 | 783 | 457 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rijo, B.; Soares Dias, A.P.; Saksiwi, N.D.; Pereira, M.F.C.; Zăvoianu, R.; Pavel, O.D.; Ferreira, O.; dos Santos, R.G. Biofuels from Pyrolysis of Third-Generation Biomass from Household and Garden Waste Composting Bin: Kinetics Analysis. Reactions 2023, 4, 295-310. https://doi.org/10.3390/reactions4020018
Rijo B, Soares Dias AP, Saksiwi ND, Pereira MFC, Zăvoianu R, Pavel OD, Ferreira O, dos Santos RG. Biofuels from Pyrolysis of Third-Generation Biomass from Household and Garden Waste Composting Bin: Kinetics Analysis. Reactions. 2023; 4(2):295-310. https://doi.org/10.3390/reactions4020018
Chicago/Turabian StyleRijo, Bruna, Ana Paula Soares Dias, Novi Dwi Saksiwi, Manuel Francisco Costa Pereira, Rodica Zăvoianu, Octavian Dumitru Pavel, Olga Ferreira, and Rui Galhano dos Santos. 2023. "Biofuels from Pyrolysis of Third-Generation Biomass from Household and Garden Waste Composting Bin: Kinetics Analysis" Reactions 4, no. 2: 295-310. https://doi.org/10.3390/reactions4020018









