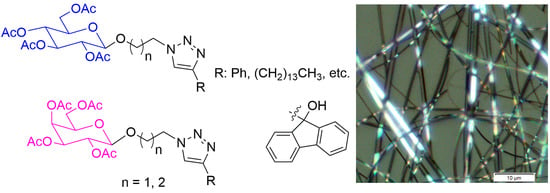
3. Experimental Details
Reagents and solvents were used as they were received from the suppliers. All purification was conducted by flash chromatography using 230–400 mesh silica gel. The solvent systems used for chromatography and for gelation test are all in volume ratios. NMR analysis was conducted using a 400 MHz Bruker NMR spectrometer. The molecular mass was measured using LCMS on an Agilent 6120B Single Quad Mass Spectrometer and LC1260 system or Shimadzu LCMS-2020 with ESI in positive ionization mode. Melting point measurements were carried out using a Stuart automatic melting point apparatus SMP40.
General method for gelation testing: About 2.0 mg of dried compound was placed in a one-dram glass vial and the corresponding solvent was added to obtain a concentration of 20.0 mg/mL. The mixture was then heated until the solids were fully dissolved, sometimes sonication was needed to dissolve the sample, then the solution was allowed to cool to room temperature and let stand for 15 min. After this period, if the sample is a clear solution, this is recorded as soluble, if solid reappeared, this is recorded as precipitate; if the sample formed a gel, then the vial is inverted and if no solvent flowing was observed, this is recorded as a stable gel, otherwise it is recorded as unstable gel. The stable gel was then serial diluted till the minimum gelation concentration, which is the concentration prior to unstable gelation, is obtained.
Metallogel testing method: In a one-dram vial, 3.0 mg of the gelator 7a (5.6 × 10−3 mmol) was dissolved in 0.5 mL DMSO:H2O (v/v 1:5) mixture. To the mixture, increasing amount of metal salt, 1.0 and 1.5 eq. with respect to the gelator were added. The final mixture was heated and allowed to cool on bench top without any disturbance. Gelation was tested by inverting the vial.
Optical microscopy studies: A small amount of the gel was placed on a clean glass slide using micro spatula, the gel was air dried but may still contain some solvents and this was observed under an Olympus BX60M optical microscope and the Olympus DP73-1-51 high performance 17MP digital camera with pixel shifting and Peltier cooled. The imaging software for image capturing is CellSens 1.11.
Atomic force microscopy studies: AFM images were acquired using Veeco Dimension 3100 Atomic Force Microscope. The tips used were Tap300-G silicon AFM probes with a resonant frequency of 300 KHz and a force constant of 40 N/m. The samples were prepared by spreading the gel on a glass plate with the help of a micro spatula which was then air dried to obtain corresponding xerogel.
FTIR studies and IR imaging microscopy: FTIR spectroscopy data was collected using Bruker Alpha FTIR spectrometer using OPUS software. Solids were used as such. For gels, a small piece of gel was placed on the plate and background scans were performed with the solvent in which the gel was prepared. IR microscopy was done using Bruker LUMOS-II installed wit ATR channel. Detector used for this study was Focal Point Array (FPA)—32 × 32 with ZnSe beam splitter. IR images were taken for the gel and its corresponding xerogel.
Rheological analyses: Rheological properties of gels were investigated by HR-2 Discovery Hybrid Rheometer from TA instrument with the TRIOS software or Anton Parr MCR 302 with RheoCompass software. The cone geometry is 25 mm Peltier plate for both and with a gap of 100 µm for HR-2 Rheometer and 1.0 mm for Anton Parr Rheometer. The experimental temperature was 25.0 °C, the sample was subjected to amplitude sweep for oscillation strain from 0.125% or 0.1% to 10%. A frequency sweep was then performed for the sample in the range of 0.1 to 100.0 rad/s for angular frequency. The results were expressed as the storage modules (G′) and loss modules (G″) as a function of the angular frequency.
PXRD analysis: Bruker D2 Phaser X-ray powder diffractometer installed with Bragg- Brentano optics and Lynx Eye position sensitive detector (PSD) was utilized. The instrument has 1 mm divergence slit, 1 mm anti-scatter air screen and 3 mm detector slit. Silicone low background sample holder was used with 2.5 cm diameter with 0.2 mm depth. The powder was ground on agate mortal with pestle to reduce the crystallite size and the sample holder was not completely covered with powder. For each sample, 2ϴ ranges from 1.5° or 2° to 35° or 60° with 0.02° step size at 2 scans/step counting time at 15 rpm. PSD opening was set at 0.25° for sample 7a and 0.5° for samples 7d and 7i. Graphs were plotted using Graph Version 12.
Fluorescent co-gels formed by compounds 7a and 7i: Fluorescence emission spectra was obtained using Shimadzu RF-6000 Spectro Fluorophotometer with excitation and emission bandwidths set at 5.0 nm and scan speed of 600 nm/min. A fluorescent co-gel was formed by using 10.0 mg of compound
7a (0.019 mmol, 10.0 eq.) and compound
7i (1.2 mg, 0.0019 mmol, 1.0 eq.) in 1.0 mL DMSO:H
2O (1:2). The fluorescence spectra were recorded at 350 nm as excitation wavelength. Control spectrum was recorded by preparing 1.2 mg/mL solution of
7i in DMSO:H
2O (1:2). Similar experiments were performed in acetonitrile solutions as well (
Figure S3).
Naproxen trapping and release experiment: A gel was prepared in a one-dram vial using compound 7a (16.0 mg) and 0.5 mg of naproxen sodium and 2.0 mL of DMSO/H2O (v/v 1:5). After a stable gel formed and the gel was left undisturbed for 15 min, 2.0 mL of water at pH 7.0 was added to the top of the gel carefully. Naproxen release from the gel was monitored by UV absorption at intervals by transferring the supernatant with a pipet to a cuvette, and after each measurement, the aqueous phase was carefully transferred back to the vial and placed on top of the gel until the next measurement. The UV spectra of the pure naproxen (0.5 mg) in 4.0 mL DMSO/H2O (v/v 1:5) was also recorded as standard.
Dye absorption study: A gel was prepared in a one-dram vial using compound 7a (16.0 mg) and 2.0 mL of DMSO/H2O (v/v 1:5). After a stable gel was formed and the gel was left at room temperature for 15 min, 2.0 mL of a toluidine blue aqueous solution (0.04 mM) was added dropwise onto the top of the gel. For dye mixture absorption study, mixture of dyes prepared by using toluidine blue (0.04 mM, 1.0 mL) and methyl orange (0.024 mM, 1.0 mL) with a total volume of 2.0 mL was added on the top of the gel prepared in a similar way using compound 7a. The adsorption of dye(s) from aqueous solution to the gel at different time intervals was monitored by UV-Vis spectroscopy.
Synthesis of sugar azide headgroup: D-glucopyranose pentaacetate
1 was synthesized following literature procedure [
30,
39]. This was followed by a glycosylation reaction to form compound
2 [
39,
40]. This compound was then treated with NaN
3 to obtain the desires azide intermediate
3 as white solid [
41]. Similar procedure was followed for the synthesis of other glucose azide with three carbon spacer and galactose azides with 2 and 3 carbon spacers [
42,
43,
44,
45].
Synthesis of sugar triazole derivatives 4a-
14h: These are synthesized using the CuAAC reactions either using CuSO
4∙5H
2O (Method A) [
31,
46] or CuI (method B). Compounds
4a, 4b, 4e, 7a, 7g, 7j, 7k, 11b, 11d, 11j were synthesized using method A and the others were synthesized using method B. The detailed methods are shown in the ESI, only the reagents and quantities and characterization data are given.
Synthesis of compound
4a [47]: Compound
3 (70 mg, 0.17 mmol), phenyl acetylene (20.4 mg, 20 µL, 0.20 mmol), CuSO
4∙5H
2O (10.0 mg, 0.04 mmol) and NaAsc (16.0 mg, 0.08 mmol). The crude product was purified with 0–2% MeOH/DCM to afford an off-white solid (86 mg, 0.165 mmol, 97%) as the desired product (R
f = 0.43 in 3% MeOH/DCM). m.p. 157.0–159.0 °C;
1H NMR (400 MHz, CDCl
3)
δ 7.94–7.84 (m, 3H), 7.49–7.41 (m, 2H), 7.38–7.31 (m, 1H), 5.19 (t,
J = 9.5 Hz, 1H), 5.12–5.01 (m, 2H), 4.75–4.68 (m, 1H), 4.63–4.53 (m, 1H). 4.49 (d,
J = 7.9 Hz, 1H), 4.34–4.25 (m, 2H), 4.16 (dd,
J = 12.4, 2.3 Hz, 1H), 3.99–3.91 (m, 1H), 3.75–3.68 (m, 1H), 2.09 (s, 3H), 2.04 (s, 3H), 2.00 (s, 3H), 1.75 (s, 3H);
13C NMR (100 MHz, CDCl
3)
δ 170.6, 170.0, 169.5, 169.4, (triazole at 147 ppm missing), 130.6, 128.8, 128.1, 125.7, 121.5, 100.6, 72.5, 72.0, 70.9, 68.3, 67.9, 61.7, 50.1, 20.7, 20.54, 20.51, 20.4. LC-MS (ESI+) calcd for C
24H
30N
3O
10 [M + H]
+ 520 found 520.
Synthesis of compound 4b: Compound 3 (100 mg, 0.24 mmol), 1-octyne (30.8 mg, 40 µL, 0.28 mmol), CuSO4∙5H2O (12.0 mg, 0.048 mmol) and NaAsc (22.0 mg, 0.11 mmol) The crude product was purified with 0–2% MeOH in DCM to afford an off-white solid (110.5 mg, 0.21 mmol, 87%) as the desired product (Rf = 0.26 in 5% MeOH/DCM). m.p. 108.0–109.0 °C; 1H NMR (400 MHz, CDCl3) δ 7.32 (s, 1H), 5.17 (t, J = 9.5 Hz, 1H), 5.07 (t, J = 9.8 Hz, 1H), 5.02–4.97 (m, 1H), 4.60–4.52 (m, 1H), 4.50–4.42 (m, 2H), 4.27–4.18 (m, 2H), 4.13 (dd, J = 12.3, 2.4 Hz, 1H), 3.95–3.87 (m, 1H), 3.73–3.65 (m, 1H), 2.76–2.61 (m, 2H), 2.08 (s, 3H), 2.02 (s, 3H), 2.00 (s, 3H), 1.95 (s, 3H), 1.71–1.61 (m, 2H), 1.41–1.26 (m, 6H), 0.88 (t, J = 6.9 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 170.5, 170.1, 169.4, 169.2, 148.4, 122.0, 100.6, 72.5, 72.0, 71.0, 68.3, 68.0, 61.8, 49.8, 31.6, 29.4, 29.0, 25.7, 22.6, 20.7, 20.5, 14.0. LC-MS (ESI+) calcd for C24H38N3O10 [M + H]+ 528 found 528.
Synthesis of compound 4d: Compound 3 (75 mg, 0.18 mmol), 1-hexadecyne (50 mg, 62 µL, 0.22 mmol), CuI (7.0 mg, 0.036 mmol) and DIEA (35.0 mg, 47.0 µL, 0.27 mmol) The crude product was purified with 0–2% MeOH in DCM to afford an off-white solid (73.6 mg, 0.12 mmol, 67%) as the desired product (Rf = 0.29 in 3% MeOH/DCM). m.p. 110.0–112.0 °C; 1H NMR (400 MHz, CDCl3) δ 7.36 (s, 1H), 5.2 (t, J = 9.5 Hz, 1H), 5.09 (t, J = 9.8 Hz, 1H), 5.02 (dd, J = 9.6, 7.9 Hz, 1H), 4.62–4.56 (m, 1H), 4.54–4.45 (m, 2H), 4.31–4.20 (m, 2H), 4.16 (dd, J = 12.4, 2.4 Hz, 1H), 3.98–3.90 (m, 1H), 3.75–3.68 (m, 1H), 2.80–2.65 (m, 2H), 2.11 (s, 3H), 2.05 (s, 3H), 2.02 (s, 3H), 1.97 (s, 3H), 1.72–1.65 (m, 2H), 1.41–1.23 (m, 22H), 0.90 (t, J = 6.8 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 170.5, 170.1, 169.4, 169.2, 148.5, 122.1, 100.6, 72.5, 72.0, 71.0, 68.3, 67.9, 61.8, 49.9, 31.9, 29.68, 29.65, 29.6, 29.5, 29.38, 29.37, 29.3, 25.7, 22.7, 20.7, 20.5, 14.1. LC-MS (ESI+) calcd for C32H54N3O10 [M + H]+ 640 found 640.
Synthesis of compound 4e: Compound 3 (75 mg, 0.18 mmol), 5-phenyl-1-pentyne (32 mg, 33 µL 0.22 mmol), CuSO4∙5H2O (9.0 mg, 0.036 mmol) and NaAsc (14.2 mg, 0.072 mmol). The crude product was purified with 0–2% MeOH in DCM to afford a white solid (98 mg, 0.17 mmol, 94%) as the desired product (Rf = 0.37 in 3% MeOH/DCM). m.p. 71.0–73.0 °C; 1H NMR (400 MHz, CDCl3) δ 7.37–7.29 (m, 2H), 7.28 (m, 1H), 7.23–7.16 (m, 3H), 5.19 (t, J = 9.5 Hz, 1H), 5.08 (t, J = 9.9 Hz, 1H), 5.01 (dd, J = 9.6, 7.9 Hz, 1H), 4.63–4.55 (m, 1H), 4.54–4.42 (m, 2H), 4.30–4.20 (m, 2H), 4.15 (dd, J = 12.4, 2.3 Hz, 1H), 3.97–3.89 (m, 1H), 3.74–3.68 (m, 1H), 2.81–2.67 (m, 4H), 2.09 (s, 5H), 2.04 (s, 3H), 2.02 (s, 3H), 1.92 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 170.5, 170.1, 169.4, 169.2, 147.8, 141.8, 128.5, 128.3, 125.8, 122.1, 100.6, 72.5, 72.0, 68.3, 67.9, 61.8, 49.9, 35.4, 31.0, 25.2, 20.5. LC-MS (ESI+) calcd for C27H36N3O10 [M + H]+ 562 found 562.
Synthesis of compound 4i: Compound 3 (60 mg, 0.14 mmol), 9-ethynyl-9-fluorenol (36 mg, 0.17 mmol), CuI (11.0 mg, 0.028 mmol) and DIEA (22.3 mg, 30 µL, 0.17 mmol). The crude product was purified with 0–2% MeOH in DCM to afford a white solid (82.5 mg, 0.13 mmol, 92%) as the desired product (Rf = 0.53 in 5% MeOH/DCM). m.p. 101.0–104.0 °C; 1H NMR (400 MHz, CDCl3) δ 7.71–7.62 (m, 4H), 7.44–7.30 (m, 5H), 5.14 (t, J = 9.5 Hz, 1H), 5.03 (t, J = 9.8 Hz, 1H), 4.94–4.88 (m, 1H), 4.56–4.48 (m, 1H), 4.46–4.38 (m, 2H), 4.23–4.13 (m, 2H), 4.13–4.06 (m, 1H), 4.00–3.92 (m, 1H), 3.68–3.61 (m, 1H), 2.05 (s, 3H), 2.02 (s, 3H), 1.99 (s, 3H), 1.81 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 170.6, 170.1, 169.4, 147.9, 147.8, 139.6, 129.50, 129.48, 128.4, 125.0, 124.9, 120.2, 100.4, 78.6, 72.5, 72.0, 70.9, 68.3, 67.5, 61.7, 50.1, 20.7, 20.5, 20.4. LC-MS (ESI+) calcd for C31H34N3O11 [M + H]+ 624 found 624.
Synthesis of compound 7a: Compound 6 (60 mg, 0.14 mmol), phenyl acetylene (18.6 mg, 20 µL, 0.18 mmol), CuSO4∙5H2O (7.0 mg, 0.028 mmol) and NaAsc (11.0 mg, 0.056 mmol). The crude product was purified with 0–30% EtOAc/hexanes to afford a white solid (53.8 mg, 0.10 mmol, 71%) as the desired product (Rf = 0.33 in 1:3 EtOAc/hexanes). m.p. 131.0–132.0 °C; 1H NMR (400 MHz, CDCl3) δ 7.84–7.81 (m, 2H), 7.80 (s, 1H), 7.45–7.39 (m, 2H), 7.36–7.30 (m, 1H), 5.22 (t, J = 9.5 Hz, 1H), 5.12–5.00 (m, 2H), 4.59–4.40 (m, 3H), 4.25 (dd, J = 12.3, 4.9 Hz, 1H), 4.13 (dd, J = 12.4, 2.4 Hz, 1H), 3.92–3.83 (m, 1H), 3.73–3.66 (m, 1H), 3.59–3.49 (m, 1H), 2.31–2.14 (m, 2H), 2.08 (s, 3H), 2.032 (s, 3H), 2.027 (s, 3H), 2.01 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 170.5, 170.2, 169.5, 169.4, 147.7, 130.6, 128.9, 128.2, 125.7, 120.2, 100.9, 72.7, 72.0, 71.3, 68.4, 65.8, 61.8, 46.6, 30.3, 20.8, 20.7, 20.6. LC-MS (ESI+) calcd for C25H32N3O10 [M + H]+ 534.2 found 534.2.
Synthesis of compound 7b: Compound 6 (70 mg, 0.16 mmol), 1-octyne (22.4 mg, 30 µL, 0.19 mmol), CuI (6.0 mg, 0.032 mmol) and DIEA (24.8 mg, 30 µL, 0.19 mmol). The crude product was purified with 0–30% EtOAc/hexanes to afford a white solid (75.6 mg, 0.14 mmol, 88%) as the desired product (Rf = 0.23 in 1:3 EtOAc/hexanes). m.p. 95.0–96.0 °C; 1H NMR (400 MHz, CDCl3) δ 7.26 (s, 1H), 5.21 (t, J = 9.5 Hz, 1H), 5.08 (t, J = 9.7 Hz, 1H), 5.04–4.98 (m, 1H), 4.50 (d, J = 7.9 Hz, 1H), 4.47–4.38 (m, 1H), 4.37–4.30 (m, 1H), 4.26 (dd, J = 12.3, 4.8 Hz, 1H), 4.13 (dd, J = 12.4, 2.3 Hz, 1H), 3.90–3.82 (m, 1H), 3.72–3.65 (m, 1H), 3.53–3.44 (m, 1H), 2.69 (t, J = 7.7 Hz, 2H), 2.24–2.10 (m, 2H), 2.07 (s, 6H), 2.02 (s, 3H), 2.00 (s, 3H), 1.70–1.61 (m, 2H), 1.42–1.24 (m, 6H), 0.88 (t, J = 6.9 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 170.5, 170.2, 169.4, 148.5, 120.9, 100.8, 72.8, 71.9, 71.3, 68.4, 66.0, 61.9, 46.4, 31.6, 30.3, 29.5, 29.0, 25.7, 22.6, 20.73, 20.69, 20.58, 20.57, 14.0. LC-MS (ESI+) calcd for C25H40N3O10 [M + H]+ 542.3 found 542.2.
Synthesis of compound 7c: Compound 6 (70 mg, 0.16 mmol), 1-dodecyne (32 mg, 42 µL, 0.19 mmol), CuI (6.0 mg, 0.032 mmol) and DIEA (24.8 mg, 30 µL, 0.19 mmol). The crude product was purified with 0–30% EtOAc/hexanes to afford an off white solid (62.6 mg, 0.10 mmol, 63%) as the desired product (Rf = 0.30 in 1:3 EtOAc/hexanes). m.p. 96.0–98.0 °C; 1H NMR (400 MHz, CDCl3) δ 7.28 (triazole 1H overlapping with CDCl3 signal), 5.24 (t, J = 9.5 Hz, 1H), 5.11 (t, J = 9.6 Hz, 1H), 5.04 (t, J = 9.6 Hz, 1H), 4.53 (d, J = 7.9 Hz, 1H), 4.50–4.41 (m, 1H), 4.40–4.32 (m, 1H), 4.29 (dd, J = 12.3, 4.8 Hz, 1H), 4.16 (dd, J = 12.4, 2.4 Hz, 1H), 3.92–3.85 (m, 1H), 3.75–3.68 (m, 1H), 3.55–3.47 (m, 1H), 2.72 (t, J = 7.5 Hz, 2H), 2.27–2.12 (m, 2H), 2.10 (s, 6H), 2.05 (s, 3H), 2.04 (s, 3H), 1.74–1.64 (m, 2H), 1.41–1.23 (m, 14H), 0.90 (t, J = 6.8 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 170.5, 170.2, 169.4, 148.5, 120.9, 100.8, 72.8, 71.9, 71.3, 68.4, 66.0, 61.9, 46.4, 31.9, 30.3, 29.59, 29.56, 29.5, 29.4, 29.3, 25.7, 22.7, 20.73, 20.69, 20.6, 14.1. LC-MS (ESI+) calcd for C29H48N3O10 [M + H]+ 598 found 598.
Synthesis of compound 7d: Compound 6 (70 mg, 0.16 mmol), 1-hexadecyne (40 mg, 50 µL, 0.19 mmol), CuI (6.0 mg, 0.032 mmol) and DIEA (24.8 mg, 30 µL, 0.19 mmol). The crude product was purified with 0–30% EtOAc/hexanes to afford a white solid (82.1 mg, 0.13 mmol, 81%) as the desired product (Rf = 0.35 in 1:3 EtOAc/hexanes). m.p. 101.0–103.0 °C; 1H NMR (400 MHz, CDCl3) δ 7.28 (triazole 1H overlapping with CDCl3 signal), 5.24 (t, J = 9.5 Hz, 1H), 5.11 (t, J = 9.9 Hz, 1H), 5.04 (dd, J = 9.6, 8.0 Hz, 1H), 4.53 (d, J = 7.9 Hz, 1H), 4.50–4.41 (m, 1H), 4.41–4.32 (m, 1H), 4.29 (dd, J = 12.3, 4.8 Hz, 1H), 4.16 (dd, J = 12.4, 2.4 Hz, 1H), 3.92–3.84 (m, 1H), 3.75–3.68 (m, 1H), 3.55–3.47 (m, 1H), 2.71 (t, J = 7.6 Hz, 2H), 2.21–2.12 (m, 2H), 2.10 (s, 6H), 2.05 (s, 3H), 2.03 (s, 3H), 1.73–1.61 (m, 2H), 1.42–1.21 (m, 24H), 0.90 (t, J = 6.7 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 170.5, 170.2, 169.4, 148.5, 120.9, 100.8, 72.8, 71.9, 71.3, 68.4, 66.0, 61.9, 46.4, 31.9, 30.3, 29.7, 29.6, 29.5, 29.4, 29.34, 29.33, 25.7, 22.7, 20.73, 20.69, 20.6, 14.1. LC-MS (ESI+) calcd for C33H56N3O10 [M + H]+ 654 found 654.
Synthesis of compound 7e: Compound 6 (70 mg, 0.16 mmol), 5-phenyl-1-pentyne (28 mg, 30 µL, 0.19 mmol), CuI (6.0 mg, 0.032 mmol) and DIEA (24.8 mg, 30 µL, 0.19 mmol). The crude product was purified with 0–30% EtOAc/hexanes to afford a white solid (68.1 mg, 0.12 mmol, 75%) as the desired product (Rf = 0.14 in 1:3 EtOAc/hexanes). m.p. 74.0–76.0 °C; 1H NMR (400 MHz, CDCl3) 7.31–7.24 (m, 3H), 7.21–7.14 (m, 3H), 5.20 (t, J = 9.5 Hz, 1H), 5.07 (t, J = 9.8 Hz, 1H), 5.04–4.98 (m, 1H), 4.49 (d, J = 7.9 Hz, 1H), 4.47–4.38 (m, 1H), 4.37–4.30 (m, 1H), 4.24 (dd, J = 12.3, 4.8 Hz, 1H), 4.12 (dd, J = 12.3, 2.4 Hz, 1H), 3.88–3.81 (m, 1H), 3.71–3.64 (m, 1H), 3.52–3.44 (m, 1H), 2.74 (t, J = 7.5 Hz, 2H), 2.69 (t, J = 7.7 Hz, 2H), 2.26–2.10 (m, 2H), 2.06 (s, 3H), 2.05 (s, 3H), 2.02 (s, 3H), 2.01–1.96 (m, 5H); 13C NMR (100 MHz, CDCl3) δ 170.5, 170.2, 169.4, 147.9, 141.9, 128.5, 128.3, 125.8, 121.1, 100.8, 72.7, 71.9, 71.3, 68.4, 66.0, 61.9, 46.4, 35.4, 31.1, 30.3, 25.2, 20.72, 20.68, 20.6. LC-MS (ESI+) calcd for C28H38N3O10 [M + H]+ 576.3, found 576.2.
Synthesis of compound 7f: Compound 6 (70 mg, 0.16 mmol), propargyl alcohol (10.7 mg, 11 µL, 0.19 mmol), CuI (6.0 mg, 0.032 mmol) and DIEA (24.8 mg, 30 µL, 0.19 mmol). The crude product was purified with 0–30% EtOAc/hexanes to afford a light brown solid (68.8 mg, 0.14 mmol, 88%) as the desired product (Rf = 0.1 in 1:3 EtOAc/hexanes). m.p. 74.0–76.0 °C; 1H NMR (400 MHz, CDCl3) δ 7.56 (s, 1H), 5.21 (t, J = 9.5 Hz, 1H), 5.09 (t, J = 9.8 Hz, 1H), 5.03–4.97 (m, 1H), 4.80 (s, 2H), 4.53–4.36 (m, 3H), 4.26 (dd, J = 12.3, 4.8 Hz, 1H), 4.17 (dd, J = 12.3, 2.4 Hz, 1H), 3.87–3.79 (m, 1H), 3.72–3.66 (m, 1H), 3.55–3.47 (m, 1H), 2.26–2.13 (m, 2H), 2.08 (s, 3H), 2.07 (s, 3H), 2.03 (s, 3H) 2.01 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 170.7, 170.2, 169.5, 169.4, 147.7, 122.3, 100.7, 72.7, 72.0, 71.3, 68.4, 65.8, 61.8, 56.6, 46.7, 30.1, 20.7, 20.6. LC-MS (ESI+) calcd for C20H30N3O11 [M + H]+ 488 found 488.
Synthesis of compound 7g: Compound 6 (70 mg, 0.16 mmol), 8-chloro-1-octyne (27.7 mg, 30 µL, 0.19 mmol), CuSO4∙5H2O (8.0 mg, 0.032 mmol) and NaAsc (13.0 mg, 0.064 mmol). The crude product was with 0–30% EtOAc/hexanes to afford a white solid (75.1 mg, 0.13 mmol, 81%) as the desired product (Rf = 0.17 in 1:3 EtOAc/hexanes). m.p. 74.0–76.0 °C; 1H NMR (400 MHz, CDCl3) δ 7.28 (s, 1H), 5.22 (t, J = 9.5 Hz, 1H), 5.09 (t, J = 9.7 Hz, 1H), 5.05–4.99 (m, 1H), 4.51 (d, J = 7.9 Hz, 1H), 4.48–4.31 (m, 2H), 4.26 (dd, J = 12.4, 4.8 Hz, 1H), 4.14 (dd, J = 12.3, 2.4 Hz, 1H), 3.90–3.82 (m, 1H), 3.73–3.66 (m, 1H), 3.57–3.45 (m, 3H), 2.71 (t, J = 7.6 Hz, 2H), 2.25–2.10 (m, 2H), 2.08 (s, 6H), 2.03 (s, 3H), 2.01 (s, 3H), 1.83–1.74 (m, 2H), 1.73–1.65 (m, 2H), 1.52–1.44 (m, 2H), 1.43–1.36 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 170.5, 170.2, 169.4, 148.1, 121.0, 100.8, 72.7, 71.9, 71.3, 68.4, 66.0, 61.9, 46.4, 45.0, 32.5, 30.3, 29.3, 28.4, 26.6, 25.5, 20.74, 20.70, 20.6. LC-MS (ESI+) calcd for C25H39ClN3O10 [M + H]+ 576.2 found 576.2.
Synthesis of compound 7h: Compound 6 (70 mg, 0.16 mmol), 1-ethynyl-1-cyclohexanol (23 mg, 24 µL, 0.19 mmol), CuI (6.0 mg, 0.032 mmol) and DIEA (24.8 mg, 30 µL, 0.19 mmol). The crude product was purified with 0–30% EtOAc/hexanes to afford an off-white solid (78.9 mg, 0.14 mmol, 88%) as the desired product (Rf = 0.10 in 1:3 EtOAc/hexanes). m.p. 120.0–122.0 °C; 1H NMR (400 MHz, CDCl3) δ 7.48 (s, 1H), 5.22 (t, J = 9.5 Hz, 1H), 5.11 (t, J = 9.9 Hz, 1H), 5.06–5.00 (m, 1H), 4.53 (d, J = 7.9 Hz, 1H), 4.51–4.44 (m, 1H), 4.43–4.36 (m, 1H), 4.28 (dd, J = 12.4, 4.7 Hz, 1H), 4.18 (dd, J = 12.3, 2.4 Hz, 1H), 3.91–3.84 (m, 1H), 3.75–3.68 (m, 1H), 3.56–3.48 (m, 1H), 2.45 (s, 1H), 2.28–2.12 (m, 2H), 2.10 (s, 3H), 2.09 (s, 3H), 2.07–1.95 (m, 8H), 1.93–1.84 (m, 2H), 1.83–1.71 (m, 2H), 1.71–1.54 (m, 3H), 1.45–1.33 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 170.7, 170.2, 169.5, 169.4, 146.5, 120.1, 100.8, 72.7, 72.0, 71.3, 69.6, 68.4, 66.0, 61.9, 46.6, 38.23, 38.17, 30.2, 25.4, 22.0, 20.74, 20.72, 20.6. LC-MS (ESI+) calcd for C25H38N3O11 [M + H]+ 556.2 found 556.2.
Synthesis of compound 7i: Compound 6 (100 mg, 0.24 mmol), 9-ethynyl-9-fluorenol (60 mg, 0.28 mmol), CuI (10.0 mg, 0.048 mmol) and DIEA (37.2 mg, 60 µL, 0.28 mmol) The crude product was purified with 0–30% EtOAc/hexanes to afford an off-white solid (121.7 mg, 0.19 mmol, 79%) as the desired product (Rf = 0.40 in 1:3 EtOAc/hexanes). m.p. 76.0–78.0 °C; 1H NMR (400 MHz, CDCl3) δ 7.68–7.59 (m, 5H), 7.42–7.36 (m, 2H), 7.34–7.28 (m, 2H), 5.16 (t, J = 9.5 Hz, 1H), 5.04 (t, J = 9.7 Hz, 1H), 4.97–4.91 (m, 1H), 4.39–4.29 (m, 3H), 4.20 (dd, J = 12.3, 4.8 Hz, 1H), 4.10–4.04 (m, 1H), 3.87–3.79 (m, 1H), 3.65–3.58 (m, 1H), 3.42–3.34 (m, 1H), 2.26–2.06 (m, 2H), 2.04–2.02 (m, 6H), 2.00 (s, 3H), 1.96 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 170.6, 170.1, 169.39, 169.38, 148.1, 148.0, 139.57, 139.55, 129.51, 129.48, 128.4, 124.8, 120.28, 120.26, 100.7, 78.7, 72.7, 71.9, 71.3, 68.3, 65.9, 61.8, 46.7, 30.0, 20.7, 20.61, 20.57. LC-MS (ESI+) calcd for C32H36N3O11 [M + H]+ 638 found 638.
Synthesis of compound 7j: Compound 6 (100 mg, 0.23 mmol), 8-nonynoic acid (42.5 mg, 0.28 mmol), CuSO4∙5H2O (12.0 mg, 0.048 mmol) and NaAsc (18.2 mg, 0.092 mmol). The crude product was purified with 0–30% EtOAc/hexanes to afford a viscous liquid (112.0 mg, 0.19 mmol, 83%) as the desired product (Rf = 0.19 in 2% MeOH in DCM). 1H NMR (400 MHz, DMSO-d6) δ 7.80 (s, 1H), 5.33–5.20 (m, 1H), 4.91 (t, J = 9.7 Hz, 1H), 4.82–4.75 (m, 2H), 4.31 (t, J = 6.8 Hz, 2H), 4.18 (dd, J = 12.3, 4.9 Hz, 1H), 4.06–3.94 (m, 2H), 3.77–3.69 (m, 2H), 2.58 (t, J = 7.5 Hz, 2H), 2.19 (t, J = 7.3 Hz, 2H), 2.08–1.97 (m, 11H), 1.95 (s, 3H), 1.65–1.42 (m, 4H), 1.39–1.22 (m, 4H); 13C NMR (100 MHz, DMSO-d6) δ 175.0, 170.5, 170.0, 169.8, 169.6, 147.4, 122.1, 99.8, 72.5, 71.4, 71.0, 68.7, 66.4, 62.2, 46.4, 34.1, 30.2, 29.3, 28.73, 28.71, 25.4, 24.9, 20.91, 20.86, 20.81, 20.7. LC-MS (ESI+) calcd for C26H40N3O12 [M + H]+ 586 found 586.
Synthesis of compound 7k: Compound 6 (150 mg, 0.35 mmol), 1,6-heptadiyne (26 µL, 0.23 mmol), CuSO4∙5H2O (12.0 mg, 0.046 mmol) and NaAsc (18.2 mg, 0.092 mmol). The crude product was purified with 10–80% EtOAc/hexanes to afford the monomer 7k as a viscous liquid (46.0 mg, 0.09 mmol, 39%), together with 115.0 mg (52%) of dimeric product. (Rf = 0.50 in 4:1 EtOAc/hexanes). 1H NMR (400 MHz, CDCl3) δ 7.36 (s, 1H), 5.24 (t, J = 9.5 Hz, 1H), 5.11 (t, J = 9.7 Hz, 1H), 5.07–5.01 (m, 1H), 4.53 (d, J = 7.9 Hz, 1H), 4.50–4.34 (m, 2H), 4.29 (dd, J = 12.4, 4.8 Hz, 1H), 4.16 (dd, J = 12.3, 2.3 Hz, 1H), 3.91–3.83 (m, 1H), 3.75–3.68 (m, 1H), 3.54–3.46 (m, 1H), 2.86 (t, J = 7.5 Hz, 2H), 2.27 (dt, J = 7.0, 2.6 Hz, 2H), 2.24–2.12 (m, 2H), 2.10 (s, 6H), 2.05 (s, 3H), 2.03 (s, 3H), 2.00 (t, J = 2.6 Hz, 1H), 1.99–1.88 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 170.6, 170.2, 169.4, 147.3, 121.6, 100.8, 83.8, 72.7, 72.0, 71.3, 68.9, 68.4, 66.0, 61.9, 46.5, 29.7, 28.0, 24.4, 20.74, 20.71, 20.6, 17.8. LC-MS (ESI+) calcd for C24H34N3O10 [M + H]+ 524 found 524.
Synthesis of compound 11a: Compound 10 (100 mg, 0.24 mmol), phenyl acetylene (29.4 mg, 32 µL, 0.28 mmol), CuI (10.0 mg, 0.048 mmol) and DIEA (46.5 mg, 63 µL, 0.36 mmol). The crude product was purified with 0–3% MeOH in DCM to afford a light brown viscous liquid (113 mg, 0.22 mmol, 92%) as the desired product (Rf = 0.35 in 5% MeOH/DCM). 1H NMR (400 MHz, CDCl3) δ 7.94–7.83 (m, 3H), 7.48–7.41 (m, 2H), 7.37–7.31 (m, 1H), 5.42–5.38 (m, 1H), 5.23 (dd, J = 10.4, 7.9 Hz, 1H), 4.99 (dd, J = 10.5, 3.4 Hz, 1H), 4.75–4.67 (m, 1H), 4.61–4.52 (m, 1H), 4.46 (d, J = 7.9 Hz, 1H), 4.36–4.27 (m, 1H), 4.22–4.09 (m, 2H), 3.98–3.87 (m, 2H), 2.16 (s, 3 H), 2.06 (s, 3 H), 1.97 (s, 3 H), 1.73 (s, 3 H); 13C NMR (100 MHz, CDCl3) δ 170.4, 170.1, 170.0, 169.7, 147.6, 130.6, 128.8, 128.1, 125.7, 121.5, 100.9, 70.9, 70.6, 68.5, 67.7, 66.9, 61.2, 50.1, 20.63, 20.59, 20.48, 20.4. LC-MS (ESI+) calcd for C24H30N3O10 [M + H]+ 520.2 found 520.2.
Synthesis of compound 11b: Compound 10 (75 mg, 0.18 mmol), 1-octyne (32 µL, 0.22 mmol), CuSO4∙5H2O (9.0 mg, 0.04 mmol) and NaAsc (16.0 mg, 0.08 mmol). The crude product was purified with 0–3% MeOH in DCM to afford clear viscous liquid (82 mg, 0.15 mmol, 83%) as the desired product (Rf = 0.35 in 5% MeOH/DCM). 1H NMR (400 MHz, CDCl3) δ 7.33 (s, 1H), 5.43–5.35 (m, 1H), 5.24–5.14 (m, 1H), 4.98 (dd, J = 10.4, 3.3 Hz, 1H), 4.62–4.54 (m, 1H), 4.51–4.41 (m, 2H), 4.27–4.19 (m, 1H), 4.20–4.08 (m, 2H), 3.97–3.86 (m, 2H), 2.78–2.61 (m, 2H), 2.16 (s, 3H), 2.05 (s, 3H), 1.98 (s, 3H), 1.94 (s, 3H), 1.73–1.61 (m, 2H), 1.43–1.23 (m, 6H), 0.88 (t, J = 6.6 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 170.3, 170.1, 170.0, 169.4, 148.3, 122.0, 101.0, 70.9, 70.7, 68.6, 67.9, 66.9, 61.2, 49.8, 31.6, 29.4, 29.0, 25.7, 22.6, 20.64, 20.61, 20.5, 14.0.LC-MS (ESI+) calcd for C24H38N3O10 [M + H]+ 528 found 528.
Synthesis of compound 11d: Compound 10 (75 mg, 0.18 mmol), 1-hexadecyne (48.9 mg, 0.22 mmol), CuSO4∙5H2O (9.0 mg, 0.04 mmol) and NaAsc (16.0 mg, 0.08 mmol). The crude product was purified with 0–30% EtOAc/hexanes to afford clear viscous liquid (62 mg, 0.10 mmol, 56%) as the desired product (Rf = 0.21 in 1:3 EtOAc/hexanes). 1H NMR (400 MHz, CDCl3) δ 7.33 (s, 1H), 5.41–5.37 (m, 1H), 5.24–5.16 (m, 1H), 4.98 (dd, J = 10.5, 3.4 Hz, 1H), 4.62–4.55 (m, 1H), 4.51–4.41 (m, 2H), 4.27–4.20 (m, 1H), 4.20–4.07 (m, 2H), 3.96–3.87 (m, 2H), 2.77–2.62 (m, 2H), 2.16 (s, 3H), 2.05 (s, 3H), 1.98 (s, 3H), 1.94 (s, 3H), 1.74–1.62 (m, 2H), 1.40–1.21 (m, 22H), 0.88 (t, J = 6.8 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 170.3, 170.1, 170.0, 169.4, 148.3, 122.0, 101.0, 70.9, 70.7, 68.6, 67.9, 66.9, 61.2, 49.8, 31.9, 29.7, 29.6, 29.5, 29.4, 29.3, 25.8, 22.7, 22.6, 20.6, 20.5, 14.1. LC-MS (ESI+) calcd for C32H54N3O10 [M + H]+ 640 found 640.
Synthesis of compound 11e: Compound 10 (75 mg, 0.18 mmol), 5-phenyl-1-pentyne (32 mg, 34 µL, 0.22 mmol), CuI (7.0 mg, 0.036 mmol) and DIEA (35.0 mg, 47 µL, 0.27 mmol). The crude product was purified with 0–30% EtOAc/hexanes to afford a clear viscous liquid (95.5 mg, 0.17 mmol, 94%) as the desired product (Rf = 0.18 in 1:3 EtOAc/hexanes). 1H NMR (400 MHz, CDCl3) δ 7.36 (s, 1H), 7.32–7.26 (m, 2H), 7.25–7.17 (m, 3H), 5.43–5.38 (m, 1H), 5.21 (dd, J = 10.5, 3.4 Hz, 1H), 5.00 (dd, J = 10.5, 3.4 Hz, 1H), 4.65–4.55 (m, 1H), 4.53–4.44 (m, 2H), 4.29–4.22 (m, 1H), 4.21–4.10 (m, 2H), 3.98–3.88 (m, 2H), 2.83–2.67 (m, 4H), 2.15 (s, 3H), 2.06 (s, 3H), 2.05–2.01 (m, 2H), 2.00 (s, 3H), 1.92 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 170.3, 170.1, 170.0, 169.4, 147.8, 141.9, 128.5, 128.3, 125.8, 122.1, 101.0, 70.9, 70.6, 68.6, 67.8, 66.9, 61.2, 49.8, 35.4, 31.0, 25.2, 20.63, 20.59, 20.5. LC-MS (ESI+) calcd for C27H36N3O10 [M + H]+ 562 found 562.
Synthesis of compound 11i: Compound 10 (100 mg, 0.24 mmol), 1,7-octadiyne (13 mg, 16 µL, 0.12 mmol), CuI (9.0 mg, 0.048 mmol) and DIEA (62.0 mg, 80 µL, 0.48 mmol). The crude product was purified with 0–30% EtOAc/hexanes to afford the monomer 11i as a viscous liquid (67.7 mg, 0.13 mmol, 54%), and 31.8 mg (0.03 mmol, 28.2%) of dimer 11j was also obtained. (Rf = 0.26 in 1:1 EtOAc/hexanes). 1H NMR (400 MHz, CDCl3) δ 7.37 (s, 1H), 5.43–5.38 (m, 1H), 5.24–5.17 (m, 1H), 5.03–4.97 (m, 1H), 4.64–4.55 (m, 1H), 4.53–4.43 (m, 1H), 4.29–4.21 (m, 1H), 4.20–4.10 (m, 2H), 3.97–3.89 (m, 2H), 2.81–2.65 (m, 2H), 2.47–2.15 (m, 5H), 2.06 (s, 3H), 1.99 (s, 3H), 1.97–1.94 (m, 4H), 1.78–1.68 (m, 2H), 1.64–1.46 (m, 4H). 13C NMR (100 MHz, CDCl3) δ 170.3, 170.1, 170.0, 169.4, 147.9, 122.1, 101.0, 84.5, 70.9, 70.6, 68.6, 68.3, 67.8, 66.9, 61.2, 49.9, 28.9, 28.4, 28.2, 25.5, 20.7, 20.63, 20.61, 20.5, 18.3. LC-MS (ESI+) calcd for C25H36N3O10 [M + Na]+ 538 found 538.
Synthesis of compound 11j: Compound 10 (100 mg, 0.24 mmol) and 1,7-octadiyne (13.6 mg, 17 µL, 0.13 mmol), CuSO4∙5H2O (11.0 mg, 0.044 mmol) and NaAsc (17.5 mg, 0.088 mmol). The crude product was purified with 0–3% MeOH in DCM to afford a viscous liquid (86.4 mg, 0.09 mmol, 82%) as the desired product (Rf = 0.31 in 5% MeOH/DCM). 1H NMR (400 MHz, CDCl3) δ 7.38 (s, 2H), 5.43–5.39 (m, 2H), 5.25–5.16 (m, 2H), 5.00 (dd, J = 10.5, 3.4 Hz, 2H), 4.66–4.55 (m, 2H), 4.54–4.42 (m, 4H), 4.31–4.22 (m, 2H), 4.22–4.09 (m, 4H), 3.99–3.85 (m, 4H), 2.83–2.62 (m, 4H), 2.17 (s, 6H), 2.06 (s, 6H), 1.99 (s, 6H), 1.95 (s, 6H), 1.86–1.64 (m, 4H), 1.56–1.42 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 170.3, 170.1, 170.0, 169.4, (triazole at 147 ppm missing), 122.6, 101.0, 70.9, 70.6, 68.5, 67.8, 66.9, 61.2, 49.9, 29.1, 28.9, 25.6, 20.7, 20.63, 20.61, 20.5. LC-MS (ESI+) calcd for C41H59N6O20 [M + H]+ 955 found 955.
Synthesis of compound 14a: Compound 13 (60 mg, 0.14 mmol) and phenyl acetylene (19 mg, 20 µL, 0.17 mmol), CuI (5.5 mg, 0.028 mmol) and DIEA (36.0 mg, 50 µL, 0.28 mmol). The crude product was purified with 0–30% EtOAc/hexanes to afford a semi-solid (68.8 mg, 0.13 mmol, 93%) as the desired product (Rf = 0.37 in 1:1 EtOAc/hexanes). 1H NMR (400 MHz, CDCl3) δ 7.85–7.81 (m, 2H), 7.79 (s, 1H), 7.46–7.40 (m, 2H), 7.36–7.30 (m, 1H), 5.42–5.38 (m, 1H), 5.28–5.21 (m, 1H), 5.03 (dd, J = 10.5, 3.4 Hz, 1H), 4.61–4.40 (m, 3H), 4.20–4.01 (m, 2H), 3.95–3.87 (m, 2H), 3.57–3.48 (m, 1H), 2.33–2.17 (m, 2H), 2.16 (s, 3H), 2.10 (s, 3H), 2.00 (s, 3H), 1.99 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 170.3, 170.2, 170.1, 169.6, 147.8, 130.6, 128.9, 128.2, 125.7, 120.1, 101.4, 70.84, 70.82, 68.9, 67.0, 65.8, 61.3, 46.6, 30.3, 20.9, 20.64, 20.59, 20.55. LC-MS (ESI+) calcd for C25H32N3O10 [M + H]+ 534 found 534.
Synthesis of compound 14b: Compound 13 (60 mg, 0.14 mmol), 1-octyne (18.7 mg, 25 µL, 0.17 mmol), CuI (5.5 mg, 0.028 mmol) and DIEA (36.0 mg, 50 µL, 0.28 mmol). The crude product was purified with 0–30% EtOAc/hexanes to afford a transparent viscous liquid (55.0 mg, 0.10 mmol, 71%) as the desired product (Rf = 0.31 in 1:1 EtOAc/hexanes). 1H NMR (400 MHz, CDCl3) δ 7.27 (s, 1H), 5.40–5.37 (m, 1H), 5.23–5.17 (m, 1H), 5.00 (dd, J = 10.5, 3.4 Hz, 1H), 4.49–4.38 (m, 2H), 4.38–4.29 (m, 1H), 4.18–4.08 (m, 2H), 3.92–3.83 (m, 2H), 3.51–3.42 (m, 1H), 2.69 (t, J = 7.7 Hz, 2H), 2.24–2.09 (m, 5H), 2.08 (s, 3H), 2.02 (s, 3H), 1.97 (s, 3H), 1.69–1.60 (m, 2H), 1.39–1.23 (m, 6H), 0.86 (t, J = 7.0 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 170.3, 170.2, 170.1, 169.6, 148.4, 120.9, 101.3, 70.8, 70.8, 68.9, 67.0, 65.9, 61.2, 46.5, 31.5, 30.3, 29.4, 28.9, 25.6, 22.5, 20.8, 20.6, 20.5, 14.0. LC-MS (ESI+) calcd for C25H40N3O10 [M + H]+ 542.2 found 542.2.
Synthesis of compound 14c: Compound 13 (60 mg, 0.14 mmol), 1-dodecyne (29 mg, 38 µL, 0.17 mmol) CuI (5.5 mg, 0.028 mmol) and DIEA (36.0 mg, 50 µL, 0.28 mmol). The crude product was purified with 0–30% EtOAc/hexanes to afford a white solid (48.9 mg, 0.08 mmol, 57%) as the desired product (Rf = 0.28 in 1:1 EtOAc/hexanes). m.p. 65.0–66.0 °C; 1H NMR (400 MHz, CDCl3) δ 7.26 (triazole 1H under CDCl3 signal), 5.43–5.38 (m, 1H), 5.26–5.19 (m, 1H), 5.03 (dd, J = 10.4, 3.5 Hz, 1H), 4.49–4.40 (m, 2H), 4.38–4.30 (m, 1H), 4.19–4.11 (m, 2H), 3.94–3.86 (m, 2H), 3.52–3.44 (m, 1H), 2.70 (t, J = 7.7 Hz, 2H), 2.24–2.11 (m, 5H), 2.10 (s, 3H), 2.04 (s, 3H), 1.99 (s, 3H), 1.71–1.62 (m, 2H), 1.41–1.21 (m, 14H), 0.88 (t, J = 6.6 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 170.3, 170.2, 170.1, 169.6, 148.5, 120.8, 101.3, 70.84, 70.78, 68.9, 67.0, 66.0, 61.2, 46.4, 31.9, 30.3, 29.59, 29.56, 29.5, 29.4, 29.3, 25.7, 22.7, 20.9, 20.63, 20.55, 14.1. LC-MS (ESI+) calcd for C29H48N3O10 [M + H]+ 598.3 found 598.4.
Synthesis of compound 14d: Compound 13 (60 mg, 0.14 mmol), 1-hexadecyne (38.5 mg, 48 µL, 0.17 mmol), CuI (5.5 mg, 0.028 mmol) and DIEA (36.0 mg, 50 µL, 0.28 mmol). The crude product was purified with 0–30% EtOAc/hexanes to afford a white solid (68.8 mg, 0.11 mmol, 79%) as the desired product (Rf = 0.43 in 3% MeOH/DCM). m.p. 77.0–78.0 °C; 1H NMR (400 MHz, CDCl3) δ 7.25 (s, 1H), 5.43–5.37 (m, 1H), 5.25–5.18 (m, 1H), 5.02 (dd, J = 10.5, 3.4 Hz, 1H), 4.51–4.39 (m, 1H), 4.38–4.29 (m, 1H), 4.19–4.09 (m, 2H), 3.96–3.84 (m, 2H), 3.52–3.43 (m, 1H), 2.69 (t, J = 7.6 Hz, 2H), 2.22–2.12 (m, 5H), 2.09 (s, 3H), 2.03 (s, 3H), 1.99 (s, 3H), 1.73–1.60 (m, 2H), 1.40–1.20 (m, 22H), 0.88 (t, J = 6.9 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 170.3, 170.2, 170.1, 169.6, 148.5, 120.8, 101.3, 70.84, 70.78, 68.9, 67.0, 66.0, 61.2, 46.4, 31.9, 30.3, 29.7, 29.6, 29.5, 29.4, 29.3, 25.7, 22.7, 20.9, 20.6, 20.5, 14.1. LC-MS (ESI+) calcd for C33H56N3O10 [M + H]+ 654.4 found 654.4.
Synthesis of compound 14e: Compound 13 (60 mg, 0.14 mmol), 5-phenyl-1-pentyne (24.5 mg, 25 µL, 0.17 mmol) CuI (5.5 mg, 0.028 mmol) and DIEA (21.7 mg, 30 µL, 0.17 mmol) The crude product was purified with 0–50% EtOAc/hexanes to afford a transparent viscous liquid (56.2 mg, 0.10 mmol, 71%) as the desired product (Rf = 0.29 in 1:1 EtOAc/hexanes). 1H NMR (400 MHz, CDCl3) δ 7.31–7.26 (m, 3H), 7.21–7.15 (m, 3H), 5.42–5.37 (m, 1H), 5.25–5.18 (m, 1H), 5.02 (dd, J = 10.5, 3.4 Hz, 1H), 4.50–4.40 (m, 2H), 4.39–4.30 (m, 1H), 4.18–4.08 (m, 2H), 3.92–3.85 (m, 2H), 3.52–3.43 (m, 1H), 2.75 (t, J = 7.7 Hz, 2H), 2.70 (t, J = 7.6 Hz, 2H), 2.24–2.11 (m, 5H), 2.08 (s, 3H), 2.06–2.01 (m, 5H), 1.99 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 170.3, 170.2, 170.1, 169.6, 147.9, 141.9, 128.5, 128.3, 125.9, 121.0, 101.3, 70.83, 70.79, 68.9, 67.0, 65.9, 61.2, 46.5, 35.4, 31.1, 30.3, 25.2, 20.9, 20.63, 20.55. LC-MS (ESI+) calcd for C28H38N3O10 [M + H]+ 576.2 found 576.2.
Synthesis of compound 14f: Compound 13 (60 mg, 0.14 mmol), 8-chloro-1-octyne (24.6 mg, 28 µL, 0.17 mmol), CuSO4∙5H2O (7.0 mg, 0.028 mmol), NaAsc (11.0 mg, 0.056 mmol). The crude product was purified with 0–50% EtOAc/hexanes to afford a semi solid (56.2 mg, 0.10 mmol, 71%) as the desired product. Rf = 0.14 in 1:1 EtOAc/hexanes; 1H NMR (400 MHz, CDCl3) δ 7.27 (s, 1H), 5.42–5.36 (m, 1H), 5.25–5.18 (m, 1H), 5.02 (dd, J = 10.5, 3.4 Hz, 1H), 4.50–4.39 (m, 2H), 4.38–4.30 (m, 1H), 4.19–4.08 (m, 2H), 3.94–3.83 (m, 2H), 3.52 (t, J = 6.7 Hz, 2H), 3.50–3.46 (m, 1H), 2.70 (t, J = 7.6 Hz, 2H), 2.23–2.10 (m, 5H), 2.09 (s, 3H), 2.03 (s, 3H), 1.98 (s, 3H), 1.81–1.73 (m, 2H), 1.73–1.62 (m, 2H), 1.52–1.43 (m, 2H), 1.43–1.34 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 169.3, 169.2, 169.1, 168.6, 147.1, 119.9, 100.3, 69.83, 69.78, 67.9, 66.0, 65.0, 60.2, 45.4, 44.0, 31.5, 29.3, 28.3, 27.4, 25.6, 24.5, 19.9, 19.6, 19.5. LC-MS (ESI+) calcd for C25H39ClN3O10 [M + H]+ 577 found 577.
Synthesis of compound 14g: Compound 13 (60 mg, 0.14 mmol), propargyl alcohol (9.5 mg, 10 µL, 0.17 mmol), CuI (5.5 mg, 0.028 mmol), DIEA (21.7.0 mg, 30 µL, 0.17 mmol). The crude was purified with 0–5% MeOH in DCM to afford a viscous liquid (55.8 mg, 0.11 mmol, 79%) as the desired product (Rf = 0.25 in 5% MeOH/DCM). 1H NMR (400 MHz, CDCl3) δ 7.56 (s, 1H), 5.39–5.35 (m, 1H), 5.20–5.14 (m, 1H), 5.00 (dd, J = 10.5, 3.4 Hz, 1H), 4.76 (s, 2H), 4.50–4.34 (m, 3H), 4.20–4.13 (m, 1H), 4.12–4.05 (m, 1H), 3.92–3.81 (m, 2H), 3.52–3.44 (m, 1H), 2.26–2.10 (m, 5H), 2.06 (s, 3H), 2.01 (s, 3H), 1.96 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 170.5, 170.2, 170.1, 169.7, 147.7, 122.3, 101.1, 70.8, 68.9, 67.1, 65.8, 61.3, 56.4, 46.8, 30.1, 20.8, 20.6, 20.5. LC-MS (ESI+) calcd for C20H30N3O11 [M + H]+ 488.2 found 488.2.
Synthesis of compound 14h: Compound 13 (70 mg, 0.16 mmol), 1-ethynyl-1-cyclohexanol (23.8 mg, 0.19 mmol), CuI (6.1 mg, 0.032 mmol) and DIEA (24.8 mg, 30 µL, 0.19 mmol). The crude was purified with 0–50% EtOAc/hexanes to afford a semi-solid (61.3 mg, 0.11 mmol, 69%) as the desired product (Rf = 0.17 in 1:1 EtOAc/hexanes). 1H NMR (400 MHz, CDCl3) δ 7.48 (s, 1H), 5.42–7.36 (m, 1H), 5.24–5.16 (m, 1H), 5.02 (dd, J = 10.5, 3.4 Hz, 1H), 4.51–4.33 (m, 3H), 4.21–4.06 (m, 2H), 3.94–3.83 (m, 2H), 3.54–3.45 (m, 1H), 2.57 (br s, 1H), 2.23–2.09 (m, 5H), 2.08 (s, 3H), 2.03 (s, 3H), 1.98–1.93 (m, 4H), 1.91–1.82 (m, 2H), 1.81–1.68 (m, 2H), 1.67–1.47 (m, 4H), 1.42–1.30 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 170.4, 170.2, 170.0, 169.6, 120.1, 101.2, 70.8, 69.5, 68.9, 67.0, 65.9, 61.3, 46.6, 38.2, 30.2, 25.4, 22.0, 20.83, 20.77, 20.69, 20.6, 20.5. LC-MS (ESI+) calcd for C25H38N3O11 [M + H]+ 556.2 found 556.2.