Facile Solvent-Free Mechanochemical Synthesis of UI3 and Lanthanoid Iodides
Abstract
:1. Introduction
2. Materials and Methods
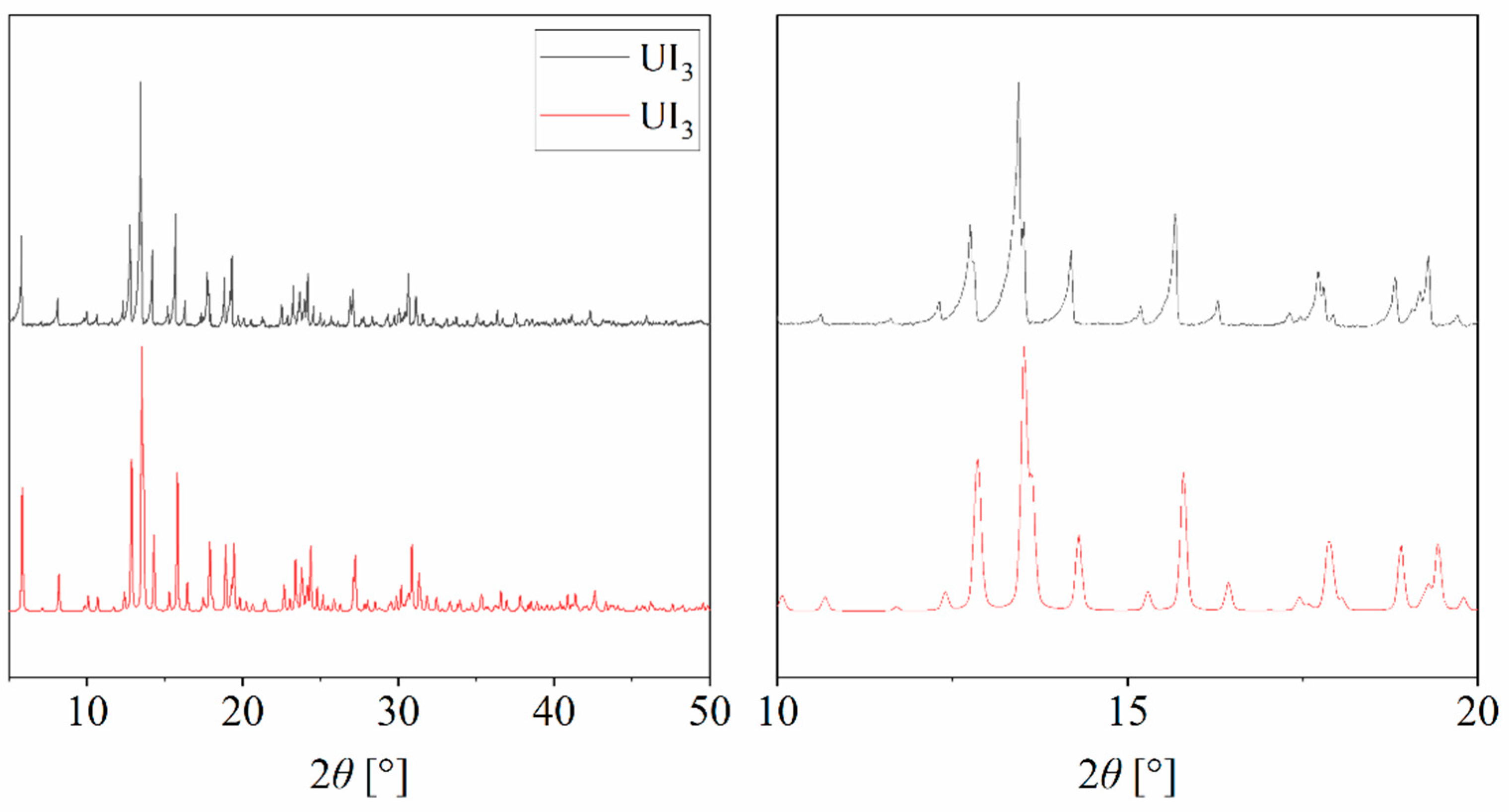
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mougel, V.; Chatelain, L.; Pécaut, J.; Caciuffo, R.; Colineau, E.; Griveau, J.-C.; Mazzanti, M. Uranium and Manganese Assembled in a Wheel-Shaped Nanoscale Single-Molecule Magnet with High Spin-Reversal Barrier. Nat. Chem. 2012, 4, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Chatelain, L.; Walsh, J.P.S.; Pécaut, J.; Tuna, F.; Mazzanti, M. Self-Assembly of a 3d-5f Trinuclear Single-Molecule Magnet from a Pentavalent Uranyl Complex. Angew. Chem. Int. Ed. 2014, 53, 13434–13438. [Google Scholar] [CrossRef] [PubMed]
- Chilton, N.F.; Goodwin, C.A.P.; Mills, D.P.; Winpenny, R.E.P. The First Near-Linear Bis(Amide) f-Block Complex: A Blueprint for a High Temperature Single Molecule Magnet. Chem. Commun. 2015, 51, 101–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, F.-S.; Day, B.M.; Chen, Y.-C.; Tong, M.-L.; Mansikkamäki, A.; Layfield, R.A. A Dysprosium Metallocene Single-Molecule Magnet Functioning at the Axial Limit. Angew. Chem. Int. Ed. 2017, 56, 11445–11449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodwin, C.A.P.; Ortu, F.; Reta, D.; Chilton, N.F.; Mills, D.P. Molecular Magnetic Hysteresis at 60 Kelvin in Dysprosocenium. Nature 2017, 548, 439–442. [Google Scholar] [CrossRef] [Green Version]
- Evans, W.J.; Fang, M.; Zucchi, G.; Furche, F.; Ziller, J.W.; Hoekstra, R.M.; Zink, J.I. Isolation of Dysprosium and Yttrium Complexes of a Three-Electron Reduction Product in the Activation of Dinitrogen, the (N2)3− Radical. J. Am. Chem. Soc. 2009, 131, 11195–11202. [Google Scholar] [CrossRef]
- Formanuik, A.; Ortu, F.; Beekmeyer, R.; Kerridge, A.; Adams, R.W.; Mills, D.P. White Phosphorus Activation by a Th(III) Complex. Dalton Trans. 2016, 45, 2390–2393. [Google Scholar] [CrossRef] [Green Version]
- Langeslay, R.R.; Fieser, M.E.; Ziller, J.W.; Furche, F.; Evans, W.J. Expanding Thorium Hydride Chemistry Through Th2+, Including the Synthesis of a Mixed-Valent Th4+ /Th3+ Hydride Complex. J. Am. Chem. Soc. 2016, 138, 4036–4045. [Google Scholar] [CrossRef]
- Falcone, M.; Chatelain, L.; Scopelliti, R.; Živković, I.; Mazzanti, M. Nitrogen Reduction and Functionalization by a Multimetallic Uranium Nitride Complex. Nature 2017, 547, 332–335. [Google Scholar] [CrossRef] [Green Version]
- Schreckenbach, G.; Shamov, G.A. Theoretical Actinide Molecular Science. Acc. Chem. Res. 2010, 43, 19–29. [Google Scholar] [CrossRef]
- Kefalidis, C.E.; Castro, L.; Perrin, L.; Rosal, I.D.; Maron, L. New Perspectives in Organolanthanide Chemistry from Redox to Bond Metathesis: Insights from Theory. Chem. Soc. Rev. 2016, 45, 2516–2543. [Google Scholar] [CrossRef] [PubMed]
- Kaltsoyannis, N. Seventeen-Coordinate Actinide Helium Complexes. Angew. Chem. Int. Ed. 2017, 56, 7066–7069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanti, J.; Lincoln, M.; Kerridge, A. Decomposition of D- and f-Shell Contributions to Uranium Bonding from the Quantum Theory of Atoms in Molecules: Application to Uranium and Uranyl Halides. Inorganics 2018, 6, 88. [Google Scholar] [CrossRef] [Green Version]
- Nonat, A.; Chan, C.F.; Liu, T.; Platas-Iglesias, C.; Liu, Z.; Wong, W.-T.; Wong, W.-K.; Wong, K.-L.; Charbonnière, L.J. Room Temperature Molecular up Conversion in Solution. Nat. Commun. 2016, 7, 11978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Weitemier, A.Z.; Zeng, X.; He, L.; Wang, X.; Tao, Y.; Huang, A.J.Y.; Hashimotodani, Y.; Kano, M.; Iwasaki, H.; et al. Near-Infrared Deep Brain Stimulation via Upconversion Nanoparticle–Mediated Optogenetics. Science 2018, 359, 679–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasegawa, Y.; Kitagawa, Y.; Nakanishi, T. Effective Photosensitized, Electrosensitized, and Mechanosensitized Luminescence of Lanthanide Complexes. NPG Asia Mater. 2018, 10, 52–70. [Google Scholar] [CrossRef] [Green Version]
- Sørensen, T.J.; Kenwright, A.M.; Faulkner, S. Bimetallic Lanthanide Complexes That Display a Ratiometric Response to Oxygen Concentrations. Chem. Sci. 2015, 6, 2054–2059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, Y.; Yu, J.; Li, Y.; Phua, S.F.Z.; Liu, G.; Lim, W.Q.; Yang, X.; Ganguly, R.; Dang, C.; Yang, C.; et al. Versatile Bimetallic Lanthanide Metal-Organic Frameworks for Tunable Emission and Efficient Fluorescence Sensing. Commun. Chem. 2018, 1, 12. [Google Scholar] [CrossRef] [Green Version]
- Maron, L.; Eisenstein, O. Do f Electrons Play a Role in the Lanthanide−Ligand Bonds? A DFT Study of Ln(NR2)3; R = H, SiH3. J. Phys. Chem. A 2000, 104, 7140–7143. [Google Scholar] [CrossRef]
- Gregson, M.; Lu, E.; Mills, D.P.; Tuna, F.; McInnes, E.J.L.; Hennig, C.; Scheinost, A.C.; McMaster, J.; Lewis, W.; Blake, A.J.; et al. The Inverse-Trans-Influence in Tetravalent Lanthanide and Actinide Bis(Carbene) Complexes. Nat. Commun. 2017, 8, 14137. [Google Scholar] [CrossRef]
- La Pierre, H.S.; Rosenzweig, M.; Kosog, B.; Hauser, C.; Heinemann, F.W.; Liddle, S.T.; Meyer, K. Charge Control of the Inverse Trans-Influence. Chem. Commun. 2015, 51, 16671–16674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaltsoyannis, N. Does Covalency Increase or Decrease across the Actinide Series? Implications for Minor Actinide Partitioning. Inorg. Chem. 2013, 52, 3407–3413. [Google Scholar] [CrossRef] [PubMed]
- Barluzzi, L.; Giblin, S.R.; Mansikkamäki, A.; Layfield, R.A. Identification of Oxidation State +1 in a Molecular Uranium Complex. J. Am. Chem. Soc. 2022, 144, 18229–18233. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, M.R.; Bates, J.E.; Ziller, J.W.; Furche, F.; Evans, W.J. Completing the Series of +2 Ions for the Lanthanide Elements: Synthesis of Molecular Complexes of Pr2+, Gd2+, Tb2+, and Lu2+. J. Am. Chem. Soc. 2013, 135, 9857–9868. [Google Scholar] [CrossRef] [PubMed]
- Langeslay, R.R.; Fieser, M.E.; Ziller, J.W.; Furche, F.; Evans, W.J. Synthesis, Structure, and Reactivity of Crystalline Molecular Complexes of the {[C5H3(SiMe3)2]3Th}1− Anion Containing Thorium in the Formal +2 Oxidation State. Chem. Sci. 2015, 6, 517–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fox, A.R.; Bart, S.C.; Meyer, K.; Cummins, C.C. Towards Uranium Catalysts. Nature 2008, 455, 341–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La Pierre, H.S.; Scheurer, A.; Heinemann, F.W.; Hieringer, W.; Meyer, K. Synthesis and Characterization of a Uranium(II) Monoarene Complex Supported by δ Backbonding. Angew. Chem. Int. Ed. 2014, 53, 7158–7162. [Google Scholar] [CrossRef]
- Guo, F.-S.; Day, B.M.; Chen, Y.-C.; Tong, M.-L.; Mansikkamäki, A.; Layfield, R.A. Magnetic Hysteresis up to 80 Kelvin in a Dysprosium Metallocene Single-Molecule Magnet. Science 2018, 362, 1400–1403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnold, P.L.; Stevens, C.J.; Bell, N.L.; Lord, R.M.; Goldberg, J.M.; Nichol, G.S.; Love, J.B. Multi-Electron Reduction of Sulfur and Carbon Disulfide Using Binuclear Uranium(III) Borohydride Complexes. Chem. Sci. 2017, 8, 3609–3617. [Google Scholar] [CrossRef] [Green Version]
- Evans, W.J.; Kozimor, S.A.; Ziller, J.W.; Fagin, A.A.; Bochkarev, M.N. Facile Syntheses of Unsolvated UI3 and Tetramethylcyclopentadienyl Uranium Halides. Inorg. Chem. 2005, 44, 3993–4000. [Google Scholar] [CrossRef]
- Girard, P.; Namy, J.L.; Kagan, H.B. Divalent Lanthanide Derivatives in Organic Synthesis. 1. Mild Preparation of Samarium Iodide and Ytterbium Iodide and Their Use as Reducing or Coupling Agents. J. Am. Chem. Soc. 1980, 102, 2693–2698. [Google Scholar] [CrossRef]
- Izod, K.; Liddle, S.T.; Clegg, W. A Convenient Route to Lanthanide Triiodide THF Solvates. Crystal Structures of LnI3(THF)4 [Ln = Pr] and LnI3(THF)3.5 [Ln = Nd, Gd, Y]. Inorg. Chem. 2004, 43, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.L.; Sattelberger, A.P.; Bott, S.G.; Vrtis, R.N. Lewis Base Adducts of Uranium Triiodide: A New Class of Synthetically Useful Precursors for Trivalent Uranium Chemistry. Inorg. Chem. 1989, 28, 1771–1773. [Google Scholar] [CrossRef]
- Windorff, C.J.; Dumas, M.T.; Ziller, J.W.; Gaunt, A.J.; Kozimor, S.A.; Evans, W.J. Small-Scale Metal-Based Syntheses of Lanthanide Iodide, Amide, and Cyclopentadienyl Complexes as Analogues for Transuranic Reactions. Inorg. Chem. 2017, 56, 11981–11989. [Google Scholar] [CrossRef] [PubMed]
- Krings, M.; Wessel, M.; Dronskowski, R. EuI2, a Low-Temperature Europium(II) Iodide Phase. Acta Crystallogr. C 2009, 65, i66–i68. [Google Scholar] [CrossRef]
- Asprey, L.B.; Keenan, T.K.; Kruse, F.H. Preparation and Crystal Data for Lanthanide and Actinide Triiodides. Inorg. Chem. 1964, 3, 1137–1141. [Google Scholar] [CrossRef] [Green Version]
- Corbett, J.D.; Simon, A. Lanthanum Triiodide (and Other Rare Earth Metal Triiodides). In Inorganic Syntheses; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1984; pp. 31–36. ISBN 978-0-470-13253-1. [Google Scholar]
- Carmichael, C.D.; Jones, N.A.; Arnold, P.L. Low-Valent Uranium Iodides: Straightforward Solution Syntheses of UI3 and UI4 Etherates. Inorg. Chem. 2008, 47, 8577–8579. [Google Scholar] [CrossRef]
- Hazin, P.N.; Huffman, J.C.; Bruno, J.W. Synthetic and Structural Studies of Pentamethylcyclopentadienyl Complexes of Lanthanum and Cerium. Organometallics 1987, 6, 23–27. [Google Scholar] [CrossRef]
- Wilke, M.; Casati, N. Insight into the Mechanochemical Synthesis and Structural Evolution of Hybrid Organic–Inorganic Guanidinium Lead(II) Iodides. Chem. Eur. J. 2018, 24, 17701–17711. [Google Scholar] [CrossRef] [PubMed]
- Wilke, M.; Casati, N. A New Route to Polyoxometalates via Mechanochemistry. Chem. Sci. 2022, 13, 1146–1151. [Google Scholar] [CrossRef]
- Katsenis, A.D.; Puškarić, A.; Štrukil, V.; Mottillo, C.; Julien, P.A.; Užarević, K.; Pham, M.-H.; Do, T.-O.; Kimber, S.A.J.; Lazić, P.; et al. In Situ X-Ray Diffraction Monitoring of a Mechanochemical Reaction Reveals a Unique Topology Metal-Organic Framework. Nat. Commun. 2015, 6, 6662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beedle, A.E.M.; Mora, M.; Davis, C.T.; Snijders, A.P.; Stirnemann, G.; Garcia-Manyes, S. Forcing the Reversibility of a Mechanochemical Reaction. Nat. Commun. 2018, 9, 3155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, D.; García, F. Main Group Mechanochemistry: From Curiosity to Established Protocols. Chem. Soc. Rev. 2019, 48, 2274–2292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ingner, F.J.L.; Giustra, Z.X.; Novosedlik, S.; Orthaber, A.; Gates, P.J.; Dyrager, C.; Pilarski, L.T. Mechanochemical Synthesis of (Hetero)Aryl Au(I) Complexes. Green Chem. 2020, 22, 5648–5655. [Google Scholar] [CrossRef]
- Alanko, G.A.; Butt, D.P. Mechanochemical Synthesis of Uranium Sesquisilicide. J. Nucl. Mater. 2014, 451, 243–248. [Google Scholar] [CrossRef]
- Woen, D.H.; Kotyk, C.M.; Mueller, T.J.; Ziller, J.W.; Evans, W.J. Tris(Pentamethylcyclopentadienyl) Complexes of Late Lanthanides Tb, Dy, Ho, and Er: Solution and Mechanochemical Syntheses and Structural Comparisons. Organometallics 2017, 36, 4558–4563. [Google Scholar] [CrossRef] [Green Version]
- Fatila, E.M.; Maahs, A.C.; Hetherington, E.E.; Cooper, B.J.; Cooper, R.E.; Daanen, N.N.; Jennings, M.; Skrabalak, S.E.; Preuss, K.E. Stoichiometric Control: 8- and 10-Coordinate Ln(Hfac)3(Bpy) and Ln(Hfac)3(Bpy)2 Complexes of the Early Lanthanides La–Sm. Dalton Trans. 2018, 47, 16232–16241. [Google Scholar] [CrossRef]
- Wegner, W.; Jaroń, T.; Grochala, W. Preparation of a Series of Lanthanide Borohydrides and Their Thermal Decomposition to Refractory Lanthanide Borides. J. Alloys Compd. 2018, 744, 57–63. [Google Scholar] [CrossRef]
- Woen, D.H.; White, J.R.K.; Ziller, J.W.; Evans, W.J. Mechanochemical C–H Bond Activation: Synthesis of the Tuckover Hydrides, (C5Me5)2Ln(μ-H)(μ-H1:H5-CH2C5Me4)Ln(C5Me5) from Solvent-Free Reactions of (C5Me5)2Ln(μ-Ph)2BPh2 with KC5Me5. J. Organomet. Chem. 2019, 899, 120885. [Google Scholar] [CrossRef]
- Szostak, M.; Procter, D.J. Beyond Samarium Diiodide: Vistas in Reductive Chemistry Mediated by Lanthanides(II). Angew. Chem. Int. Ed. 2012, 51, 9238–9256. [Google Scholar] [CrossRef]
- Procter, D.J.; Flowers, R.A.; Skrydstrup, T. Organic Synthesis Using Samarium Diiodide; RSC Publishing: London, UK, 2009; ISBN 978-1-84755-110-8. [Google Scholar]
- Boisson, C.; Berthet, J.C.; Lance, M.; Nierlich, M.; Ephirtikhine, M. Novel Ring-Opening Reaction of Tetrahydrofuran Promoted by a Cationic Uranium Amide Compound. Chem. Commun. 1996, 18, 2129–2130. [Google Scholar] [CrossRef]
- Avens, L.R.; Bott, S.G.; Clark, D.L.; Sattelberger, A.P.; Watkin, J.G.; Zwick, B.D. A Convenient Entry into Trivalent Actinide Chemistry: Synthesis and Characterization of AnI3(THF)4 and An[N(SiMe3)2]3 (An = U, Np, Pu). Inorg. Chem. 1994, 33, 2248–2256. [Google Scholar] [CrossRef]
- Rudel, S.S.; Deubner, H.L.; Scheibe, B.; Conrad, M.; Kraus, F. Facile Syntheses of Pure Uranium(III) Halides: UF3, UCl3, UBr3, and UI3. Z. Für Anorg. Allg. Chem. 2018, 644, 323–329. [Google Scholar] [CrossRef]
- Bärnighausen, H.; Schulz, N. Die Kristallstruktur der monoklinen Form von Europium(II)jodid EuJ2. Acta Crystallogr. B 1969, 25, 1104–1110. [Google Scholar] [CrossRef]
- Simler, T.; Feuerstein, T.J.; Yadav, R.; Gamer, M.T.; Roesky, P.W. Access to Divalent Lanthanide NHC Complexes by Redox-Transmetallation from Silver and CO2 Insertion Reactions. Chem. Commun. 2019, 55, 222–225. [Google Scholar] [CrossRef]
- Silantyeva, L.I.; Ilichev, V.A.; Shavyrin, A.S.; Yablonskiy, A.N.; Rumyantcev, R.V.; Fukin, G.K.; Bochkarev, M.N. Unexpected Findings in a Simple Metathesis Reaction of Europium and Ytterbium Diiodides with Perfluorinated Mercaptobenzothiazolates of Alkali Metals. Organometallics 2020, 39, 2972–2983. [Google Scholar] [CrossRef]
- Bannister, R.D.; Levason, W.; Light, M.E.; Reid, G. Tertiary Phosphine Oxide Complexes of Lanthanide Diiodides and Dibromides. Polyhedron 2018, 154, 259–262. [Google Scholar] [CrossRef]
- Casely, I.J.; Liddle, S.T.; Blake, A.J.; Wilson, C.; Arnold, P.L. Tetravalent Cerium Carbene Complexes. Chem. Commun. 2007, 47, 5037–5039. [Google Scholar] [CrossRef]
- Gregson, M.; Lu, E.; McMaster, J.; Lewis, W.; Blake, A.J.; Liddle, S.T. A Cerium(IV)–Carbon Multiple Bond. Angew. Chem. Int. Ed. 2013, 52, 13016–13019. [Google Scholar] [CrossRef]
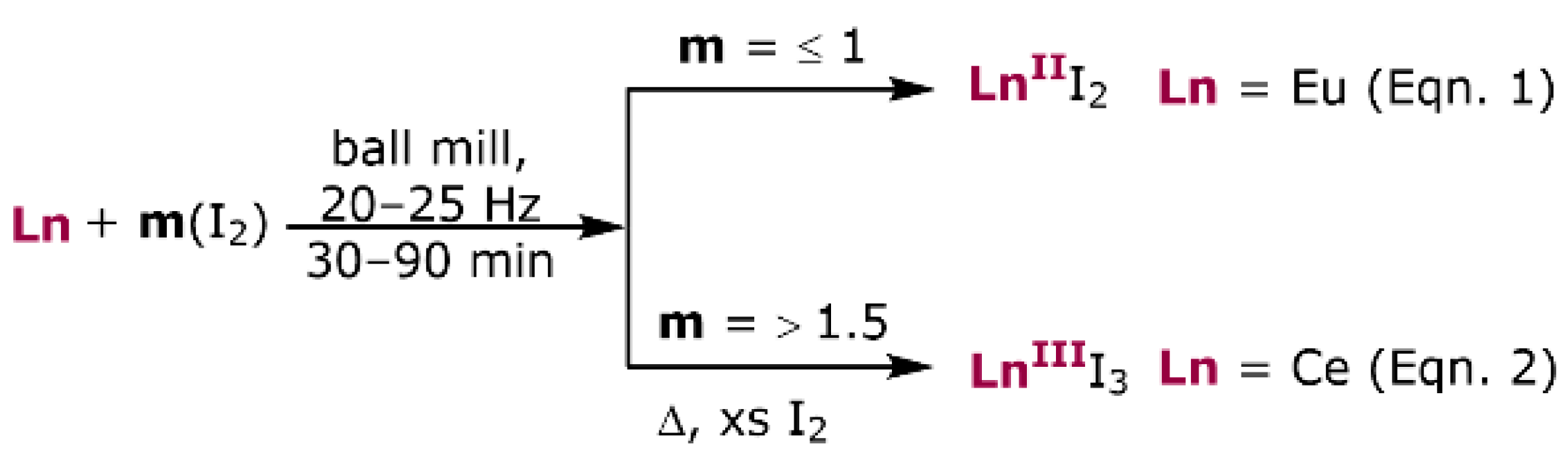
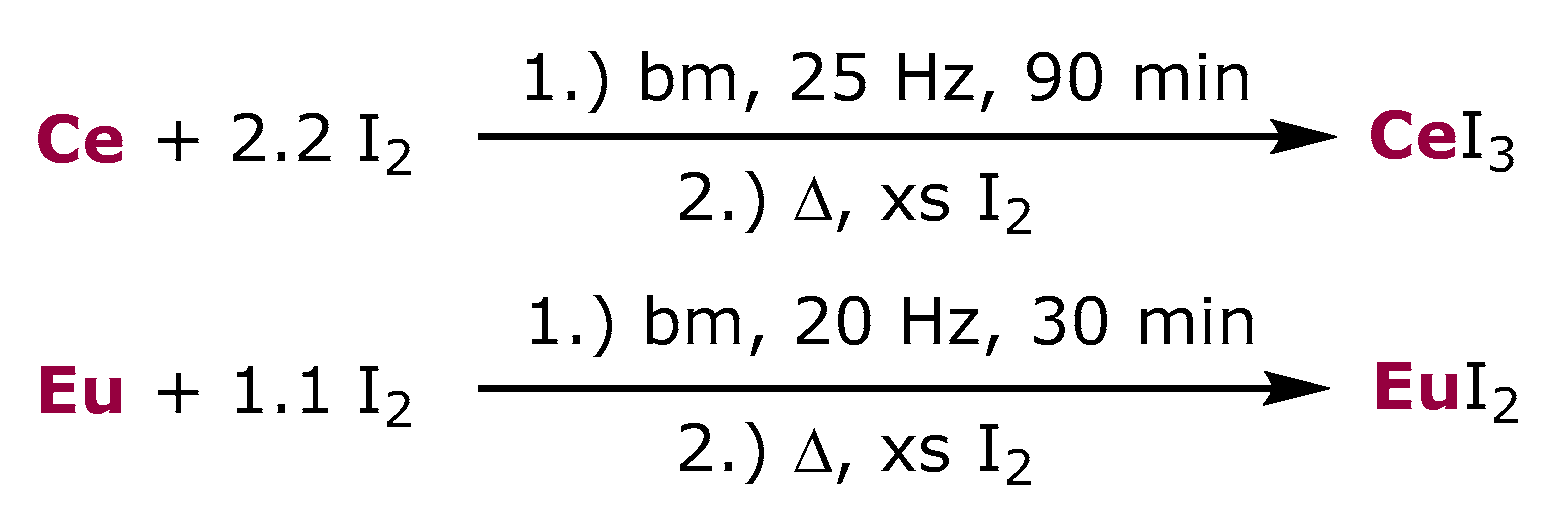



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Werner, D.; Badea, D.; Schönzart, J.; Eimermacher, S.; Bätz, P.; Wickleder, M.S.; Zegke, M. Facile Solvent-Free Mechanochemical Synthesis of UI3 and Lanthanoid Iodides. Chemistry 2022, 4, 1672-1678. https://doi.org/10.3390/chemistry4040108
Werner D, Badea D, Schönzart J, Eimermacher S, Bätz P, Wickleder MS, Zegke M. Facile Solvent-Free Mechanochemical Synthesis of UI3 and Lanthanoid Iodides. Chemistry. 2022; 4(4):1672-1678. https://doi.org/10.3390/chemistry4040108
Chicago/Turabian StyleWerner, Daniel, Désirée Badea, Jasmin Schönzart, Sophia Eimermacher, Philipp Bätz, Mathias S. Wickleder, and Markus Zegke. 2022. "Facile Solvent-Free Mechanochemical Synthesis of UI3 and Lanthanoid Iodides" Chemistry 4, no. 4: 1672-1678. https://doi.org/10.3390/chemistry4040108




