Structural Characterization, Antioxidant, and Antiviral Activity of Sulfated Polysaccharide (Fucoidan) from Sargassum asperifolium (Turner) J. Agardh
Abstract
:1. Introduction
2. Materials and Methodology
2.1. Algal Material
2.2. Collection Site
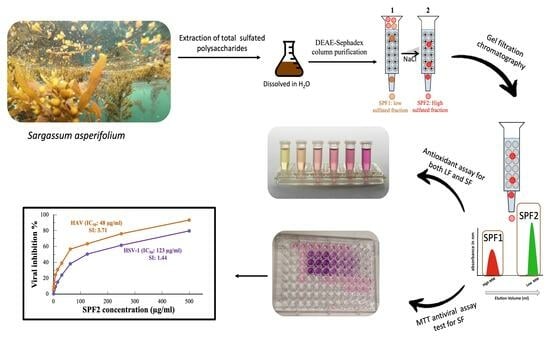
2.3. Extraction
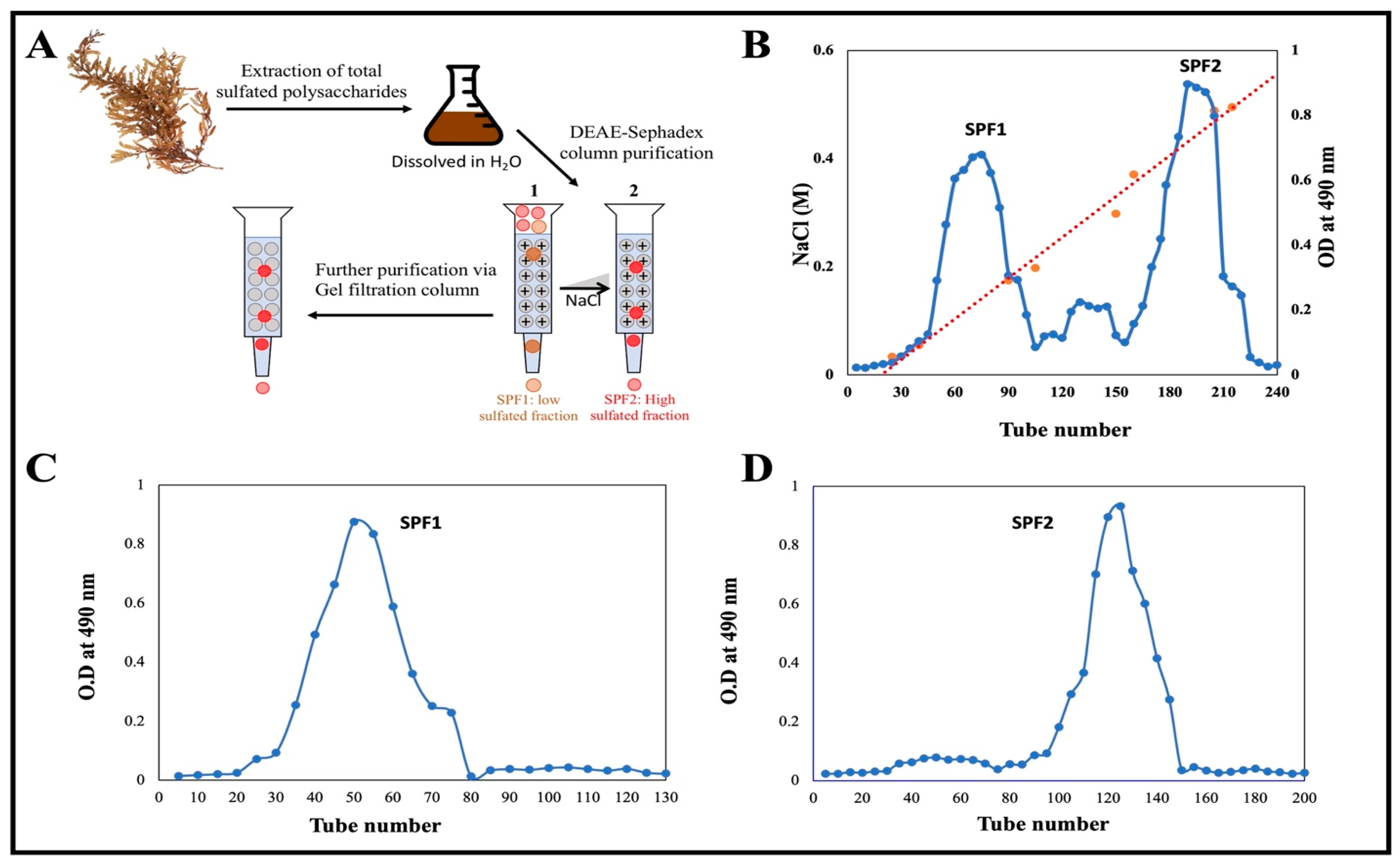
2.4. Fractionation and Purification of Polysaccharide
2.5. Monosaccharide Analysis
2.6. Determination of Total Sulfate Content
2.7. Determination of Relative Molecular Weight of Both SPF1 and SPF2
2.8. Fourier Transform Infrared (FT-IR) Spectroscopy
2.9. 1H and 13C Nuclear Magnetic Resonance (1H and 13C NMR) Spectroscopy
2.10. DPPH Free Radical Scavenging Activity
2.11. Antiviral Activity of SPF2
2.11.1. Mammalian Cell Line
2.11.2. Virus Propagation
2.11.3. Cytotoxicity Evaluation Using Viability Assay
2.11.4. Antiviral Assay
2.12. Statistical Analysis
3. Results and Discussion
3.1. Extraction and Purification of the Sulfated Polysaccharide via Chromatography
3.2. Monosaccharide Compositions of Purified Polysaccharides SPF1 and SPF2
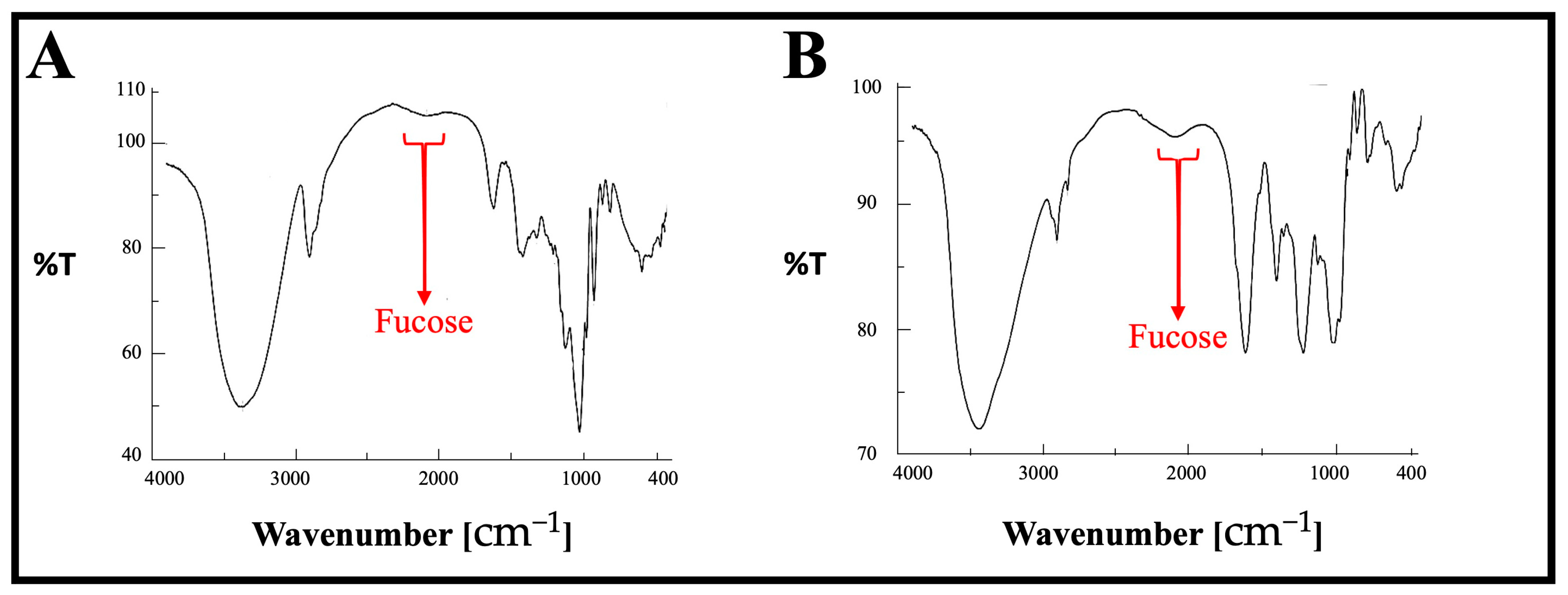
3.3. FT-IR Analysis
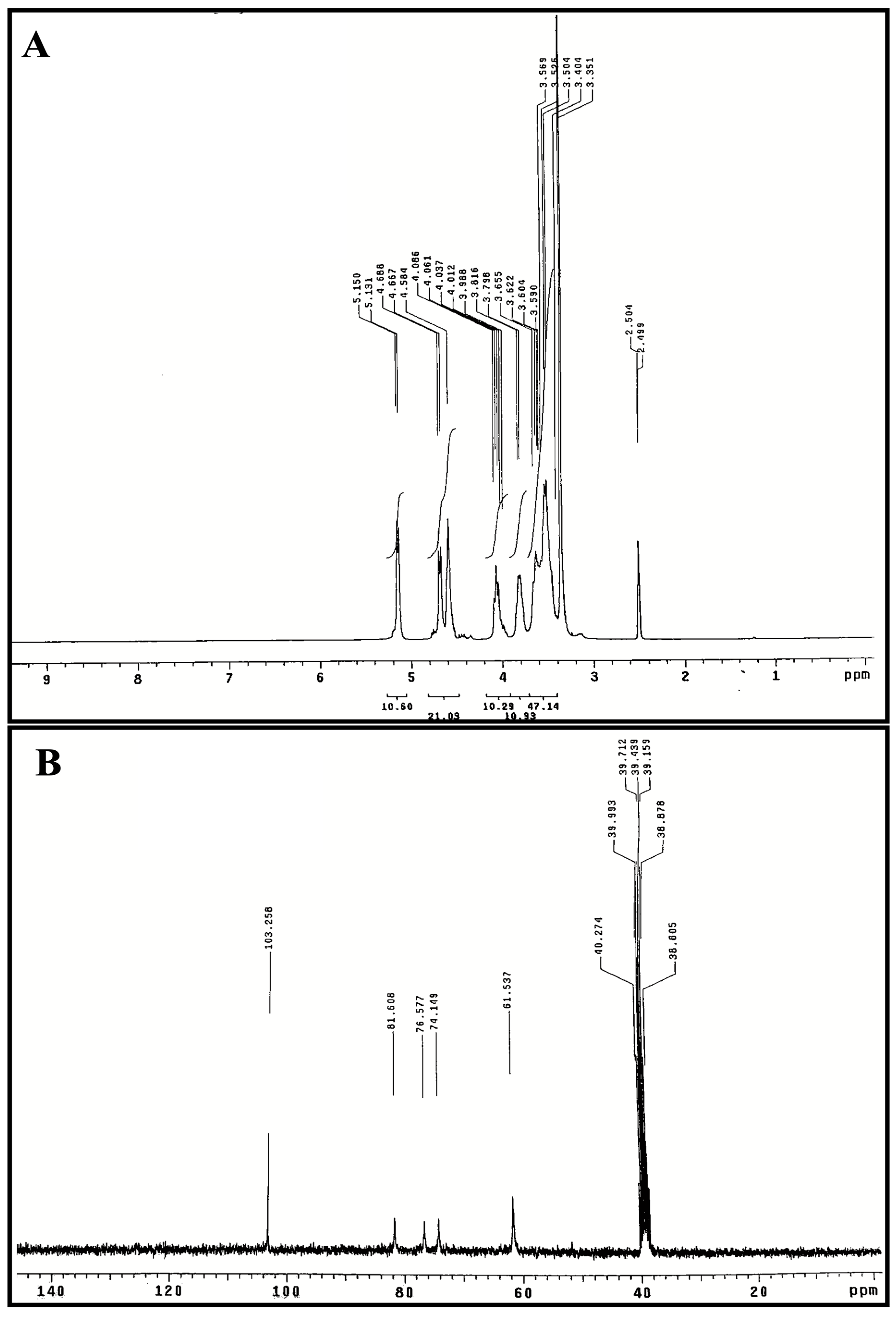
3.4. 1H and 13C NMR Spectroscopy
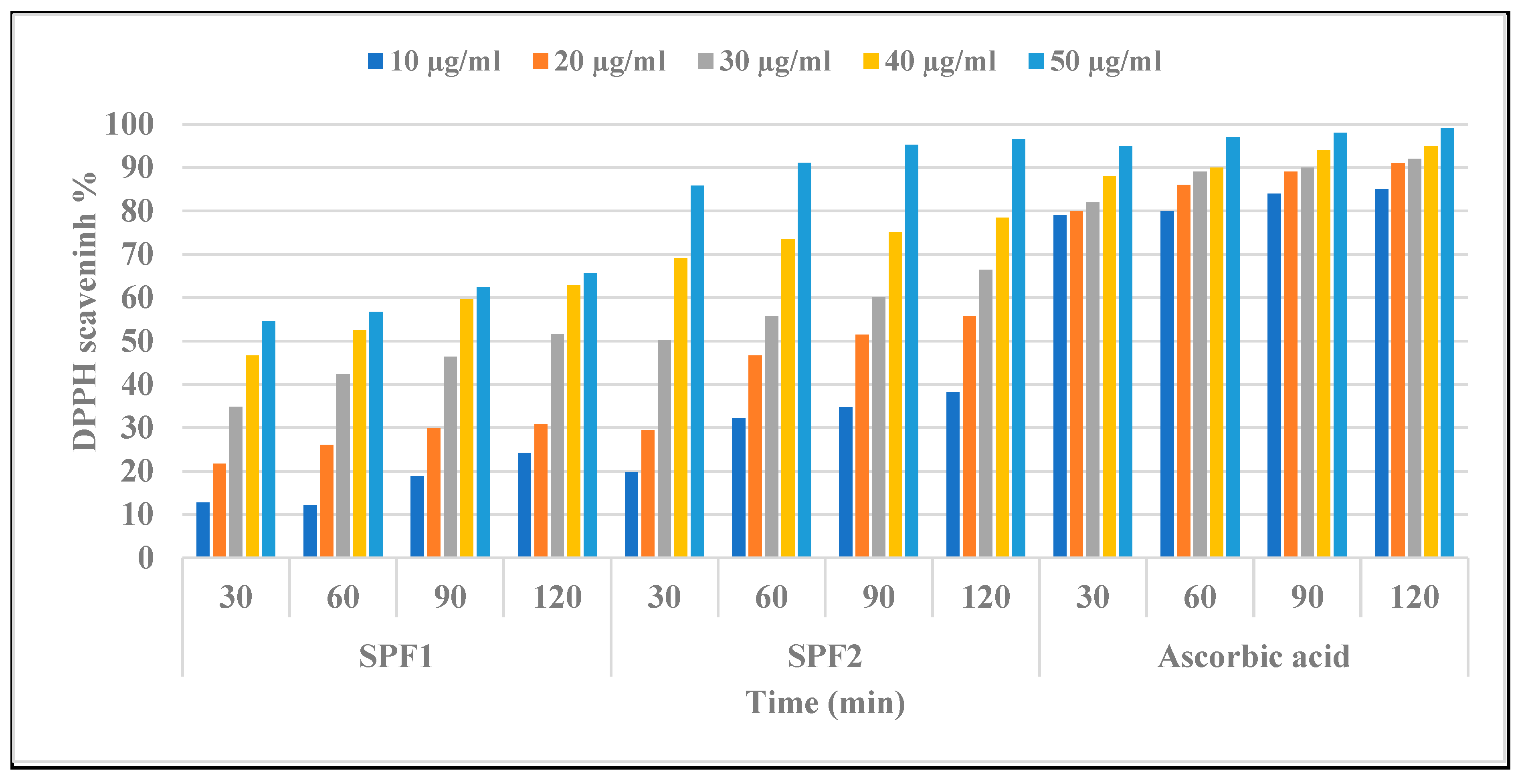
3.5. Assessment of In Vitro Antioxidant Activity of SPF1 and SPF2 Polysaccharides
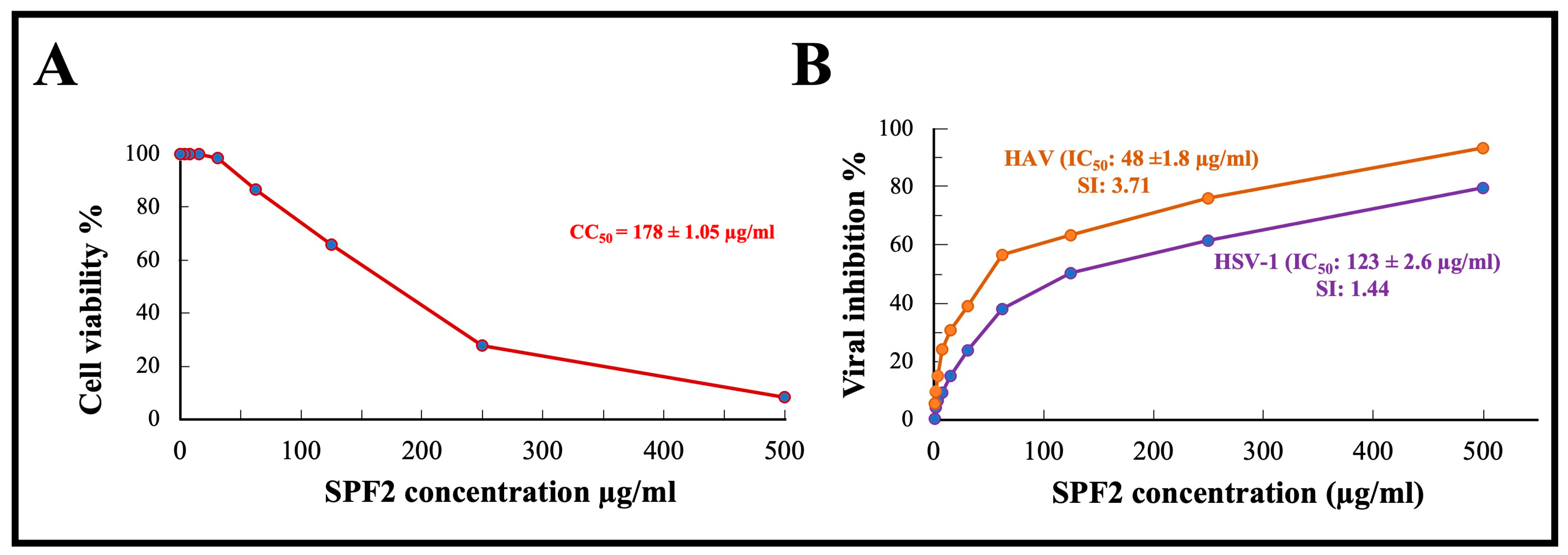
3.6. In Vitro Cytotoxicity of SPF2 Polysaccharide
3.7. Antiviral Activity of SPF2 Polysaccharide
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Di, Y.; Ye, J.; Wei, W. Study on the public psychological states and its related factors during the outbreak of coronavirus disease 2019 (COVID-19) in some regions of China. Psychol. Health Med. 2021, 26, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Hans, N.; Naik, S.N.; Malik, A. Platform Molecules from Algae by Using Supercritical CO2 and Subcritical Water Extraction. In Handbook of Algal Technologies and Phytochemicals: Volume I: Food, Health and Nutraceutical Applications; Ravishankar, G., Ambati, R.R., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 229–243. [Google Scholar]
- Khalid, S.; Abbas, M.; Saeed, F.; Bader-Ul-Ain, H.; Ansar Rasul Suleria, H. Therapeutic potential of seaweed bioactive compounds. In Seaweed Biomaterials; Maiti, S., Ed.; IntechOpen: Rijeka, Croatia, 2018; pp. 7–25. [Google Scholar]
- Mayer, A.M.S.; Rodríguez, A.D.; Berlinck, R.G.S.; Hamann, M.T. Marine pharmacology in 2005-6: Marine compounds with anthelmintic, antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems, and other miscellaneous mechanisms of action. Biochim. Biophys. Acta 2009, 1790, 283–308. [Google Scholar] [PubMed]
- Deniaud-Bouët, E.; Hardouin, K.; Potin, P.; Kloareg, B.; Hervé, C. A review about brown algal cell walls and fucose-containing sulfated polysaccharides: Cell wall context, biomedical properties and key research challenges. Carbohydr. Polym. 2017, 175, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Muthukumar, J.; Chidambaram, R.; Sukumaran, S. Sulfated polysaccharides and its commercial applications in food industries—A review. J. Food Sci. Technol. 2021, 58, 2453–2466. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L. Seaweeds as source of bioactive substances and skin care therapy—Cosmeceuticals, algotheraphy, and thalassotherapy. Cosmetics 2018, 5, 68. [Google Scholar] [CrossRef]
- Kim, K.J.; Lee, O.H.; Lee, B.Y. Fucoidan, a sulfated polysaccharide, inhibits adipogenesis through the mitogen-activated protein kinase pathway in 3T3-L1 preadipocytes. Life Sci. 2010, 86, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Andri Frediansyah, S.S. The antiviral activity of iota-, kappa-, and lambda-carrageenan against COVID-19: A critical review. Clin. Epidemiol. Glob. Health 2021, 12, 100826. [Google Scholar] [CrossRef]
- Jabeen, M.; Dutot, M.; Fagon, R.; Verrier, B.; Monge, C. Seaweed sulfated polysaccharides against respiratory viral infections. Pharmaceutics 2021, 13, 733. [Google Scholar] [CrossRef]
- Reynolds, D.; Huesemann, M.; Edmundson, S.; Sims, A.; Hurst, B.; Cady, S.; Beirne, N.; Freeman, J.; Berger, A.; Gao, S. Viral inhibitors derived from macroalgae, microalgae, and cyanobacteria: A review of antiviral potential throughout pathogenesis. Algal Res. 2021, 57, 102331. [Google Scholar] [CrossRef]
- Hu, T.; Liu, D.; Chen, Y.; Wu, J.; Wang, S. Antioxidant activity of sulfated polysaccharide fractions extracted from Undaria pinnitafida in vitro. Int. J. Biol. Macromol. 2020, 46, 193–198. [Google Scholar] [CrossRef]
- Lim, S.J.; Mustapha, W.A.W.; Maskat, M.Y.; Latip, J.; Badri, K.H.; Hassan, O. Chemical properties and toxicology studies of fucoidan extracted from Malaysian Sargassum binderi. Food Sci. Biotechnol. 2016, 25, 23–29. [Google Scholar] [CrossRef]
- Heald-Sargent, T.; Gallagher, T. Ready, set, fuse! The coronavirus spike protein and acquisition of fusion competence. Viruses 2012, 4, 557–580. [Google Scholar] [CrossRef] [PubMed]
- Belouzard, S.; Millet, J.K.; Licitra, B.N.; Whittaker, G.R. Mechanisms of coronavirus cell entry mediated by the viral spike protein. Viruses 2012, 4, 1011–1033. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.-L.; Li, Y.; Ni, L.-Q.; Li, Y.-X.; Cui, Y.-S.; Jiang, S.-L.; Xie, E.-Y.; Du, J.; Deng, F.; Dong, C.-X. Structural characterization and antiviral activity of two fucoidans from the brown algae Sargassum henslowianum. Carbohydr. Polym. 2020, 229, 115487. [Google Scholar] [CrossRef] [PubMed]
- Laine, R.F.; Albecka, A.; van de Linde, S.; Rees, E.J.; Crump, C.M.; Kaminski, C.F. Structural analysis of herpes simplex virus by optical super-resolution imaging. Nat. Commun. 2015, 6, 5980–5990. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, X.-H.; Luo, Z.; Liu, L.-F.; Yan, C.; Yan, C.-Y.; Chen, G.-D.; Gao, H.; Duan, W.-J.; Kurihara, H.; et al. Traditional chinese medicine as a potential source for HSV-1 therapy by acting on virus or the susceptibility of host. Int. J. Mol. Sci. 2018, 19, 3266–3289. [Google Scholar] [CrossRef] [PubMed]
- Richards, C.M.; Case, R.; Hirst, T.R.; Hill, T.J.; Williams, N.A. Protection against recurrent ocular herpes simplex virus type 1 disease after therapeutic vaccination of latently infected mice. J. Virol. 2003, 77, 6692–6699. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.A.; Harris, E.A. Herpes simplex virus type 1 and other pathogens are key causative factors in sporadic alzheimer’s disease. J. Alzheimers Dis. 2015, 48, 319–353. [Google Scholar] [CrossRef]
- Cristina, J.; Costa-Mattioli, M. Genetic variability and molecular evolution of hepatitis A virus. Virus Res. 2007, 127, 151–157. [Google Scholar] [CrossRef]
- Mohamed, S.F.; Agili, F.A. Antiviral sulphated polysaccharide from brown algae Padina pavonia characterization and structure elucidation. Int. J. ChemTech Res. 2013, 5, 1469–1476. [Google Scholar]
- Asker, M.S.; Mohamed, S.F.; El-Sayed, O.H. Chemical structure and antiviral activity of water-soluble sulfated polysaccharides from Sargassum latifolium. J. Appl. Sci. Res. 2007, 3, 1178–1185. [Google Scholar]
- Josephine, A.; Nithya, K.; Amudha, G.; Veena, C.K.; Preetha, S.P.; Varalakshmi, P. Role of sulphated polysaccharides from Sargassum wightii in cyclosporine A-induced oxidative liver injury in rats. BMC Pharmacol. 2008, 8, 4–12. [Google Scholar] [CrossRef]
- Mattio, L.; Payri, C.E. 190 years of Sargassum taxonomy, facing the advent of DNA phylogenies. Bot. Rev. 2011, 77, 31–70. [Google Scholar] [CrossRef]
- Available online: https://www.google.com/maps/place/Farasan+Island/@17.1544381,33.3202851,5z/data=!4m6!3m5!1s0x160853c66cd55563:0xb3938bc011e607f3!8m2!3d16.705833!4d41.983333!16zL20vMGJibjQ3?entry=tts (accessed on 23 June 2023).
- Silva, T.M.A.; Alves, L.G.; de Queiroz, K.C.S.; Santos, M.G.L.; Marques, C.T.; Chavante, S.F.; Rocha, H.A.O.; Leite, E.L. Partial characterization and anticoagulant activity of a heterofucan from the brown seaweed Padina gymnospora. Braz. J. Med. Biol. Res. 2005, 38, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Y.; Chen, Y.-C. Extraction and characterization of fucoidan from six brown macroalgae. J. Mar. Sci. Technol. 2016, 24, 319–328. [Google Scholar]
- Trabelsi, I.; Slima, S.B.; Ktari, N.; Bardaa, S.; Elkaroui, K.; Abdeslam, A.; Ben Salah, R. Purification, composition and biological activities of a novel heteropolysaccharide extracted from Linum usitatissimum L. seeds on laser burn wound. Int. J. Biol. Macromol. 2020, 144, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Sudhamani, S.R.; Tharanathan, R.N.; Prasad, M.S. Isolation and characterization of an extracellular polysaccharide from Pseudomonas caryophylli CFR 1705. Carbohydr. Polym. 2004, 56, 423–427. [Google Scholar] [CrossRef]
- El-Newary, S.A.; Ibrahim, A.Y.; Asker, M.S.; Mahmoud, M.G.; El Awady, M.E. Production, characterization and biological activities of acidic exopolysaccharide from marine Bacillus amyloliquefaciens 3MS 2017. Asian Pac. J. Trop. Med. 2017, 10, 652–662. [Google Scholar] [CrossRef]
- Kolmert, Å.; Wikström, P.; Hallberg, K.B. A fast and simple turbidimetric method for the determination of sulfate in sulfate-reducing bacterial cultures. J. Microbiol. Methods 2000, 41, 179–184. [Google Scholar] [CrossRef]
- Jun, H.-I.; Lee, C.-H.; Song, G.-S.; Kim, Y.-S. Characterization of the pectic polysaccharides from pumpkin peel. LWT J. Food Sci. Technol. 2006, 39, 554–561. [Google Scholar] [CrossRef]
- Wang, H.; Ooi, E.V.; Ang, P.O. Antiviral polysaccharides isolated from Hong Kong brown seaweed Hydroclathrus clathratus. Sci Chin. C Life Sci. 2007, 50, 611–618. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Gao, Q.; Feng, M.; Yang, B.; Ren, J.; Gu, L.; Cui, C.; Zhao, M. Structural characterisation of polysaccharides from Tricholoma matsutake and their antioxidant and antitumour activities. Food Chem. 2013, 138, 2242–2249. [Google Scholar] [CrossRef] [PubMed]
- Bulu, M.; Dhrubo, J.S.; Beduin, M.; Amit, K.N. Extraction, characterization, haematocompatibility and antioxidant activity of linseed polysaccharide. Carbohydr. Polym. Technol. Appl. 2023, 5, 100321–100330. [Google Scholar]
- Venkatesan, M.; Arumugam, V.; Pugalendi, R.; Ramachandran, K.; Sengodan, K.; Vijayan, S.R.; Sundaresan, U.; Ramachandran, S.; Pugazhendhi, A. Antioxidant, anticoagulant and mosquitocidal properties of water-soluble polysaccharides (WSPs) from Indian seaweeds. Process Biochem. 2019, 84, 196–204. [Google Scholar] [CrossRef]
- Al-Salahi, R.; Alswaidan, I.; Marzouk, M. Cytotoxicity evaluation of a new set of 2-aminobenzo[de]iso-quinoline-1,3-diones. Int. J. Mol. Sci. 2014, 15, 22483–22491. [Google Scholar] [CrossRef] [PubMed]
- Randazzo, W.; Piqueras, J.; Rodríguez-Díaz, J.; Aznar, R.; Sánchez, G. Improving efficiency of viability-qPCR for selective detection of infectious HAV in food and water samples. J. Appl. Microbiol. 2018, 124, 958–964. [Google Scholar] [CrossRef]
- Pintó, R.M.; Diez, J.M.; Bosch, A. Use of the colonic carcinoma cell line CaCo-2 for in vivo amplification and detection of enteric viruses. J. Med. Virol. 1994, 44, 310–315. [Google Scholar] [CrossRef]
- Vijayan, P.; Raghu, C.; Ashok, G.; Dhanaraj, S.A.; Suresh, B. Antiviral activity of medicinal plants of Nilgiris. Ind. J. Med. Res. 2004, 120, 24–29. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Shehabeldine, A.M.; Elbahnasawy, M.A.; Hasaballah, A.I. Green phytosynthesis of silver nanoparticles using Echinochloa stagnina extract with reference to their antibacterial, cytotoxic, and larvicidal activities. Bionanoscience 2021, 11, 526–538. [Google Scholar] [CrossRef]
- Al-Salahi, R.; Alswaidan, I.; Ghabbour, H.A.; Ezzeldin, E.; Elaasser, M.; Marzouk, M. Docking and antiherpetic activity of 2-aminobenzo[de]-isoquinoline-1,3-diones. Molecules 2015, 20, 5099–5111. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.M.; Hsiung, G.D. Evaluation of new antiviral agents: I. In vitro perspectives. Antivir. Res. 1989, 11, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Keivan, Z.; Moloud, A.Z.; Kohzad, S.; Zahra, R. Antiviral activity of Aloe vera against herpes simplex virus type 2: An in vitro study. Afr. J. Adv. Biotechnol. 2007, 6, 1770–1773. [Google Scholar] [CrossRef]
- Ale, M.T.; Maruyama, H.; Tamauchi, H.; Mikkelsen, J.D.; Meyer, A.S. Fucoidan from Sargassum sp. and Fucus vesiculosus reduces cell viability of lung carcinoma and melanoma cells in vitro and activates natural killer cells in mice in vivo. Int. J. Biol. Macromol. 2011, 49, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, H.R.; Biller, P.; Ross, A.B.; Adams, J.M.M. The seasonal variation of fucoidan within three species of brown macroalgae. Algal Res. 2017, 22, 79–86. [Google Scholar] [CrossRef]
- Rioux, L.E.; Turgeon, S.L.; Beaulieu, M. Characterization of polysaccharides extracted from brown seaweeds. Carbohydr. Polym. 2007, 69, 530–537. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Wu, T.-C.; Hsieh, S.-L.; Tsai, Y.-H.; Yeh, C.-W.; Huang, C.-Y. Antioxidant activity and growth inhibition of human colon cancer cells by crude and purified fucoidan preparations extracted from Sargassum cristaefolium. J. Food Drug Anal. 2015, 23, 766–777. [Google Scholar] [CrossRef]
- Béress, A.; Wassermann, O.; Tahhan, S.; Bruhn, T.; Béress, L.; Kraiselburd, E.N.; Gonzalez, L.V.; de Motta, G.E.; Chavez, P.I. A new procedure for the isolation of anti-HIV compounds (polysaccharides and polyphenols) from the marine alga Fucus vesiculosus. J. Nat. Prod. 1993, 56, 478–488. [Google Scholar] [CrossRef]
- Lee, S.-H.; Ko, C.-I.; Ahn, G.; You, S.; Kim, J.-S.; Heu, M.S.; Kim, J.; Jee, Y.; Jeon, Y.-J. Molecular characteristics and anti-inflammatory activity of the fucoidan extracted from Ecklonia Cava. Carbohydr. Polym. 2012, 89, 599–606. [Google Scholar] [CrossRef]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef]
- Synytsya, A.; Kim, W.-J.; Kim, S.-M.; Pohl, R.; Synytsya, A.; Kvasnička, F.; Čopíková, J.; Il Park, Y. Structure and antitumour activity of fucoidan isolated from sporophyll of Korean brown seaweed Undaria pinnatifida. Carbohydr. Polym. 2010, 81, 41–48. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, Y.; Men, Y.; Zhang, J.; Liu, H.; Sun, Y. Structural characterization and immunomodulatory activity of exopolysaccharides from submerged culture of Auricularia auricula-judae. Int. J. Biol. Macromol. 2018, 115, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Zhang, Y.; Zhang, H.; Li, J.; Tao, W.; Linhardt, R.J.; Chen, S.; Ye, X. Physicochemical properties and conformations of water-soluble peach gums via different preparation methods. Food Hydrocoll. 2018, 95, 571–579. [Google Scholar] [CrossRef]
- Ke, H.; Bao, T.; Chen, W. Polysaccharide from Rubus chingii Hu affords protection against palmitic acid-induced lipotoxicity in human hepatocytes. Int. J. Biol. Macromol. 2019, 133, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, W.; Rui, X.; Chen, X.; Jiang, M.; Dong, M. Structural characterization and bioactivity of released exopolysaccharides from Lactobacillus plantarum 70810. Int. J. Biol. Macromol. 2014, 67, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Patankar, M.S.; Oehninger, S.; Barnett, T.; Williams, R.L.; Clark, G.F. A revised structure for fucoidan may explain some of its biological activities. J. Biol. Chem. 1993, 268, 21770–21776. [Google Scholar] [CrossRef]
- Feng, F.; Zhou, Q.; Yang, Y.; Zhao, F.; Du, R.; Han, Y.; Xiao, H.; Zhou, Z. Characterization of highly branched dextran produced by Leuconostoc citreum B-2 from pineapple fermented product. Int. J. Biol. Macromol. 2018, 113, 45–50. [Google Scholar] [CrossRef]
- Rani, R.P.; Anandharaj, M.; Sabhapathy, P.; Ravindran, A.D. Physiochemical and biological characterization of novel exopolysaccharide produced by Bacillus tequilensis FR9 isolated from chicken. Int. J. Biol. Macromol. 2017, 96, 1–10. [Google Scholar] [CrossRef]
- Das, D.; Goyal, A. Characterization and biocompatibility of glucan: A safe food additive from probiotic Lactobacillus plantarum DM5. J. Sci. Food Agric. 2014, 94, 683–690. [Google Scholar] [CrossRef]
- Bilan, M.I.; Grachev, A.A.; Ustuzhanina, N.E.; Shashkov, A.S.; Nifantiev, N.E.; Usov, A.I. A highly regular fraction of a fucoidan from the brown seaweed Fucus distichus. Carbohydr. Res. 2004, 339, 511–517. [Google Scholar] [CrossRef]
- Hu, X.; Pang, X.; Wang, P.G.; Chen, M. Isolation and characterization of an antioxidant exopolysaccharide produced by Bacillus sp. S-1 from Sichuan Pickles. Carbohydr. Polym. 2019, 204, 9–16. [Google Scholar] [CrossRef]
- Mulloy, B.; Mourão, P.A.S.; Gray, E. Structure/function studies of anticoagulant sulphated polysaccharides using NMR. J. Biotechnol. 2000, 77, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.M.; Amer, M.S. Characterization and biological properties of sulfated polysaccharides of Corallina officinalis and Pterocladia capillacea. Acta Bot. Bras. 2020, 34, 623–632. [Google Scholar] [CrossRef]
- Zhong, R.; Chen, Y.; Ling, J.; Xia, Z.; Zhan, Y.; Sun, E.; Shi, Z.; Feng, L.; Jia, X.; Song, J.; et al. The toxicity and metabolism properties of herba epimedii flavonoids on laval and adult zebrafish. Evid. Based Complement. Altern. Med. 2019, 2019, 3745051–3745060. [Google Scholar]
- Sae-Lao, T.; Tohtong, R.; Bates, D.O.; Wongprasert, K. Sulfated galactans from red seaweed Gracilaria fisheri target EGFR and inhibit cholangiocarcinoma cell proliferation. Am. J. Chin. Med. 2017, 45, 615–633. [Google Scholar] [CrossRef]
- Song, S.; Peng, H.; Wang, Q.; Liu, Z.; Dong, X.; Wen, C.; Ai, C.; Zhang, Y.; Wang, Z.; Zhu, B. Inhibitory activities of marine sulfated polysaccharides against SARS-CoV-2. Food Funct. 2020, 11, 7415–7420. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, K.C.S.; Medeiros, V.P.; Queiroz, L.S.; Abreu, L.R.D.; Rocha, H.A.O.; Ferreira, C.V.; Jucá, M.B.; Aoyama, H.; Leite, E.L. Inhibition of reverse transcriptase activity of HIV by polysaccharides of brown algae. Biomed. Pharmacother. 2008, 62, 303–307. [Google Scholar] [CrossRef]
- Dwi, L.W.; Ningsih, A.T.; Ita, W.; Rexie, M.; Anicia, H.; Christel, M.; Nathalie, B. The potential of cytotoxin and antiviral in Sargassum polycystum and Sargassum ilicifolium’s polysaccharides extract. Indones. J. Mar. Sci. 2020, 25, 91–96. [Google Scholar]
- Gilles, B.; Edgar, C.; Romain, B.; Christel, M.; Nathalie, B.; Yolanda, F.; Daniel, R. Antiviral and cytotoxic activities of polysaccharides extracted from four tropical seaweed species. Nat. Prod. Commun. 2017, 12, 807–811. [Google Scholar]
- Hugo, P.; Kévin, H.; Gilles, B.; Christel, M.; Stéphane, C.; Yolanda, F.; Daniel, R.; Nathalie, B. Sulfated polysaccharides from seaweed strandings as renewable source for potential antivirals against Herpes simplex virus 1. Mar. Drugs 2022, 20, 116–138. [Google Scholar]

| SP | Molecular Mass Moments (g/mol or Da) | Monosugars Molar Ratio | Uronic Acid % | Sulfate % | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mw | Mn | PI * | Glucuronic Acid | Glucose | Mannose | Fucose | |||
| SPF1 | 5.49 × 105 | 4.95 × 105 | 1.1 | 1.8 | 2.8 | 1.9 | 1.0 | 21.08 | 9.65 |
| SPF2 | 3.46 × 105 | 1.97 × 105 | 1.75 | 1.6 | 2.5 | 1.0 | 1.8 | 25.34 | 15.32 |
| Sample | Frequency (cm−1) of Spectra | ||||||
|---|---|---|---|---|---|---|---|
| 1229–1252 | 825–840 | 3405–3445 | 2924.08 | 2121–2143 | 1410–1456 | 1035–1070 | |
| SPF1 | 1219.76 | 819.59 | 3373.85 | 2926.45 | 2113.6 | 1431.89 | 1030.77 |
| SPF2 | 1237.11 | 820.563 | 3433.64 | 2919.7 | 2116.49 | 1425.14 | 1032.69 |
| Functional group | S=O | C-O-S | O-H | C-H | Fucose | CO within COOH | C-O-C glucosides |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ageeli, A.A.; Mohamed, S.F. Structural Characterization, Antioxidant, and Antiviral Activity of Sulfated Polysaccharide (Fucoidan) from Sargassum asperifolium (Turner) J. Agardh. Chemistry 2023, 5, 2756-2771. https://doi.org/10.3390/chemistry5040176
Ageeli AA, Mohamed SF. Structural Characterization, Antioxidant, and Antiviral Activity of Sulfated Polysaccharide (Fucoidan) from Sargassum asperifolium (Turner) J. Agardh. Chemistry. 2023; 5(4):2756-2771. https://doi.org/10.3390/chemistry5040176
Chicago/Turabian StyleAgeeli, Abeer A., and Sahera Fathalla Mohamed. 2023. "Structural Characterization, Antioxidant, and Antiviral Activity of Sulfated Polysaccharide (Fucoidan) from Sargassum asperifolium (Turner) J. Agardh" Chemistry 5, no. 4: 2756-2771. https://doi.org/10.3390/chemistry5040176