New Treatment Option for Clinical and Subclinical Mastitis in Dairy Cows Using Acoustic Pulse Technology (APT)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Layout
2.2. Acoustic Pulse Technology and Antibiotic Treatment Protocols
2.3. Recovery from Inflammation
2.4. Statistical Analyses
2.4.1. Subclinical Mastitis
- (i)
- Log SCC was analyzed from the data of the milk recording days (0, 30, 60, and 90 days after subclinical mastitis diagnosis) by PROC MIXED procedure of SAS with the general form: log SCC = Intercept + Herd + Lactation number + Group + Time + Group × Time + error, where: Herd = 4 dairy farms; Lactation number = 1, 2, 3, or more; Group = 2 experimental groups (APT vs. NT); Time = 4 different time points.
- (ii)
- Daily MY was tested throughout the study period (90 days) by PROC MIXED procedure of SAS with the general form: Daily MY = Intercept + Herd + Lactation number + Group + Day from treatment + Group × Day from treatment + error, where: Herd = 4 dairy farms; Lactation number = 1, 2, 3 or more lactations; Group = 2 experimental groups (APT vs. NT). All variables were considered as fixed effects except for days from treatment, which was considered a continuous effect.
- (iii)
- Because subclinical mastitis was diagnosed at different times during the lactation and because of the strong effect of DIM on milk level, another test was performed to check the effect of time of infection on MY. This analysis included period of mastitis: 0–50 days, 51–120 days, 121–220 days. The statistical model was produced by PROC MIXED procedure of SAS with the general form: Daily MY = Intercept + Herd + Lactation number + Group + Day from treatment + Period + Group × Day from treatment + Group × Period + error, where: Herd = 4 dairy farms; Lactation number = 1, 2, 3, or more lactations; Group = 2 experimental groups (APT vs. NT); Period = the 3 periods of mastitis. All variables were considered fixed effects except for days from treatment which was considered a continuous effect.
2.4.2. Clinical Mastitis
- (i)
- Chi-square test was used for univariate examination of the association between recovered vs. non-recovered cows and type of bacteria causing the mastitis.
- (ii)
- Milk yield of the clinical mastitis group was total milk produced by the APT or AB cows at different time points during the study (0, 30, 60, and 90 days). At each time point, the sum of MY by all cows in the group was calculated and the percentage of change from time 0 was calculated. The statistical model tested the difference in milk % compared to time 0 (which was considered as 100%) at every time point in each experimental group. For the statistical analysis, we used the PROC MIXED procedure of SAS with the general form: % of milk produced in each group = Intercept + Herd + Lactation number + Group + Time + Group × Time + error, where: Herd = 7 dairy farms; Lactation number = 1, 2, 3 or more lactations; Group = 2 experimental groups (APT vs. AB); Time = 4 different time points.
3. Results
3.1. Cows with Subclinical Mastitis
3.1.1. Recovery from Mastitis
3.1.2. Milk Yield
3.2. Cows with Clinical Mastitis
3.2.1. Recovery
3.2.2. Milk Yield
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, C.; Holfeld, J.; Schaden, W.; Orgill, D.; Ogawa, R. Mechanotherapy: Revisiting physical therapy and recruiting mechanobiology for a new era in medicine. Trends Mol. Med. 2013, 19, 555–564. [Google Scholar] [CrossRef]
- Rohringer, S.; Holnthoner, W.; Hackl, M.; Weihs, A.M.; Rünzler, D.; Skalicky, S.; Karbiener, M.; Scheideler, M.; Pröll, J.; Gabriel, C.; et al. Molecular and cellular effects of in vitro shockwave treatment on lymphatic endothelial cells. PLoS ONE 2014, 9, e114806. [Google Scholar] [CrossRef]
- d’Agostino, M.C.; Craig, K.; Tibalt, E.; Respizzi, S. Shock wave as biological therapeutic tool: From mechanical stimulation to recovery and healing, through mechanotransduction. Int. J. Surg. 2015, 24, 147–153. [Google Scholar] [CrossRef] [PubMed]
- de Girolamo, L.; Stanco, D.; Galliera, E.; Vigano, M.; Lovati, A.B.; Marazzi, M.G.; Romeo, P.; Sansone, V. Soft-focused extracorporeal shock waves increase the expression of tendon-specific markers and the release of anti-inflammatory cytokines in an adherent culture model of primary human tendon cells. Ultrasound Med. Biol. 2014, 40, 1204–1215. [Google Scholar] [CrossRef]
- Ito, K.; Fukumoto, Y.; Shimokawa, H. Extracorporeal shock wave therapy for ischemic cardiovascular disorders. Am. J. Cardiovasc. Drugs 2011, 11, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Holfeld, J.; Tepeköylü, C.; Blunder, S.; Lobenwein, D.; Kirchmair, E.; Dietl, M.; Kozaryn, R.; Lener, D.; Theurl, M.; Paulus, P.; et al. Low energy shock wave therapy induces angiogenesis in acute hind-limb ischemia via VEGF receptor 2 phosphorylation. PLoS ONE 2014, 9, e103982. [Google Scholar] [CrossRef]
- Tara, S.; Miyamoto, M.; Takagi, G.; Kirinoki-Ichikawa, S.; Tezuka, A.; Hada, T.; Takagi, I. Low-energy extracorporeal shock wave therapy improves microcirculation blood flow of ischemic limbs in patients with peripheral arterial disease: Pilot study. J. Nippon Med. Sch. 2014, 81, 19–27. [Google Scholar] [CrossRef] [Green Version]
- Waugh, C.; Morrissey, D.; Jones, E.; Riley, G.; Langberg, H.; Screen, H. In vivo biological response to extracorporeal shockwave therapy in human tendinopathy: Response of tendinopathy to shockwave therapy. Eur. Cells Mater. 2015, 29, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.A.; Stojadinovic, A.; Anam, K.; Amare, M.; Naik, S.; Peoples, G.E.; Tadaki, D.; Elster, E.A. Extracorporeal shock wave therapy suppresses the early proinflammatory immune response to a severe cutaneous burn injury. Int. Wound J. 2009, 6, 11–21. [Google Scholar] [CrossRef]
- Shao, P.L.; Chiu, C.C.; Yuen, C.M.; Chua, S.; Chang, L.T.; Sheu, J.J.; Sun, C.K.; Wu, C.J.; Wang, C.J.; Yip, H.K. Shock wave therapy effectively attenuates inflammation in rat carotid artery following endothelial denudation by balloon catheter. Cardiology 2010, 115, 130–144. [Google Scholar] [CrossRef]
- Mantovani, A.; Biswas, S.K.; Galdiero, M.R.; Sica, A.; Locati, M. Macrophage plasticity and polarization in tissue repair and remodeling. J. Pathol. 2013, 229, 176–185. [Google Scholar] [CrossRef]
- Yip, L.; Woehrle, T.; Corriden, R.; Hirsh, M.; Chen, Y.; Inoue, Y.; Ferrari, V.; Insel, P.A.; Junger, W.G. Autocrine regulation of T-cell activation by ATP release and P2X7 receptors. FASEB J. 2009, 23, 1685–1693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, T.; Junger, W.G.; Yuan, C.; Jin, A.; Zhao, Y.; Zheng, X.; Zeng, Y.; Liu, J. Shockwaves increase T-cell proliferation and IL-2 expression through ATP release, P2X7 receptors, and FAK activation. Am. J. Physiol. Cell Physiol. 2010, 298, C457–C464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porcherie, A.; Cunha, P.; Trotereau, A.; Roussel, P.; Gilbert, F.B.; Rainard, P.; Germon, P. Repertoire of Escherichia coli agonists sensed by innate immunity receptors of the bovine udder and mammary epithelial cells. Vet. Res. 2012, 43, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Blum, S.E.; Heller, E.D.; Jacoby, S.; Krifuks, O.; Leitner, G. Comparison of the immune responses associated with experimental bovine mastitis caused by different strains of Escherichia coli. J. Dairy Res. 2017, 84, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Oviedo-Boyso, J.; Valdez-Alarcón, J.J.; Cajero-Juárez, M.; Ochoa-Zarzosa, A.; López-Meza, J.E.; Bravo-Patino, A.; Baizabal-Aguirre, V.M. Innate immune response of bovine mammary gland to pathogenic bacteria responsible for mastitis. J. Infect. 2007, 54, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Blum, S.E.; Heller, E.D.; Jacoby, S.; Krifuks, O.; Merin, U.; Silanikove, N.; Lavon, Y.; Leitner, G. Physiological response of mammary glands to Escherichia coli infection: A conflict between glucose need for milk production and immune response. Sci. Rep. 2020, 10, 9602. [Google Scholar] [CrossRef]
- Akers, R.M.; Nickerson, S.C. Mastitis and its impact on structure and function in the ruminant mammary gland. J. Mammary Gland Biol. Neoplasia 2011, 16, 275–289. [Google Scholar] [CrossRef]
- Leitner, G.; Merin, U.; Silanikove, N. Effects of glandular bacterial infection and stage of lactation on milk clotting parameters: Comparison among cows, goats and sheep. Int. Dairy J. 2011, 21, 279–285. [Google Scholar] [CrossRef]
- Leitner, G.; Blum, S.E.; Krifuks, O.; Edery, N.; Merin, U. Correlation between milk bacteriology, cytology and mammary tissue histology in cows: Cure from the pathogen or recovery from the inflammation. Pathogens 2020, 9, 364. [Google Scholar] [CrossRef]
- Akers, R.M. A 100-year review: Mammary development and lactation. J. Dairy Sci. 2017, 100, 10332–10352. [Google Scholar] [CrossRef]
- Leitner, G.; Zilberman, D.; Papirov, E.; Shefy, S. Assessment of acoustic pulse therapy (APT), a non-antibiotic treatment for dairy cows with clinical and subclinical mastitis. PLoS ONE 2018, 13, e0199195. [Google Scholar] [CrossRef] [PubMed]
- Afimilk. AfiFarm Herd Management Data-Acquisition System. Available online: http://www.afimilk.com (accessed on 12 June 2020).
- SCR. SCR Dairy Cow Monitoring and Herd Management systems. Available online: http://www.scrdairy.com (accessed on 12 June 2020).
- Weigel, K.A.; Palmer, R.W.; Caraviello, D.Z. Investigation of factors affecting voluntary and involuntary culling in expanding herds in Wisconsin using survival analysis. J. Dairy Sci. 2003, 86, 1482–1486. [Google Scholar] [CrossRef]
- De Vries, A. Cow longevity economics: The cost benefit of keeping the cow in the herd. In Proceedings of the Cow Longevity Conference, Stockholm, Sweden, 27–28 August 2013. [Google Scholar]
- De Vries, A. Economic trade-offs between genetic improvement and longevity in dairy cattle. J. Dairy Sci. 2017, 100, 4184–4192. [Google Scholar] [CrossRef]
- Beaudeau, F.; Seegers, H.; Ducrocq, V.; Fourichon, C.; Bareille, N. Effect of health disorders on culling in dairy cows: A review and critical discussion. Ann. Zootech. 2000, 49, 293–311. [Google Scholar] [CrossRef]
- Cha, E.; Bar, D.; Hertl, J.A.; Tauer, L.W.; Bennett, G.; González, R.N.; Schukken, Y.H.; Welcome, F.L.; Gröhn, Y.T. The cost and management of different types of clinical mastitis in dairy cows estimated by dynamic programming. J. Dairy Sci. 2011, 94, 4476–4487. [Google Scholar] [CrossRef] [Green Version]
- Cha, E.; Hertl, J.A.; Bar, D.; Gröhn, Y.T. The cost of different types of lameness in dairy cows calculated by dynamic programming. Prevent. Vet. Med. 2010, 97, 1–8. [Google Scholar] [CrossRef]
- Halasa, T.; Huijps, K.; Østerås, O.; Hogeveen, H. Economic effects of bovine mastitis and mastitis management: A review. Vet. Q. 2007, 29, 18–31. [Google Scholar] [CrossRef]
- Economou, V.; Gousia, P. Agriculture and food animals as a source of antimicrobial-resistant bacteria. Infect. Drug Resist. 2015, 8, 49–61. [Google Scholar] [CrossRef] [Green Version]
- Magouras, I.; Carmo, L.P.; Stärk, K.D.; Schüpbach-Regula, G. Antimicrobial usage and-resistance in livestock: Where should we focus? Front. Vet. Sci. 2017, 4, 148. [Google Scholar] [CrossRef] [Green Version]
- Pyörälä, S. Treatment of mastitis during lactation. Ir. Vet. J. 2009, 62, S40–S44. [Google Scholar] [CrossRef] [Green Version]


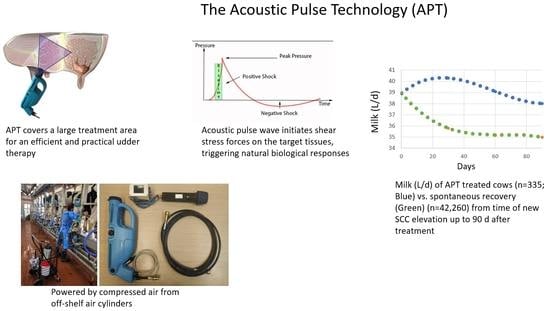
 ) or no treatment (NT) as a control (n = 59;
) or no treatment (NT) as a control (n = 59;  ). The trend line for MY was significantly higher (P < 0.024) in the APT vs. NT group.
). The trend line for MY was significantly higher (P < 0.024) in the APT vs. NT group.
 ) or no treatment (NT) as a control (n = 59;
) or no treatment (NT) as a control (n = 59;  ). The trend line for MY was significantly higher (P < 0.024) in the APT vs. NT group.
). The trend line for MY was significantly higher (P < 0.024) in the APT vs. NT group.
 ) or not treated (NT;
) or not treated (NT;  ) during different periods of lactation. (A) Period 1—from parturition to 50 days postpartum, APT (n = 16) vs. NT (n = 24). (B) Period 2—51–120 days postpartum, APT (n = 23) vs. NT (n = 13). (C) Period 3—121–220 days postpartum, APT (n = 16) vs. NT (n = 18).
) during different periods of lactation. (A) Period 1—from parturition to 50 days postpartum, APT (n = 16) vs. NT (n = 24). (B) Period 2—51–120 days postpartum, APT (n = 23) vs. NT (n = 13). (C) Period 3—121–220 days postpartum, APT (n = 16) vs. NT (n = 18).
 ) or not treated (NT;
) or not treated (NT;  ) during different periods of lactation. (A) Period 1—from parturition to 50 days postpartum, APT (n = 16) vs. NT (n = 24). (B) Period 2—51–120 days postpartum, APT (n = 23) vs. NT (n = 13). (C) Period 3—121–220 days postpartum, APT (n = 16) vs. NT (n = 18).
) during different periods of lactation. (A) Period 1—from parturition to 50 days postpartum, APT (n = 16) vs. NT (n = 24). (B) Period 2—51–120 days postpartum, APT (n = 23) vs. NT (n = 13). (C) Period 3—121–220 days postpartum, APT (n = 16) vs. NT (n = 18).
 ) vs. antibiotics (AB;
) vs. antibiotics (AB;  ).
).
 ) vs. antibiotics (AB;
) vs. antibiotics (AB;  ).
).
| Group | APT | NT | P [F] |
|---|---|---|---|
| Lactation | 2.18 ± 0.10 | 2.20 ± 0.11 | 0.91 |
| Days in milk | 103.1 ± 10.50 | 99.4 ± 10.70 | 0.81 |
| Milk yield (L/day) | 41 ± 91.17 | 42.6 ± 1.18 | 0.69 |
| log SCC | 6.06 ± 0.04 | 6.02 ± 0.04 | 0.50 |
| Period | APT vs. Control (%) | Assumed MY (L/Day) | Difference in MY (L/Day) (APT vs. NT) | Day from Treatment to 305 Days | Total Milk (L) (APT vs. NT) |
|---|---|---|---|---|---|
| 1 (up to 50 days) | 7.65 | 50 | 3.83 | 250 | 956.3 |
| 2 (51–120 days) | 8.90 | 40 | 3.56 | 200 | 712.0 |
| 3 (121–220 days) | −0.16 | 30 | −0.04 | 150 | −7.2 |
| Total | 5.46 | 40 | 2.45 | 200 | 553.7 |
| Group | APT | AB | P [F] |
|---|---|---|---|
| Lactation | 2.26 ± 0.18 | 2.47 ± 0.10 | 0.08 |
| Days in milk | 126.0 ± 11.30 | 113.5 ± 11.60 | 0.46 |
| Milk yield (L/day) | 38.1 ± 1.14 | 41.6 ± 1.60 | 0.13 |
| log SCC | 6.62 ± 0.03 | 6.52 ± 0.04 | 0.41 |
| Group | Bacteria | APT | AB | P [F] |
|---|---|---|---|---|
| Total tested | 46/59 (78.0%) | 49/59 (83.1%) | NS | |
| Recovered | E. coli | 18/21 (85.7%) | 3/17 (17.6%) | <0.001 |
| Streptococci | 12/18 (66.0%) | 8/18 (44.4%) | 0.02 | |
| Other 1 | 7/7 (100%) | 8/14 (57.1%) | 0.01 | |
| Total recovery | 27/46 (80.5%) | 19/49 (38.7%) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leitner, G.; Papirov, E.; Gilad, D.; Haran, D.; Arkin, O.; Zuckerman, A.; Lavon, Y. New Treatment Option for Clinical and Subclinical Mastitis in Dairy Cows Using Acoustic Pulse Technology (APT). Dairy 2021, 2, 256-269. https://doi.org/10.3390/dairy2020022
Leitner G, Papirov E, Gilad D, Haran D, Arkin O, Zuckerman A, Lavon Y. New Treatment Option for Clinical and Subclinical Mastitis in Dairy Cows Using Acoustic Pulse Technology (APT). Dairy. 2021; 2(2):256-269. https://doi.org/10.3390/dairy2020022
Chicago/Turabian StyleLeitner, Gabriel, Eduard Papirov, Dan Gilad, Doron Haran, Oded Arkin, Alon Zuckerman, and Yaniv Lavon. 2021. "New Treatment Option for Clinical and Subclinical Mastitis in Dairy Cows Using Acoustic Pulse Technology (APT)" Dairy 2, no. 2: 256-269. https://doi.org/10.3390/dairy2020022