pH, the Fundamentals for Milk and Dairy Processing: A Review
Abstract
:1. Introduction
2. Understanding pH
2.1. The Hydrogen Ion
2.2. Acid—Base Reactions
2.3. The pH–Temperature Relationship
2.4. Hydrogen Ion Activity and pH in Non-Aqueous Solutions
3. Measuring pH
3.1. Measurement Approaches and Types of Probes
3.2. Monitoring pH
3.2.1. Off-Line and At-Line Measurements
3.2.2. On-Line Measurements
3.2.3. In-Line/In-Situ Measurements
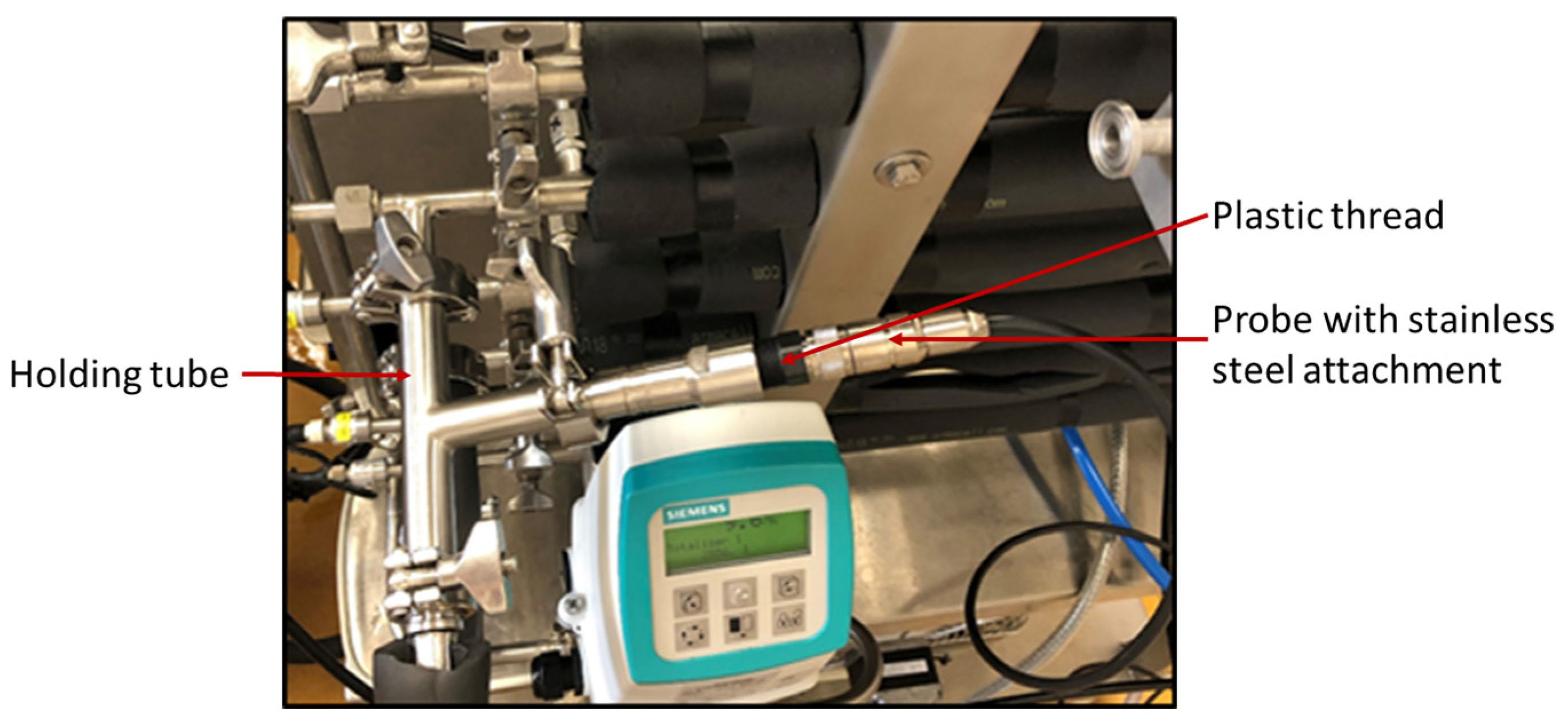
4. Industrial Applications of In-Line pH Measurement under Challenging Environmental Conditions
Considerations for Choosing the Right Probe
5. pH in Dairy Systems
5.1. Effects of Solids Content and Temperature on Milk pH during Dairy Processing
5.2. Addition of Salts
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Karastogianni, S.; Girousi, S.; Sotiropoulos, S. pH: Principles and Measurement. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 333–338. [Google Scholar]
- Salvo, P.; Melai, B.; Calisi, N.; Paoletti, C.; Bellagambi, F.; Kirchhain, A.; Trivella, M.G.; Fuoco, R.; Di Francesco, F. Graphene-based devices for measuring pH. Sens. Actuators B Chem. 2018, 256, 976–991. [Google Scholar] [CrossRef]
- Orouji, A.; Abbasi-Moayed, S.; Ghasemi, F.; Hormozi-Nezhad, M.R. A wide-range pH indicator based on colorimetric patterns of gold@ silver nanorods. Sens. Actuators B Chem. 2022, 358, 131479. [Google Scholar] [CrossRef]
- Martinsen, O.G.; Grimnes, S. Bioimpedance and Bioelectricity Basics; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Calio, P.B.; Li, C.; Voth, G.A. Resolving the structural debate for the hydrated excess proton in water. J. Am. Chem. Soc. 2021, 143, 18672–18683. [Google Scholar] [CrossRef] [PubMed]
- Yang, J. Deuterium: Discovery and Applications in Organic Chemistry; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Bleam, W. Chapter 1—Element Abundance. In Soil and Environmental Chemistry, 2nd ed.; Bleam, W., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 1–38. [Google Scholar]
- Katz, J.J. Deuterium and Tritium. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons: New York, NY, USA, 1998. [Google Scholar]
- Iglesias, F.C.; Barber, D.H. Heavy Water Reactor Fuel Design and Performance. In Encyclopedia of Materials: Science and Technology; Buschow, K.H.J., Cahn, R.W., Flemings, M.C., Ilschner, B., Kramer, E.J., Mahajan, S., Veyssière, P., Eds.; Elsevier: Oxford, UK, 2001; pp. 3741–3745. [Google Scholar]
- Carroll, J. Chapter 1—Introduction. In Natural Gas Hydrates, 4th ed.; Carroll, J., Ed.; Gulf Professional Publishing: Boston, MA, USA, 2020; pp. 1–26. [Google Scholar]
- Rondinini, S. pH measurements in non-aqueous and aqueous–organic solvents–definition of standard procedures. Anal. Bioanal. Chem. 2002, 374, 813–816. [Google Scholar] [CrossRef]
- Streng, W.H.; Hsi, S.K.; Helms, P.E.; Tan, H.G.H. General treatment of pH–solubility profiles of weak acids and bases and the effects of different acids on the solubility of a weak base. J. Pharm. Sci. 1984, 73, 1679–1684. [Google Scholar] [CrossRef]
- Butler, J.N. Ionic Equilibrium: Solubility and pH Calculations; John Wiley & Sons: Hoboken, NJ, USA, 1998. [Google Scholar]
- Upreti, P.; Bühlmann, P.; Metzger, L.E. Influence of Calcium and Phosphorus, Lactose, and Salt-to-Moisture Ratio on Cheddar Cheese Quality: pH Buffering Properties of Cheese. J. Dairy Sci. 2006, 89, 938–950. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.; Sayed, M.; Sohail, M.; Shah, L.A.; Raja, M.A. Advanced oxidation and reduction processes. Adv. Water Purif. Tech. 2019, 135–164. [Google Scholar] [CrossRef]
- Holt, C. The milk salts and their interaction with casein. Adv. Dairy Chem. 1997, 3, 233–256. [Google Scholar]
- Lucey, J.; Horne, D. Milk salts: Technological significance. In Advanced Dairy Chemistry; McSweeney, P.F.P., Ed.; Springer: New York, NY, USA, 2009; pp. 351–389. [Google Scholar]
- Ayyampalayam, S.N. Modeling the Temperature Dependance of pKa and Integration of Chemical Process Models Using SPARC. Ph.D. Thesis, University of Georgia, Athens, GA, USA, 2004. [Google Scholar]
- Reijenga, J.; Van Hoof, A.; Van Loon, A.; Teunissen, B. Development of methods for the determination of pKa values. Anal. Chem. Insights 2013, 8, S12304. [Google Scholar] [CrossRef] [Green Version]
- ISO 8968-4:2016; Milk and Milk Products–Determination of Nitrogen Content–Part 4: Determination of Protein and Non-Protein Nitrogen Content and True Protein Content Calculation (Reference Method). ISO: Geneva, Switzerland, 2016.
- Bolan, N.S.; Kandaswamy, K. pH. In Encyclopedia of Soils in the Environment; Hillel, D., Ed.; Elsevier: Oxford, UK, 2005; pp. 196–202. [Google Scholar]
- Himmel, D.; Goll, S.K.; Leito, I.; Krossing, I. A Unified pH Scale for All Phases. Angew. Chem. Int. Ed. 2010, 49, 6885–6888. [Google Scholar] [CrossRef]
- Ugo, P.; Daniele, S.; Mazzocchin, G.-A.; Bontempelli, G. Acid-base equilibria in organic solvents: Part 3. An Absolute pH Scale from Proton Basicity Evaluated by Cyclic Voltammetry. Anal. Chim. Acta 1988, 208, 207–217. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Fara, D.C.; Yang, H.; Tämm, K.; Tamm, T.; Karelson, M. Quantitative measures of solvent polarity. Chem. Rev. 2004, 104, 175–198. [Google Scholar] [CrossRef]
- Kahlert, H.; Leito, I. Generalization of Acid-Base Diagrams Based on the Unified pH-Scale. ChemPhysChem 2019, 20, 1779–1785. [Google Scholar] [CrossRef] [Green Version]
- Deleebeeck, L.; Snedden, A.; Nagy, D.; Szilágyi Nagyné, Z.; Roziková, M.; Vičarová, M.; Heering, A.; Bastkowski, F.; Leito, I.; Quendera, R.; et al. Unified pH Measurements of Ethanol, Methanol, and Acetonitrile, and Their Mixtures with Water. Sensors 2021, 21, 3935. [Google Scholar] [CrossRef]
- Lainela, S.; Leito, I.; Heering, A.; Capitaine, G.; Anes, B.; Camões, F.; Stoica, D. Toward Unified pH of Saline Solutions. Water 2021, 13, 2522. [Google Scholar] [CrossRef]
- Heering, A.; Bastkowski, F.; Seitz, S. Glass electrode half-cells for measuring unified pH in ethanol–water mixtures. J. Sens. Sens. Syst. 2020, 9, 383–389. [Google Scholar]
- Radtke, V.; Stoica, D.; Leito, I.; Camões, F.; Krossing, I.; Anes, B.; Roziková, M.; Deleebeeck, L.; Veltzé, S.; Näykki, T.; et al. A unified pH scale for all solvents: Part I—Intention and reasoning (IUPAC Technical Report). Pure Appl. Chem. 2021, 93, 1049–1060. [Google Scholar] [CrossRef]
- Erxleben, S.W.; Pelan, E.; Wolf, B. Effect of ethanol on the stability of sodium caseinate stabilised emulsions. Food Hydrocoll. 2021, 121, 107058. [Google Scholar] [CrossRef]
- Yuqing, M.; Jianrong, C.; Keming, F. New technology for the detection of pH. J. Biochem. Biophys. Methods 2005, 63, 1–9. [Google Scholar] [CrossRef]
- Galster, H. pH Measurement; VCH (Verlagsgesellschaft): New York, NY, USA, 1991. [Google Scholar]
- Webster, D. pH—Principles and measurement. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Oxford, UK, 2003; pp. 4501–4507. [Google Scholar]
- Johnson, E.; King, J. The determination of the acidity of milk. Analyst 1951, 76, 504–509. [Google Scholar] [CrossRef]
- Allan, D.; Heacock, H. Determining the accuracy of colorimetric pH testing compared to potentiometric methods. BCIT Environ. Public Health J. 2017. [Google Scholar] [CrossRef]
- Herber, S.; Olthuis, W.; Bergveld, P.; Berg, A. Exploitation of a pH-sensitive hydrogel for CO2 detection. In Proceedings of the Eurosensors XVII, European Conference on Solid-State Transducers, Guimaraes, Portugal, 21–14 September 2003; pp. 21–24. [Google Scholar]
- Horváth, V.; Horvai, G. ION-SELECTIVE ELECTRODES | Solid-State. In Encyclopedia of Analytical Science, 2nd ed.; Worsfold, P., Townshend, A., Poole, C., Eds.; Elsevier: Oxford, UK, 2005; pp. 502–508. [Google Scholar]
- Kohler, H.-H.; Haider, C.; Woelki, S. Selectivity and dynamic behavior of glass electrodes. Adv. Colloid Interface Sci. 2005, 114, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Kitabayashi, H.; Ito, K.; Nasu, H.; Ishihara, A.; Nishio, Y. Effect of heat-treatment on the pH sensitivity of stainless-steel electrodes as pH sensors. Heliyon 2019, 5, e01239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.-R.; Ren, Q.-Q.; Yuan, X.-J.; Wen, W.; Chen, W.; Zhan, D.-P. Iridium oxide based coaxial pH ultramicroelectrode. Electrochem. Commun. 2014, 40, 35–37. [Google Scholar] [CrossRef]
- Yamamoto, K.; Shi, G.; Zhou, T.; Xu, F.; Zhu, M.; Liu, M.; Kato, T.; Jin, J.-Y.; Jin, L. Solid-state pH ultramicrosensor based on a tungstic oxide film fabricated on a tungsten nanoelectrode and its application to the study of endothelial cells. Anal. Chim. Acta 2003, 480, 109–117. [Google Scholar] [CrossRef]
- Nyein, H.Y.Y.; Gao, W.; Shahpar, Z.; Emaminejad, S.; Challa, S.; Chen, K.; Fahad, H.M.; Tai, L.-C.; Ota, H.; Davis, R.W. A wearable electrochemical platform for noninvasive simultaneous monitoring of Ca2+ and pH. ACS Nano 2016, 10, 7216–7224. [Google Scholar] [CrossRef] [Green Version]
- Guinovart, T.; Valdés-Ramírez, G.; Windmiller, J.R.; Andrade, F.J.; Wang, J. Bandage-based wearable potentiometric sensor for monitoring wound pH. Electroanalysis 2014, 26, 1345–1353. [Google Scholar] [CrossRef]
- Hashimoto, T.; Miwa, M.; Nasu, H.; Ishihara, A.; Nishio, Y. pH sensors using 3d-block metal oxide-coated stainless steel electrodes. Electrochim. Acta 2016, 220, 699–704. [Google Scholar] [CrossRef] [Green Version]
- Munir, M.T.; Wilson, D.I.; Depree, N.; Boiarkina, I.; Prince-Pike, A.; Young, B.R. Real-time product release and process control challenges in the dairy milk powder industry. Curr. Opin. Food Sci. 2017, 17, 25–29. [Google Scholar] [CrossRef]
- Tajammal Munir, M.; Yu, W.; Young, B.R.; Wilson, D.I. The current status of process analytical technologies in the dairy industry. Trends Food Sci. Technol. 2015, 43, 205–218. [Google Scholar] [CrossRef]
- O’Shea, N.; O’Callaghan, T.F.; Tobin, J.T. The application of process analytical technologies (PAT) to the dairy industry for real time product characterization—Process viscometry. Innov. Food Sci. Emerg. Technol. 2019, 55, 48–56. [Google Scholar] [CrossRef]
- Bychkov, A.Y.; Bénézeth, P.; Pokrovsky, O.S.; Shvarov, Y.V.; Castillo, A.; Schott, J. Experimental determination of calcite solubility and the stability of aqueous Ca– and Na–carbonate and –bicarbonate complexes at 100–160 °C and 1–50 bar pCO2 using in-situ pH measurements. Geochim. Cosmochim. Acta 2020, 290, 352–365. [Google Scholar] [CrossRef]
- Palmer, D.A.; Bénézeth, P.; Wesolowski, D.J. Aqueous high-temperature solubility studies. I. The solubility of boehmite as functions of ionic strength (to 5 molal, NaCl), temperature (100–290 °C), and pH as determined by in-situ measurements. Geochim. Cosmochim. Acta 2001, 65, 2081–2095. [Google Scholar] [CrossRef]
- Wen, K.; Hu, C.; Wu, W.; Shvedova, K.; Born, S.C.; Takizawa, B.; Mascia, S. Proof-of-Concept Design of an In-Line pH Neutralization System with Coarse and Fine Adjustments for the Continuous Manufacturing of Pharmaceuticals. Org. Process Res. Dev. 2021, 25, 1853–1861. [Google Scholar] [CrossRef]
- Aydogdu, T.; O’Mahony, J.A.; Huppertz, T.; Magan, J.B.; McCarthy, N.A. Measuring pH of skim milk and milk permeate at ultra-high temperatures at laboratory and pilot scale. Int. Dairy J. 2023, 139, 105565. [Google Scholar] [CrossRef]
- Aydogdu, T.; O’Mahony, J.A.; McCarthy, N.A. Measurement of pH at high temperature in milk protein solutions. Int. Dairy J. 2022, 131, 105383. [Google Scholar] [CrossRef]
- Sanjuan, B.; Béchu, E.; Braibant, G.; Lebert, F. High Temperature-High Pressure Rated Sensors and Tools Useful for Geothermal Purposes. Bibliographical Review; Final Report, BRGM/RP-57342-FR; BRGM: Orléans, France, 2009; 44p.
- Inda, Y.; Yamashita, K.; Umegaki, T.; Greenblatt, M. High temperature pH sensitivities of stabilized zirconia films and ceria ceramics. Solid State Ion. 1996, 86, 1121–1124. [Google Scholar] [CrossRef]
- Jung, Y.-J.; Yeon, J.-W. Fabrication and evaluation of a new high-temperature pH sensor for use in PWR nuclear power plants. Bull. Korean Chem. Soc. 2010, 31, 2939–2942. [Google Scholar] [CrossRef] [Green Version]
- Truche, L.; Bazarkina, E.F.; Berger, G.; Caumon, M.-C.; Bessaque, G.; Dubessy, J. Direct measurement of CO2 solubility and pH in NaCl hydrothermal solutions by combining in-situ potentiometry and Raman spectroscopy up to 280 °C and 150 bar. Geochim. Cosmochim. Acta 2016, 177, 238–253. [Google Scholar] [CrossRef]
- Van Dijk, H.J.M.; Hersevoort, A. The properties of casein micelles. V: The determination of heat-induced calcium phosphate precipitations in milk. Ned. Melk Zuiveltijdschr. 1992, 46, 69–76. [Google Scholar]
- Van Slyke, D.D. On the measurement of buffer values and on the relationship of buffer value to the dissociation constant of the buffer and the concentration and reaction of the buffer solution. J. Biol. Chem. 1922, 52, 525–570. [Google Scholar] [CrossRef]
- Yadav, M.; Singh, V. Studies on calcium and phosphorus content of buffalo milk. I. Variation due to stage of lactation. Milchwissenschaft 1970, 25, 529–531. [Google Scholar]
- Gaucheron, F. The minerals of milk. Reprod. Nutr. Dev. 2005, 45, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuijse, H.; Huppertz, T. Heat-induced changes in milk salts: A review. Int. Dairy J. 2021, 126, 105220. [Google Scholar] [CrossRef]
- Kelleher, C.M.; Aydogdu, T.; Murphy, K.M.; O’Mahony, J.A.; Kelly, A.L.; O’Callaghan, D.J.; McCarthy, N.A. The effect of protein profile and preheating on denaturation of whey proteins and development of viscosity in milk protein beverages during heat treatment. Int. J. Dairy Technol. 2020, 73, 494–501. [Google Scholar] [CrossRef]
- Aydogdu, T.; Ho, Q.T.; Ahrné, L.; O’Mahony, J.A.; McCarthy, N.A. The influence of milk minerals and lactose on heat stability and age-thickening of milk protein concentrate systems. Int. Dairy J. 2021, 118, 105037. [Google Scholar] [CrossRef]
- Dash, K.K.; Fayaz, U.; Dar, A.H.; Shams, R.; Manzoor, S.; Sundarsingh, A.; Deka, P.; Khan, S.A. A comprehensive review on heat treatments and related impact on the quality and microbial safety of milk and milk-based products. Food Chem. Adv. 2022, 1, 100041. [Google Scholar] [CrossRef]
- Raikos, V. Effect of heat treatment on milk protein functionality at emulsion interfaces. A review. Food Hydrocoll. 2010, 24, 259–265. [Google Scholar] [CrossRef]
- McCarthy, N.A.; Magan, J.B.; Kelleher, C.M.; Kelly, A.L.; O’Mahony, J.A.; Murphy, E.G. Heat treatment of milk: Effect on concentrate viscosity, powder manufacture and end-product functionality. Int. Dairy J. 2022, 128, 105289. [Google Scholar] [CrossRef]
- Boiani, M.; Fenelon, M.; FitzGerald, R.J.; Kelly, P.M. Use of 31P NMR and FTIR to investigate key milk mineral equilibria and their interactions with micellar casein during heat treatment. Int. Dairy J. 2018, 81, 12–18. [Google Scholar] [CrossRef] [Green Version]
- Havea, P.; Singh, H.; Creamer, L.K. Heat-induced aggregation of whey proteins: Comparison of cheese WPC with acid WPC and relevance of mineral composition. J. Agric. Food Chem. 2002, 50, 4674–4681. [Google Scholar] [CrossRef]
- Pouliot, Y.; Boulet, M.; Paquin, P. Observations on the heat-induced salt balance changes in milk I. Effect of heating time between 4 and 90 °C. J. Dairy Res. 1989, 56, 185–192. [Google Scholar] [CrossRef]
- Sauer, A.; Moraru, C.I. Heat stability of micellar casein concentrates as affected by temperature and pH. J. Dairy Sci. 2012, 95, 6339–6350. [Google Scholar] [CrossRef] [Green Version]
- Schiffer, S.; Scheidler, E.; Kiefer, T.; Kulozik, U. Effect of Temperature, Added Calcium and pH on the Equilibrium of Caseins between Micellar State and Milk Serum. Foods 2021, 10, 822. [Google Scholar] [CrossRef]
- Morr, C.V. Effect of Heat upon Size and Composition of Proteins Sedimented from Normal and Concentrated Skimmilk1. J. Dairy Sci. 1965, 48, 29–33. [Google Scholar] [CrossRef]
- Pouliot, Y.; Boulet, M.; Paquin, P. Observations on the heat-induced salt balance changes in milk II. Reversibility on cooling. J. Dairy Res. 1989, 56, 193–199. [Google Scholar] [CrossRef]
- Wahlgren, N.M.; Dejmek, P.; Drakenberg, T. A 43Ca and 31P NMR study of the calcium and phosphate equilibria in heated milk solutions. J. Dairy Res. 1990, 57, 355–364. [Google Scholar] [CrossRef]
- Schmitt, M.; Saulnier, F.; Malhautier, L.; Linden, G. Effect of temperature on the salt balance of milk studied by capillary ion electrophoresis. J. Chromatogr. A 1993, 640, 419–424. [Google Scholar] [CrossRef]
- Brule, G.; Real del Sol, E.; Fauquant, J.; Fiaud, C. Mineral Salts Stability in Aqueous Phase of Milk: Influence of Heat Treatments. J. Dairy Sci. 1978, 61, 1225–1232. [Google Scholar] [CrossRef]
- Fox, P. Heat-induced changes in milk preceding coagulation. J. Dairy Sci. 1981, 64, 2127–2137. [Google Scholar] [CrossRef]
- Le Ray, C.; Maubois, J.-L.; Gaucheron, F.; Brulé, G.; Pronnier, P.; Garnier, F. Heat stability of reconstituted casein micelle dispersions: Changes induced by salt addition. Le Lait 1998, 78, 375–390. [Google Scholar] [CrossRef] [Green Version]
- Augustin, M.-A.; Clarke, P.T. Effects of added salts on the heat stability of recombined concentrated milk. J. Dairy Res. 1990, 57, 213–226. [Google Scholar] [CrossRef]
- Sweetsur, A.W.M.; Muir, D.D. The use of permitted additives and heat-treatment to optimize the heat-stability of skim milk and concentrated skim milk. Int. J. Dairy Technol. 1980, 33, 101–105. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, Y.; Wang, J. Comparative study on the heat stability of goat milk and cow milk. Indian J. Anim. Res. 2016, 50, 610–613. [Google Scholar] [CrossRef]
- Singh, J.; Dean, A.; Prakash, S.; Bhandari, B.; Bansal, N. Ultra high temperature stability of milk protein concentrate: Effect of mineral salts addition. J. Food Eng. 2021, 300, 110503. [Google Scholar] [CrossRef]
- Crowley, S.V.; Kelly, A.L.; O’Mahony, J.A. Fortification of reconstituted skim milk powder with different calcium salts: Impact of physicochemical changes on stability to processing. Int. J. Dairy Technol. 2014, 67, 474–482. [Google Scholar] [CrossRef]
- Renhe, I.R.T.; Indris, L.M.; Corredig, M. Effect of calcium chelators on heat stability and heat-induced changes of milk microfiltered concentrates. Int. Dairy J. 2018, 82, 4–10. [Google Scholar] [CrossRef]
- Karlsson, M.A.; Lundh, Å.; Innings, F.; Höjer, A.; Wikström, M.; Langton, M. The effect of calcium, citrate, and urea on the stability of ultra-high temperature treated milk: A full factorial designed study. Foods 2019, 8, 418. [Google Scholar] [CrossRef] [Green Version]
- de Kort, E.; Minor, M.; Snoeren, T.; van Hooijdonk, T.; van der Linden, E. Effect of calcium chelators on physical changes in casein micelles in concentrated micellar casein solutions. Int. Dairy J. 2011, 21, 907–913. [Google Scholar] [CrossRef]
- de Kort, E.; Minor, M.; Snoeren, T.; van Hooijdonk, T.; van der Linden, E. Effect of calcium chelators on heat coagulation and heat-induced changes of concentrated micellar casein solutions: The role of calcium-ion activity and micellar integrity. Int. Dairy J. 2012, 26, 112–119. [Google Scholar] [CrossRef]
- McCarthy, N.A.; Power, O.; Wijayanti, H.B.; Kelly, P.M.; Mao, L.; Fenelon, M.A. Effects of calcium chelating agents on the solubility of milk protein concentrate. Int. J. Dairy Technol. 2017, 70, 415–423. [Google Scholar] [CrossRef]
- Chen, L.; Liu, H. Effect of emulsifying salts on the physicochemical properties of processed cheese made from Mozzarella. J. Dairy Sci. 2012, 95, 4823–4830. [Google Scholar] [CrossRef]
- Guinee, T.; O’Callaghan, D. Effect of increasing the protein-to-fat ratio and reducing fat content on the chemical and physical properties of processed cheese product. J. Dairy Sci. 2013, 96, 6830–6839. [Google Scholar] [CrossRef] [Green Version]

| pH | H+ Concentration (mol/L) | OH− Concentration (mol/L) | |
|---|---|---|---|
| Acidic | 0 | 1.0 | 0.00000000000001 |
| 1 | 0.1 | 0.0000000000001 | |
| 2 | 0.01 | 0.000000000001 | |
| 3 | 0.001 | 0.00000000001 | |
| 4 | 0.0001 | 0.0000000001 | |
| 5 | 0.00001 | 0.000000001 | |
| 6 | 0.000001 | 0.00000001 | |
| Neutral | 7 | 0.0000001 | 0.0000001 |
| Alkaline | 8 | 0.00000001 | 0.000001 |
| 9 | 0.000000001 | 0.00001 | |
| 10 | 0.0000000001 | 0.0001 | |
| 11 | 0.00000000001 | 0.001 | |
| 12 | 0.000000000001 | 0.01 | |
| 13 | 0.0000000000001 | 0.1 | |
| 14 | 0.00000000000001 | 1.0 |
| Acid | Chemical Formula | pKa1 | pKa2 | pKa3 | |
|---|---|---|---|---|---|
| Strong acid | Hydrochloric Acid | HCl | −7.0 | - | - |
| Weak acids | Phosphoric Acid | H3PO4 | 2.1 | 7.1 | 12.4 |
| Citric Acid | HOC(CO2H)(CH2CO2H)2 | 3.1 | 4.8 | 6.4 | |
| Lactic Acid | CH3CH(OH)COOH | 3.9 | - | - | |
| Acetic Acid | CH3COOH | 4.8 | - | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aydogdu, T.; O’Mahony, J.A.; McCarthy, N.A. pH, the Fundamentals for Milk and Dairy Processing: A Review. Dairy 2023, 4, 395-409. https://doi.org/10.3390/dairy4030026
Aydogdu T, O’Mahony JA, McCarthy NA. pH, the Fundamentals for Milk and Dairy Processing: A Review. Dairy. 2023; 4(3):395-409. https://doi.org/10.3390/dairy4030026
Chicago/Turabian StyleAydogdu, Tugce, James A. O’Mahony, and Noel A. McCarthy. 2023. "pH, the Fundamentals for Milk and Dairy Processing: A Review" Dairy 4, no. 3: 395-409. https://doi.org/10.3390/dairy4030026





