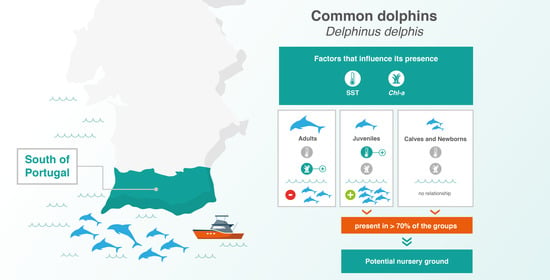
Oceanographic Determinants of the Abundance of Common Dolphins (Delphinus delphis) in the South of Portugal
Abstract
:1. Introduction
2. Material and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Neumann, D.R. The activity budget of free-ranging common dolphins (Delphinus delphis) in the Northwestern Bay of Plenty, New Zealand. Aquat. Mamm. 2001, 27, 121–136. [Google Scholar]
- Hastie, G.; Swift, R.; Slesser, G.; Thompson, P.; Turrel, W. Environmental models for predicting oceanic dolphin habitat in the Northeast Atlantic. ICES J. Mar. Sci. 2005, 62, 760–770. [Google Scholar] [CrossRef]
- Selzer, L.A.; Payne, P.M. The distribution of white-sided (Lagenorhynchus acutus) and common dolphins (Delphinus delphis) vs. environmental features of the continental shelf of the Northeastern United States. Mar. Mammal Sci. 1988, 4, 141–153. [Google Scholar] [CrossRef]
- Baumgartner, M.F.; Mullin, K.D.; May, L.N.; Leming, T.D. Cetacean habitats in the Northern Gulf of Mexico—Statistical data included. Fish. Bull. 2001, 99, 219–239. [Google Scholar] [CrossRef]
- Gowans, S.; Whitehead, H. Distribution and habitat partitioning by small odontocetes in the gully, a submarine canyon on the scotian shelf. Can. J. Zool. 1995, 73, 1599–1608. [Google Scholar] [CrossRef]
- Baumgartner, M.F. The distribution of Risso’s dolphin (Grampus griseus) with respect to the Physiography of the Northern Gulf of Mexico. Mar. Mammal Sci. 1997, 13, 614–638. [Google Scholar] [CrossRef]
- Reilly, S.B. Seasonal changes in distribution and habitat differences among dolphins in the Eastern Tropical Pacific. Mar. Ecol. Prog. Ser. 1990, 66, 1–11. [Google Scholar] [CrossRef]
- Polacheck, T. Relative abundance, distribution and inter-specific relationship of cetacean schools in the Eastern Tropical Pacific. Mar. Mammal Sci. 1987, 3, 54–77. [Google Scholar] [CrossRef]
- Cockcroft, V.G.; Peddemors, V.M. seasonal distribution and density of common dolphins Delphinus delphis off the south-east coast of Southern Africa. S. Afr. J. Mar. Sci. 1990, 9, 371–377. [Google Scholar] [CrossRef]
- Young, D.D.; Cockcroft, V.G. Diet of common dolphins Delphinus delphis off the south-east coast of Southern Africa: Opportunism or specialization? J. Zool. 1994, 234, 41–53. [Google Scholar] [CrossRef]
- Amaral, A.R.; Beheregaray, L.B.; Bilgmann, K.; Freitas, L.; Robertson, K.M.; Sequeira, M.; Stockin, K.A.; Coelho, M.M.; Möller, L.M. Influences of past climatic changes on historical population structure and demography of a cosmopolitan marine predator, the common dolphin (genus Delphinus). Mol. Ecol. 2012, 21, 4854–4871. [Google Scholar] [CrossRef] [PubMed]
- Henderson, E.E.; Forney, K.A.; Barlow, J.P.; Hildebrand, J.A.; Douglas, A.B.; Calambokidis, J.; Sydeman, W.J. Effects of fluctuations in sea-surface temperature on the occurrence of small cetaceans off Southern California. Fish. Bull. 2014, 112, 159–177. [Google Scholar] [CrossRef]
- Ballance, L.T.; Pitman, R.L.; Fiedler, P.C. Oceanographic influences on seabirds and cetaceans of the eastern tropical pacific: A review. Prog. Oceanogr. 2006, 69, 360–390. [Google Scholar] [CrossRef]
- Bilgmann, K.; Möller, L.M.; Harcourt, R.G.; Gales, R.; Beheregaray, L.B. Common dolphins subject to fisheries impacts in southern australia are genetically differentiated: Implications for conservation. Anim. Conserv. 2008, 11, 518–528. [Google Scholar] [CrossRef]
- Valdez, F.P.; Corrigan, S.; Möller, L.; Bilgmann, K.; Beheregaray, L.B.; Allen, S. Fine-Scale genetic structure in short-beaked common dolphins (Delphinus delphis) along the East Australian current. Mar. Biol. 2010, 158, 113–126. [Google Scholar] [CrossRef]
- Alcaraz, D.; Paruelo, J.; Cabello, J. Identification of current ecosystem functional types in the Iberian Peninsula. Glob. Ecol. Biogeogr. 2006, 15, 200–212. [Google Scholar] [CrossRef]
- Moura, A.E.; Sillero, N.; Rodrigues, A. Common dolphin (Delphinus delphis) habitat preferences using data from two platforms of opportunity. Acta Oecologica 2012, 38, 24–32. [Google Scholar] [CrossRef]
- Bograd, S.J.; Rykaczewski, R.R.; García-Reyes, M.; Sydeman, W.J.; Bakun, A.; Miller, A.J.; Black, B.A. Anticipated effects of climate change on coastal upwelling ecosystems. Curr. Clim. Chang. Rep. 2015, 1, 85–93. [Google Scholar] [CrossRef] [Green Version]
- Cravo, A.; Relvas, P.; Cardeira, S.; Rita, F.; Madureira, M.; Sánchez, R. An upwelling filament off southwest iberia: Effect on the chlorophyll a and nutrient export. Cont. Shelf Res. 2010, 30, 1601–1613. [Google Scholar] [CrossRef]
- Cravo, A.; Relvas, P.; Cardeira, S.; Rita, F. Nutrient and chlorophyll a transports during an upwelling event in the NW margin of the gulf of cadiz. J. Mar. Syst. 2013, 128, 208–221. [Google Scholar] [CrossRef]
- García Lafuente, J.; Ruiz, J. The gulf of cádiz pelagic ecosystem: A review. Prog. Oceanogr. 2007, 74, 228–251. [Google Scholar] [CrossRef]
- Silva, M.A.; Sequeira, M. Patterns in the mortality of common dolphins (Delphinus delphis) on the portuguese coast, using stranding records, 1975–1998. Aquat. Mamm. 2005, 29, 88–98. [Google Scholar] [CrossRef]
- Beare, D.; Pierce, G.J.; Patterson, I.A.P.; Reid, R.J.; Learmonth, J.A.; Reid, D.G.; Santos, M.B.; Ross, H.M. Variability in the diet of harbor porpoises (Phocoena phocoena) in scottish waters 1992–2003. Mar. Mammal Sci. 2006, 20, 1–27. [Google Scholar] [CrossRef]
- Cañadas, A. Towards Conservation of Dolphins in the Alborán Sea. Hacia La Conservación de Los Delfines En El Mar de Alborán. Ph.D. Thesis, Universidad Autónoma de Madrid, Madrid, Spain, 2006. [Google Scholar]
- De Stephanis, R.; Cornulier, T.; Verborgh, P.; Sierra, J.S.; Gimeno, N.P.; Guinet, C. Summer spatial distribution of cetaceans in the strait of gibraltar in relation to the oceanographic context. Mar. Ecol. Prog. Ser. 2008, 353, 275–288. [Google Scholar] [CrossRef]
- Verborgh, P.; De Stephanis, R.; Pérez, S.; Jaget, Y.; Barbraud, C.; Guinet, C. Survival rate, abundance, and residency of long-finned pilot whales in the strait of gibraltar. Mar. Mammal Sci. 2009, 25, 523–536. [Google Scholar] [CrossRef]
- Brito, C.; Vieira, N.; Sá, E.; Carvalho, I. Cetaceans’ occurrence off the west central portugal coast: A compilation of data from whaling, observations of opportunity and boat-based surveys. Environ. Res. 2009, 2, 2–5. [Google Scholar]
- Forcada, J.; Hammond, P. Geographical variation in abundance of striped and common dolphins of the western mediterranean. J. Sea Res. 1998, 39, 313–325. [Google Scholar] [CrossRef]
- Peddemors, V.M. Delphinids of Southern Africa: A review of their distribution, status and life history. J. Cetacean Res. Manag. 1999, 1, 157–165. [Google Scholar]
- Cañadas, A.; Sagarminaga, R.; García-Tiscar, S. Cetacean distribution related with depth and slope in the mediterranean waters off Southern Spain. Deep. Res. Part I Oceanogr. Res. Pap. 2002, 49, 2053–2073. [Google Scholar] [CrossRef]
- Evans, P.G.H.; Hammond, P.S. Monitoring cetaceans in european waters. Mamm. Rev. 2004, 34, 131–156. [Google Scholar] [CrossRef]
- Evans, P. Dolphins; Whittet Books Limited: London, UK, 1994. [Google Scholar]
- Gannier, A. Les Cétacés de Méditerranée Nord-Occidentale: Estimation de Leur Abondance et Mise En Relation de La Variation Saisonnière de Leur Distribution Avec l’écologie Du Milieu. Ph.D. Thesis, Ecole Pratique des Hautes Etudes, Montpellier, France, 1995. [Google Scholar]
- Santos, M.; German, I.; Correia, D.; Read, F.; Martinez Cedeira, J.; Caldas, M.; López, A.; Velasco, F.; Pierce, J. Long-Term variation in common dolphin diet in relation to prey abundance. Mar. Ecol. Prog. Ser. 2013, 481, 249–268. [Google Scholar] [CrossRef]
- Pusineri, C.; Magnin, V.; Meynier, L.; Spitz, J.; Hassani, S.; Ridoux, V. Food and feeding ecology of the common dolphin (Delphinus delphis) in the oceanic northeast atlantic and comparison with its diet in neritic areas. Mar. Mammal Sci. 2007, 23, 30–47. [Google Scholar] [CrossRef]
- Spitz, J.; Mourocq, E.; Léauté, J.-P.; Quéro, J.-C.; Ridoux, V. Prey selection by the common dolphin: Fulfilling high energy requirements with high quality food. J. Exp. Mar. Biol. Ecol. 2010, 390, 73–77. [Google Scholar] [CrossRef] [Green Version]
- Gannier, A. Present distribution of common dolphin Delphinus delphis in French mediterranean and adjacent waters as obtained from small boat surveys. Aquat. Conserv. Mar. Freshw. Ecosyst. 2018, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Vinding, K.; Bester, M.; Kirkman, S.P.; Chivell, W.; Elwen, S.H. The Use of Data from a platform of opportunity (Whale Watching) to study coastal cetaceans on the southwest coast of South Africa. Tour. Mar. Environ. 2015, 11, 33–54. [Google Scholar] [CrossRef]
- Shane, S.H. Behavior and ecology of the bottlenose dolphin at Sanibel Island, Florida. Bottlenose Dolphin 1990, 245–265. [Google Scholar]
- Neumann, D.R.; Orams, M.B. Behaviour and ecology of common dolphins (Delphinus delphis) and the impact of tourism in mercury bay, North Island, New Zealand. Sci. Conserv. 2005, 5–40. [Google Scholar]
- Wood, S.N. Generalized Additive Models: An Introduction with R, 2nd ed.; CRC Press: New York, NY, USA, 2017. [Google Scholar] [CrossRef]
- Marra, G.; Wood, S.N. Practical variable selection for generalized additive models. Comput. Stat. Data Anal. 2011, 55, 2372–2387. [Google Scholar] [CrossRef]
- Kämpf, J.; Chapman, P. Upwelling Systems of the World; Springer: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Stockin, K.A.; Pierce, G.J.; Binedell, V.; Wiseman, N.; Orams, M.B. Factors affecting the occurrence and demographics of common dolphins (Delphinus sp.) in the Hauraki Gulf, New Zealand. Aquat. Mamm. 2008, 34, 200–211. [Google Scholar] [CrossRef] [Green Version]
- Würsig, B. Occurrence and group organization of Atlantic bottlenose porpoises (Tursiops truncatus) in an argentine bay. Biol. Bull. 1978, 154, 348–359. [Google Scholar] [CrossRef]
- Evans, P.G.H. The Natural History of Whales and Dolphins; Christopher Helm Mammal Serie: London, UK, 1987. [Google Scholar]
- Bearzi, G.; Notarbartolo-Di-Sciara, G.; Politi, E. Social ecology of bottlenose dolphins in the Kvarneric (Northern Adriatic Sea). Mar. Mammal Sci. 1997, 13, 650–668. [Google Scholar] [CrossRef]
- Baptista, V.; Silva, P.L.; Relvas, P.; Teodósio, M.A.; Leitão, F. Sea surface temperature variability along the portuguese coast since 1950. Int. J. Climatol. 2018, 38, 1145–1160. [Google Scholar] [CrossRef]
- Cañadas, A.; Vázquez, J.A. Common dolphins in the alboran sea: Facing a reduction in their suitable habitat due to an increase in sea surface temperature. Deep Sea Res. Part II Top. Stud. Oceanogr. 2017, 141, 306–318. [Google Scholar] [CrossRef]
- Cañadas, A.; Hammond, P.S. Abundance and habitat preferences of the short-beaked common dolphin Delphinus delphis in the southwestern mediterranean: Implications for conservation. Endanger. Species Res. 2008, 4, 309–331. [Google Scholar] [CrossRef]
- Hammond, P.S.; Bearzi, G.; Bjorge, A.; Forney, K.; Karczmarski, L.; Kasuya, T.; Perrin, W.F.; Scott, M.D.; Wang, J.Y.; Wells, R.S.; et al. Delpinus delphis. In The IUCN Red List of Threatened Species; International Union for Conservation of Nature and Natural Resources: Gland, Switzerland, 2008; p. e.T6336A12649851. [Google Scholar] [CrossRef]
- Würsig, B.; Reeves, R.R.; Ortega-Ortiz, J.G. Global climate change and marine mammals. In Marine Mammals; Evans, P.G.H., Raga, J.A., Eds.; Springer US: Boston, MA, USA, 2002; pp. 589–608. [Google Scholar] [CrossRef]



| April | May | June | July | August | September | October | November | Total | |
|---|---|---|---|---|---|---|---|---|---|
| No. sightings 2010 | 0 | 0 | 29 | 145 | 164 | 72 | 27 | 0 | 437 |
| Effort (hours) 2010 | 0 | 0 | 49.5 | 508.5 | 658.5 | 216 | 28.5 | 0 | 1461 |
| No. sightings 2011 | 13 | 7 | 17 | 64 | 95 | 62 | 31 | 0 | 289 |
| Effort (hours) 2011 | 21 | 18 | 28.5 | 102 | 174 | 76.5 | 43.5 | 0 | 463.5 |
| No. sightings 2012 | 1 | 8 | 24 | 71 | 119 | 43 | 27 | 0 | 293 |
| Effort (hours) 2012 | 13.5 | 99 | 78 | 195 | 250.5 | 72 | 49.5 | 0 | 757.5 |
| No. sightings 2013 | 0 | 15 | 43 | 144 | 168 | 67 | 58 | 0 | 495 |
| Effort (hours) 2013 | 9 | 19.5 | 117 | 280.5 | 271.5 | 130.5 | 42 | 0 | 870 |
| No. sightings 2014 | 17 | 27 | 91 | 120 | 93 | 31 | 34 | 2 | 415 |
| Effort (hours) 2014 | 28.5 | 70.5 | 147 | 192 | 276 | 156 | 121.5 | 4.5 | 996 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro, J.; Couto, A.; Borges, F.O.; Cid, A.; Laborde, M.I.; Pearson, H.C.; Rosa, R. Oceanographic Determinants of the Abundance of Common Dolphins (Delphinus delphis) in the South of Portugal. Oceans 2020, 1, 165-173. https://doi.org/10.3390/oceans1030012
Castro J, Couto A, Borges FO, Cid A, Laborde MI, Pearson HC, Rosa R. Oceanographic Determinants of the Abundance of Common Dolphins (Delphinus delphis) in the South of Portugal. Oceans. 2020; 1(3):165-173. https://doi.org/10.3390/oceans1030012
Chicago/Turabian StyleCastro, Joana, Ana Couto, Francisco O. Borges, André Cid, Marina I. Laborde, Heidi C. Pearson, and Rui Rosa. 2020. "Oceanographic Determinants of the Abundance of Common Dolphins (Delphinus delphis) in the South of Portugal" Oceans 1, no. 3: 165-173. https://doi.org/10.3390/oceans1030012