Graphene-Oxide-Coated CoP2@C Anode Enables High Capacity of Lithium-Ion Batteries
Abstract
:1. Introduction
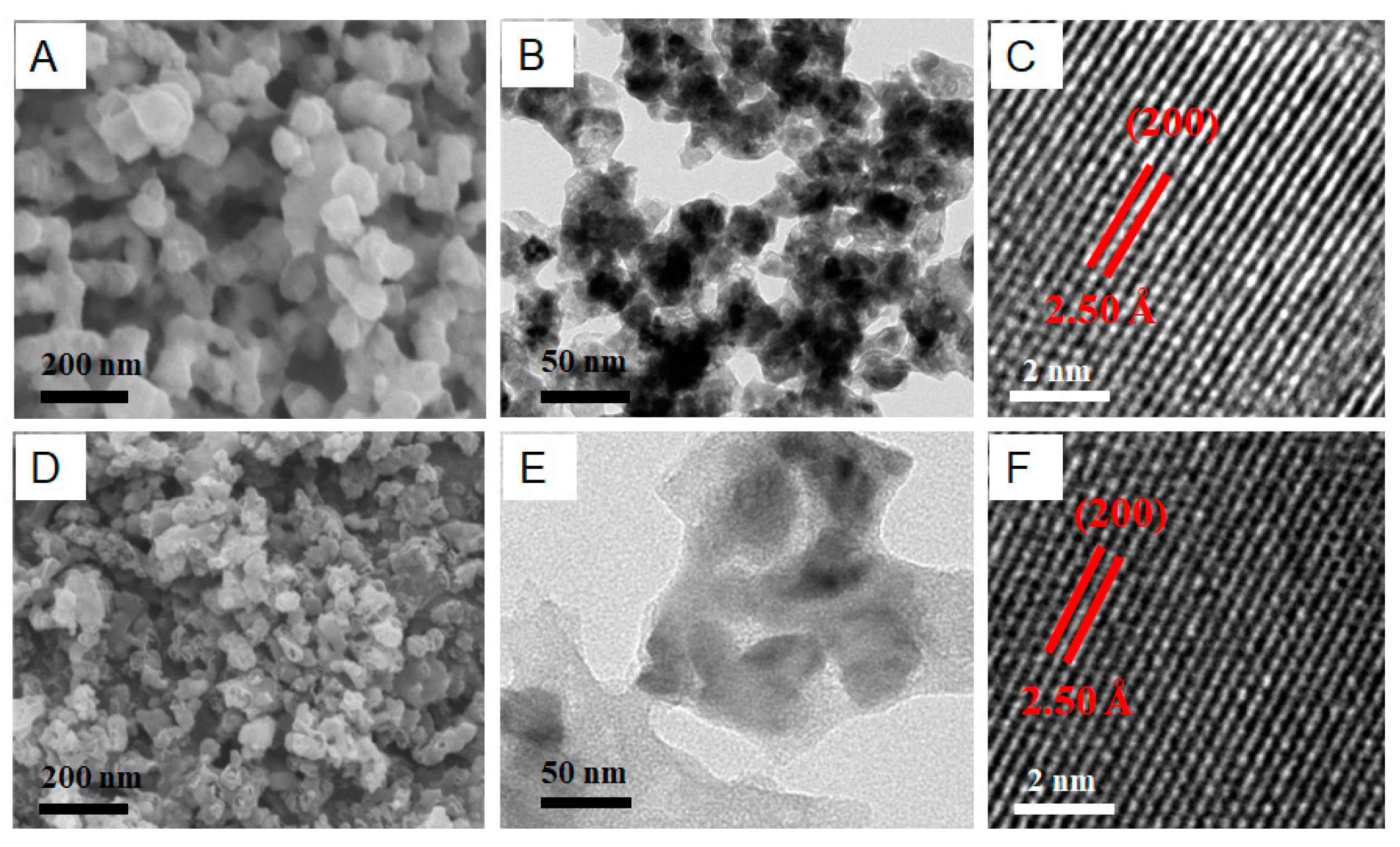
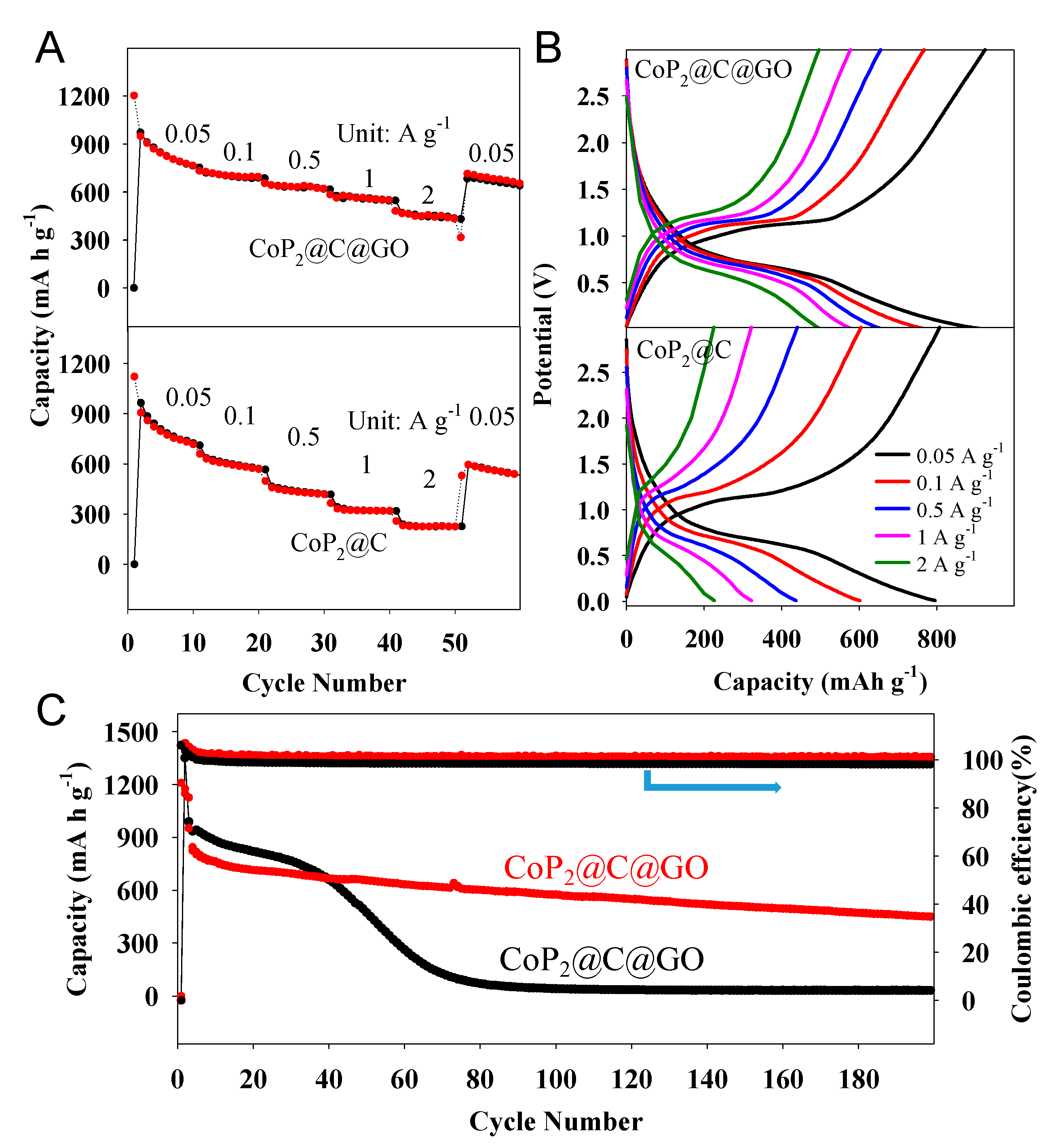
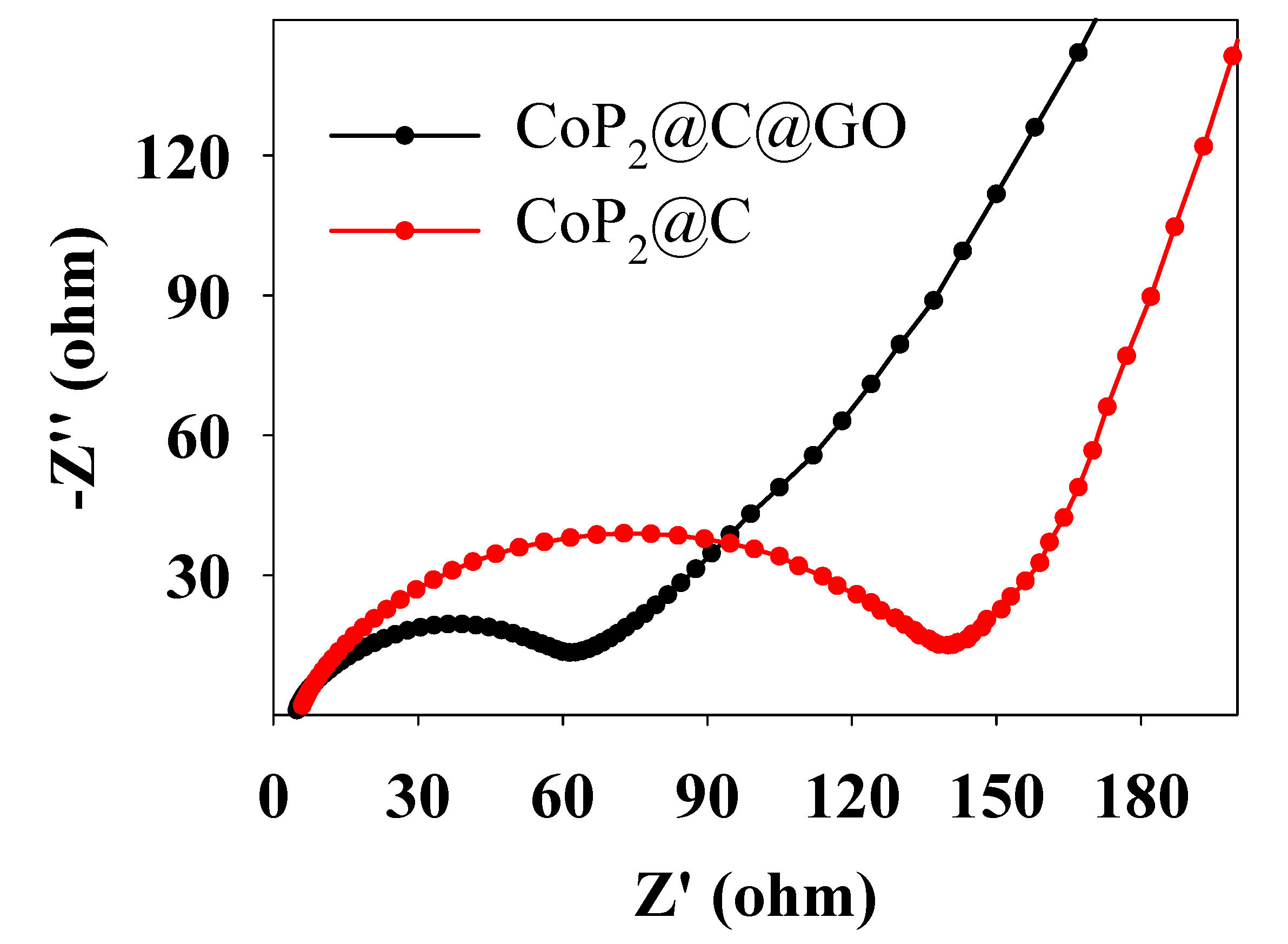
2. Results
3. Materials and Methods
3.1. Chemicals
3.2. Synthesis of ZIF-67
3.3. Synthesis of CoP2@C
3.4. Synthesis of GO
3.5. Synthesis of CoP2@C@GO
3.6. Materials Characterization
3.7. Coin Cell Assembly
3.8. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, D.Q.; Ren, J.F.; Li, C.X.; Luo, B.; Wang, L.; Li, Y.Y. An Advanced Design Concept of Mansion-like Freestanding Silicon Anodes with Improved Lithium Storage Performances. Chin. J. Struct. Chem. 2022, 41, 2205055–2205062. [Google Scholar] [CrossRef]
- Lin, J.J.; Zhou, Y.; Wen, J.B.; Si, W.J.; Gao, H.C.; Wang, G.M.; Kang, X.W. Pyrrole derivatives as interlayer modifier of Li-S batteries: Modulation of electrochemical performance by molecular perturbation. J. Energy Chem. 2022, 75, 164–172. [Google Scholar] [CrossRef]
- Liang, Z.L.; Yang, Y.; Li, H.; Liu, L.Y.; Shi, Z.C. Lithium Storage Performance of Hard Carbons Anode Materials Prepared by Different Precursors. J. Electrochem. 2021, 27, 177–184. [Google Scholar] [CrossRef]
- Kim, Y.; Hwang, H.; Yoon, C.S.; Kim, M.G.; Cho, J. Reversible Lithium Intercalation in Teardrop-Shaped Ultrafine SnP0.94 Particles: An Anode Material for Lithium-Ion Batteries. Adv. Mater. 2007, 19, 92–96. [Google Scholar] [CrossRef]
- Boyanov, S.; Zitoun, D.; Ménétrier, M.; Jumas, J.C.; Womes, M.; Monconduit, L. Comparison of the Electrochemical Lithiation/Delitiation Mechanisms of FePx (x = 1, 2, 4) Based Electrodes in Li-Ion Batteries. J. Phys. Chem. C 2009, 113, 21441–21452. [Google Scholar] [CrossRef]
- Hall, J.W.; Membreno, N.; Wu, J.; Celio, H.; Jones, R.A.; Stevenson, K.J. Low-Temperature Synthesis of Amorphous FeP2 and Its Use as Anodes for Li Ion Batteries. J. Am. Chem. Soc. 2012, 134, 5532–5535. [Google Scholar] [CrossRef]
- Park, C.-M.; Sohn, H.-J. Tetragonal Zinc Diphosphide and Its Nanocomposite as an Anode for Lithium Secondary Batteries. Chem. Mater. 2008, 20, 6319–6324. [Google Scholar] [CrossRef]
- Xiang, J.Y.; Wang, X.L.; Zhong, J.; Zhang, D.; Tu, J.P. Enhanced rate capability of multi-layered ordered porous nickel phosphide film as anode for lithium ion batteries. J. Power Sources 2011, 196, 379–385. [Google Scholar] [CrossRef]
- Carenco, S.; Surcin, C.; Morcrette, M.; Larcher, D.; Mézailles, N.; Boissière, C.; Sanchez, C. Improving the Li-Electrochemical Properties of Monodisperse Ni2P Nanoparticles by Self-Generated Carbon Coating. Chem. Mater. 2012, 24, 688–697. [Google Scholar] [CrossRef]
- Cabana, J.; Monconduit, L.; Larcher, D.; Palacín, M.R. Beyond Intercalation-Based Li-Ion Batteries: The State of the Art and Challenges of Electrode Materials Reacting Through Conversion Reactions. Adv. Mater. 2010, 22, E170–E192. [Google Scholar] [CrossRef]
- Kim, S.O.; Manthiram, A. Phosphorus-Rich CuP2 Embedded in Carbon Matrix as a High-Performance Anode for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 16221–16227. [Google Scholar] [CrossRef]
- Kwon, H.T.; Kim, J.H.; Jeon, K.J.; Park, C.M. CoxP compounds: Electrochemical conversion/partial recombination reaction and partially disproportionated nanocomposite for Li-ion battery anodes. RSC Adv. 2014, 4, 43227–43234. [Google Scholar] [CrossRef]
- Jiao, G.H.; Gu, Y.; Wang, J.; Wu, D.J.; Tao, S.; Chu, S.Q.; Liu, Y.S.; Qian, B.; Chu, W.S. Porous CoP/C@MCNTs hybrid composite derived from metal-organic frameworks for high-performance lithium-ion batteries. J. Mater. Sci. 2019, 54, 3273–3283. [Google Scholar] [CrossRef]
- Wang, X.X.; Na, Z.L.; Yin, D.M.; Wang, C.L.; Wu, Y.M.; Huang, G.; Wang, L.M. Phytic Acid-Assisted Formation of Hierarchical Porous CoP/C Nanoboxes for Enhanced Lithium Storage and Hydrogen Generation. ACS Nano 2018, 12, 12238–12246. [Google Scholar] [CrossRef]
- Cui, Y.H.; Xue, M.Z.; Fu, Z.W.; Wang, X.L.; Liu, X.J. Nanocrystalline CoP thin film as a new anode material for lithium ion battery. J. Alloys Compd. 2013, 555, 283–290. [Google Scholar] [CrossRef]
- Jiang, J.T.; Zhu, K.; Fang, Y.Z.; Wang, H.Z.; Ye, K.; Yan, J.; Wang, G.L.; Cheng, K.; Zhou, L.M.; Cao, D.X. Coralloidal carbon-encapsulated CoP nanoparticles generated on biomass carbon as a high-rate and stable electrode material for lithium-ion batteries. J. Colloid Interface Sci. 2018, 530, 579–585. [Google Scholar] [CrossRef]
- Chen, M.N.; Zeng, P.Y.; Zhao, Y.Y.; Fang, Z. CoP nanoparticles enwrapped in N-doped carbon nanotubes for high performance lithium-ion battery anodes. Front. Mater. Sci. 2018, 12, 214–224. [Google Scholar] [CrossRef]
- Han, Z.; Wang, B.B.; Liu, X.J.; Wang, G.; Wang, H.; Bai, J.T. Peapod-like one-dimensional (1D) CoP hollow nanorods embedded into graphene networks as an anode material for lithium-ion batteries. J. Mater. Sci. 2018, 53, 8445–8459. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, Y.; Chen, W.L.; Yan, Y.W.; Xue, L.H.; Zhang, W.X. CoP3@PPy microcubes as anode for lithium-ion batteries with improved cycling and rate performance. Chem. Eng. J. 2018, 347, 455–461. [Google Scholar] [CrossRef]
- Xia, G.; Su, J.; Li, M.; Jiang, P.; Yang, Y.; Chen, Q. A MOF-derived self-template strategy toward cobalt phosphide electrodes with ultralong cycle life and high capacity. J. Mater. Chem. A 2017, 5, 10321–10327. [Google Scholar] [CrossRef]
- Shen, J.H.; Wang, Y.; Yao, Y.F.; Hou, L.; Gu, K.L.; Zhu, Y.H.; Li, C.Z. Flexible Free-Standing Hierarchical Carbon-Coated CoP2 Nanosheets for High-Performance Lithium-Ion Batteries. ACS Appl. Energ. Mater. 2018, 1, 7253–7262. [Google Scholar] [CrossRef]
- Khatib, R.; Dalverny, A.L.; Saubanère, M.; Gaberscek, M.; Doublet, M.L. Origin of the Voltage Hysteresis in the CoP Conversion Material for Li-Ion Batteries. J. Phys. Chem. C 2013, 117, 837–849. [Google Scholar] [CrossRef]
- Xu, L.; Thompson, C.V. Mechanisms of the cyclic (de)lithiation of RuO2. J. Mater. Chem. A 2020, 8, 21872–21881. [Google Scholar] [CrossRef]
- Xu, L.; Chon, M.J.; Mills, B.; Thompson, C.V. Mechanical stress and morphology evolution in RuO2 thin film electrodes during lithiation and delithiation. J. Power Sources 2022, 552, 232260. [Google Scholar] [CrossRef]
- Song, H.R.; Xue, S.D.; Chen, S.M.; Wang, Z.J.; Song, Y.L.; Li, J.W.; Song, Z.B.; Yang, L.Y.; Pan, F. Polymeric Wetting Matrix for a Stable Interface between Solid-state Electrolytes and Li Metal Anode. Chin. J. Struct. Chem. 2022, 41, 2205048–2205054. [Google Scholar] [CrossRef]
- Ma, D.J.; Zhu, Q.L.; Li, X.T.; Gao, H.C.; Wang, X.F.; Kang, X.W.; Tian, Y. Unraveling the Impact of Ether and Carbonate Electrolytes on the Solid-Electrolyte Interface and the Electrochemical Performances of ZnSe@C Core-Shell Composites as Anodes of Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2019, 11, 8009–8017. [Google Scholar] [CrossRef]
- Gao, H.C.; Ning, S.L.; Zhou, Y.; Men, S.; Kang, X.W. Polyacrylonitrile-induced formation of core-shell carbon nanocages: Enhanced redox kinetics towards polysulfides by confined catalysis in Li-S batteries. Chem. Eng. J. 2021, 408, 127323. [Google Scholar] [CrossRef]
- Yang, J.; Gao, H.C.; Men, S.; Shi, Z.Q.; Lin, Z.; Kang, X.W.; Chen, S.W. CoSe2 Nanoparticles Encapsulated by N-Doped Carbon Framework Intertwined with Carbon Nanotubes: High-Performance Dual-Role Anode Materials for Both Li- and Na-Ion Batteries. Adv. Sci. 2018, 5, 1800763. [Google Scholar] [CrossRef]
- Men, S.; Zheng, H.; Ma, D.J.; Huang, X.L.; Kang, X.W. Unraveling the stabilization mechanism of solid electrolyte interface on ZnSe by rGO in sodium ion battery. J. Energy Chem. 2021, 54, 124–130. [Google Scholar] [CrossRef]
- Chen, M.; Zhao, J.M.; Sun, C.A.F. High-volumetric-capacity WSe2 Anode for Potassium-ion Batteries. Chin. J. Struct. Chem. 2021, 40, 926–932. [Google Scholar] [CrossRef]
- Xu, X.J.; Liu, J.; Hu, R.Z.; Liu, J.W.; Ouyang, L.Z.; Zhu, M. Self-Supported CoP Nanorod Arrays Grafted on Stainless Steel as an Advanced Integrated Anode for Stable and Long-Life Lithium-Ion Batteries. Chem.-Eur. J. 2017, 23, 5198–5204. [Google Scholar] [CrossRef]
- Hu, Y.J.; Yin, Y.H.; Zhang, M.; Wu, Z.P.; Shen, Z.R. In-situ Growth of Carbon Nanosheets Intercalated with TiO2 for Improving Electrochemical Performance and Stability of Lithium-ion Batteries. Chin. J. Struct. Chem. 2021, 40, 1513–1524. [Google Scholar] [CrossRef]
- Chai, S.W.; Xiao, X.; Li, Y.B.; An, C.H. Graphene-Coated 1D MoTe2 Nanorods as Anode for Enhancing Lithium-Ion Battery Performance. Chin. J. Struct. Chem. 2022, 41, 2208018–2208024. [Google Scholar] [CrossRef]
- Gan, Y.M.; Zhu, J.J.; Zhang, Q.X.; Wang, C.Y.; Guan, L.H.; Zhao, Y. Boosting Stable and Fast Potassium Storage of Iron Sulfide through Rational Yolk-Shell Design and Ni Doping. Chin. J. Struct. Chem. 2022, 41, 2205030–2205036. [Google Scholar] [CrossRef]
- Liu, J.D.Y.; Yu, X.; Bao, J.Z.; Lin, Y.X. A Polyanionic, Quasi-zero-strain and Open-framework K0.76V0.55Nb0.45OPO4 for Sodium-ion Batteries. Chin. J. Struct. Chem. 2021, 40, 1631–1638. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Z.D.; Fu, Y.R.; Liu, Q.; Xiao, G. Ca2Nb2O7 as a Novel Open-framework Anode Material for Potassium-ion Batteries. Chin. J. Struct. Chem. 2021, 40, 233–238. [Google Scholar] [CrossRef]
- Teng, X.; Niu, Y.; Gong, S.; Liu, X.; Chen, Z. Selective CO2 Reduction to Formate on Heterostructured Sn/SnO2 Nanoparticles Promoted by Carbon Layer Networks. J. Electrochem. 2022, 28, 2108441. [Google Scholar] [CrossRef]
- Wu, R.; Wang, D.P.; Zhou, K.; Srikanth, N.; Wei, J.; Chen, Z. Porous cobalt phosphide/graphitic carbon polyhedral hybrid composites for efficient oxygen evolution reactions. J. Mater. Chem. A 2016, 4, 13742–13745. [Google Scholar] [CrossRef]
- Wang, J.-Y.; Tong, X.-F.; Peng, Q.-F.; Guan, Y.-P.; Wang, W.-K.; Wang, A.-B.; Liu, N.-Q.; Huang, Y.-Q. Efficient Interface Enabled by Nano-Hydroxyapatite@Porous Carbon for Lithium-Sulfur Batteries. J. Electrochem. 2022, 28, 2219008. [Google Scholar] [CrossRef]
- Long, X.Y.; Meng, J.Z.; Gu, J.B.; Ling, L.Q.; Li, Q.W.; Liu, N.; Wang, K.W.; Li, Z.Q. Interfacial Engineering of NiFeP/NiFe-LDH Heterojunction for Efficient Overall Water Splitting. Chin. J. Struct. Chem. 2022, 41, 2204046–2204053. [Google Scholar] [CrossRef]
- Li, L.H.; Tian, T.; Cai, Q.R.; Shih, C.J.; Santos, E.J.G. Asymmetric electric field screening in van der Waals heterostructures. Nat. Commun. 2018, 9, 1271. [Google Scholar] [CrossRef]
- Chen, S.; Zheng, S.-S.; Zheng, L.-M.; Zhang, Y.-H.; Wang, Z.-L. Optimized Electrochemical Performance of Si@C Prepared by Hydrothermal Reaction and Glucose Carbon Source. J. Electrochem. 2022, 28, 2112221. [Google Scholar] [CrossRef]
- Yang, D.; Zhu, J.; Rui, X.; Tan, H.; Cai, R.; Hoster, H.E.; Yu, D.Y.W.; Hng, H.H.; Yan, Q. Synthesis of Cobalt Phosphides and Their Application as Anodes for Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2013, 5, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Gao, H.C.; Ma, D.J.; Zou, J.S.; Lin, Z.; Kang, X.W.; Chen, S.W. High-performance Li-Se battery cathode based on CoSe2-porous carbon composites. Electrochim. Acta 2018, 264, 341–349. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, C.; Li, W.; Yang, Q. One-pot synthesis of carbon-coated Ni5P4 nanoparticles and CoP nanorods for high-rate and high-stability lithium-ion batteries. J. Mater. Chem. A 2015, 3, 23345–23351. [Google Scholar] [CrossRef]
- Cai, W.; Yan, C.; Yao, Y.-X.; Xu, L.; Xu, R.; Jiang, L.-L.; Huang, J.-Q.; Zhang, Q. Rapid Lithium Diffusion in Order@Disorder Pathways for Fast-Charging Graphite Anodes. Small Struct. 2020, 1, 2000010. [Google Scholar] [CrossRef]
- Ge, X.; Li, Z.; Yin, L. Metal-organic frameworks derived porous core/shellCoP@C polyhedrons anchored on 3D reduced graphene oxide networks as anode for sodium-ion battery. Nano Energy 2017, 32, 117–124. [Google Scholar] [CrossRef]
- Li, J.; Zhang, T.; Han, C.; Li, H.; Shi, R.; Tong, J.; Li, B. Crystallized lithium titanate nanosheets prepared via spark plasma sintering for ultra-high rate lithium ion batteries. J. Mater. Chem. A 2019, 7, 455–460. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, L.; Ge, X.; Li, C.; Dong, S.; Wang, C.; Yin, L. Core-shell structured CoP/FeP porous microcubes interconnected by reduced graphene oxide as high performance anodes for sodium ion batteries. Nano Energy 2017, 32, 494–502. [Google Scholar] [CrossRef]
- Rhee, D.Y.; Kim, J.; Moon, J.; Park, M.-S. Off-stoichiometric TiO2−x-decorated graphite anode for high-power lithium-ion batteries. J. Alloys Compd. 2020, 843, 156042. [Google Scholar] [CrossRef]
- Von Lim, Y.; Huang, S.; Zhang, Y.; Kong, D.; Wang, Y.; Guo, L.; Zhang, J.; Shi, Y.; Chen, T.P.; Ang, L.K.; et al. Bifunctional porous iron phosphide/carbon nanostructure enabled high-performance sodium-ion battery and hydrogen evolution reaction. Energ. Storage Mater. 2018, 15, 98–107. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Z.; Li, W.; Zhong, X.; Gu, L.; Yu, Y. Nitridation Br-doped Li4Ti5O12 anode for high rate lithium ion batteries. J. Power Sources 2014, 266, 323–331. [Google Scholar] [CrossRef]
- Zhang, X.-H.; Jiang, R.; Fan, C.-Y.; Xie, D.; Li, B.; Zhang, J.-P.; Wu, X.-L. Engineering All-Purpose Amorphous Carbon Nanotubes with High N/O-Co-Doping Content to Bridge the Alkali-Ion Batteries and Li Metal Batteries. Small 2021, 17, 2006566. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Xie, H.; Dou, Z.; Hao, Z.; Huang, Q.; Guo, Z.; Wang, C.; Miao, K.; Kang, X. Graphene-Oxide-Coated CoP2@C Anode Enables High Capacity of Lithium-Ion Batteries. Electrochem 2023, 4, 473-484. https://doi.org/10.3390/electrochem4040031
Zhang W, Xie H, Dou Z, Hao Z, Huang Q, Guo Z, Wang C, Miao K, Kang X. Graphene-Oxide-Coated CoP2@C Anode Enables High Capacity of Lithium-Ion Batteries. Electrochem. 2023; 4(4):473-484. https://doi.org/10.3390/electrochem4040031
Chicago/Turabian StyleZhang, Wei, Hangxuan Xie, Zirui Dou, Zhentao Hao, Qianhui Huang, Ziqi Guo, Chao Wang, Kanghua Miao, and Xiongwu Kang. 2023. "Graphene-Oxide-Coated CoP2@C Anode Enables High Capacity of Lithium-Ion Batteries" Electrochem 4, no. 4: 473-484. https://doi.org/10.3390/electrochem4040031





