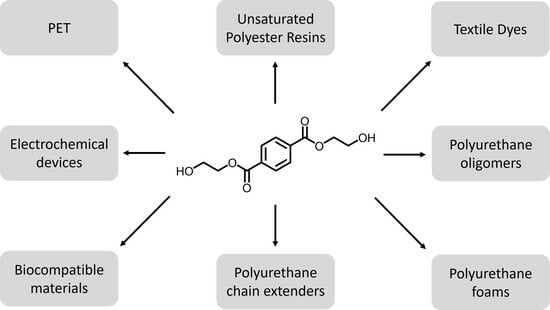
Envisioning a BHET Economy: Adding Value to PET Waste
Abstract
:1. Introduction
2. Life-Cycle and Economic Analyses
3. BHET as a Feedstock
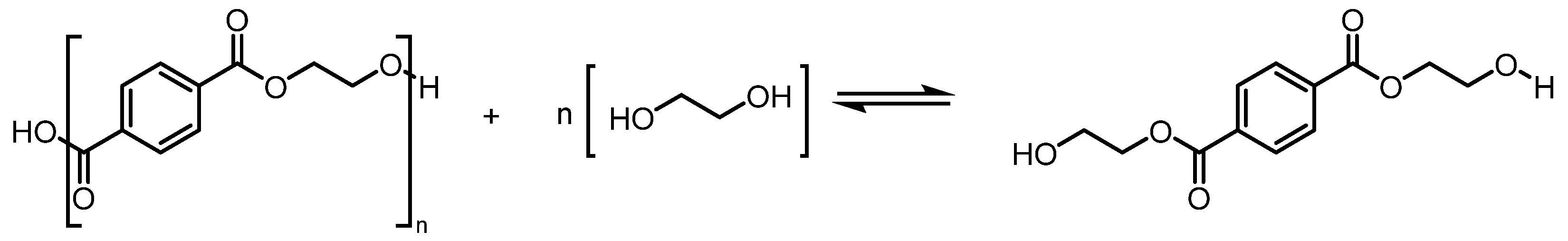
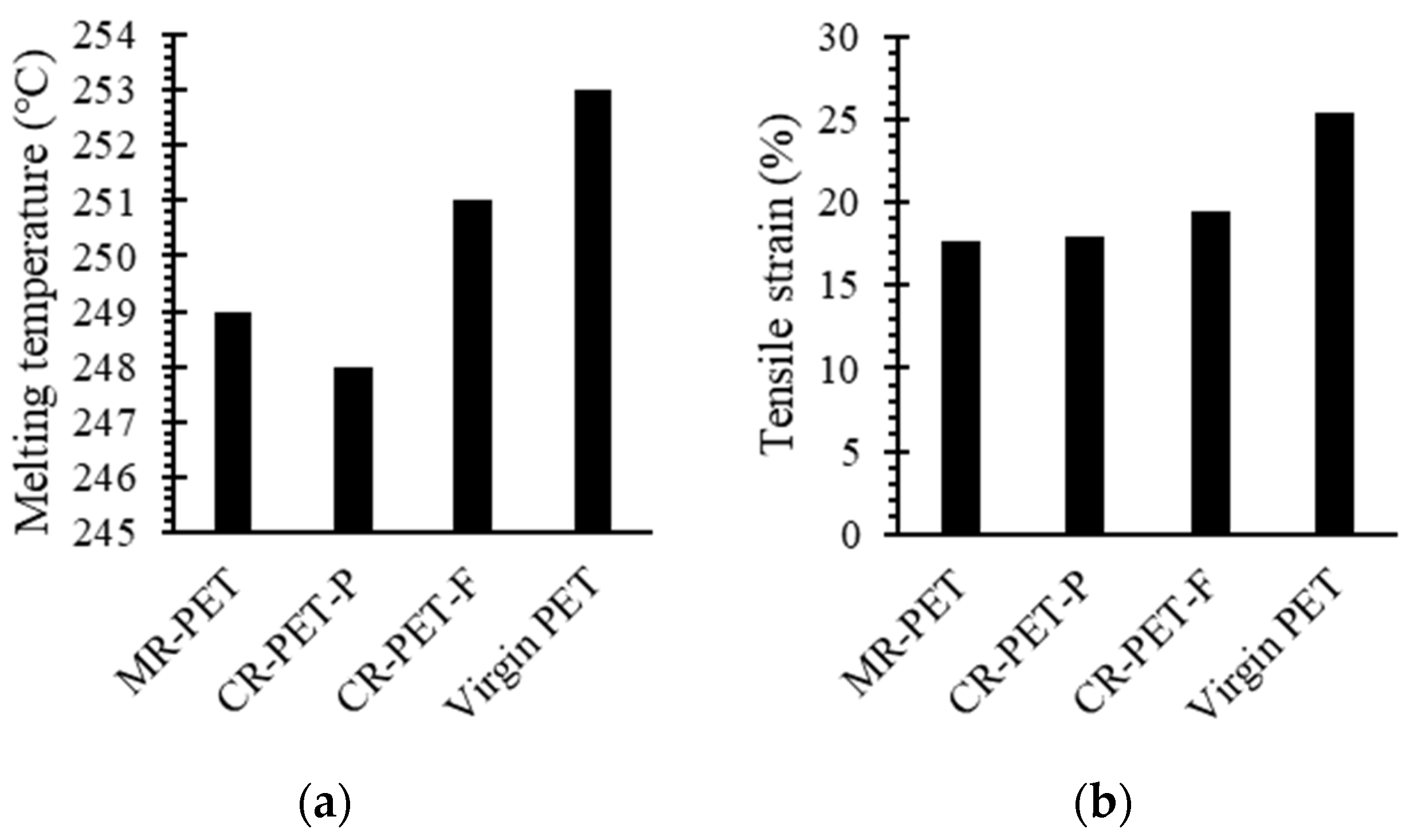
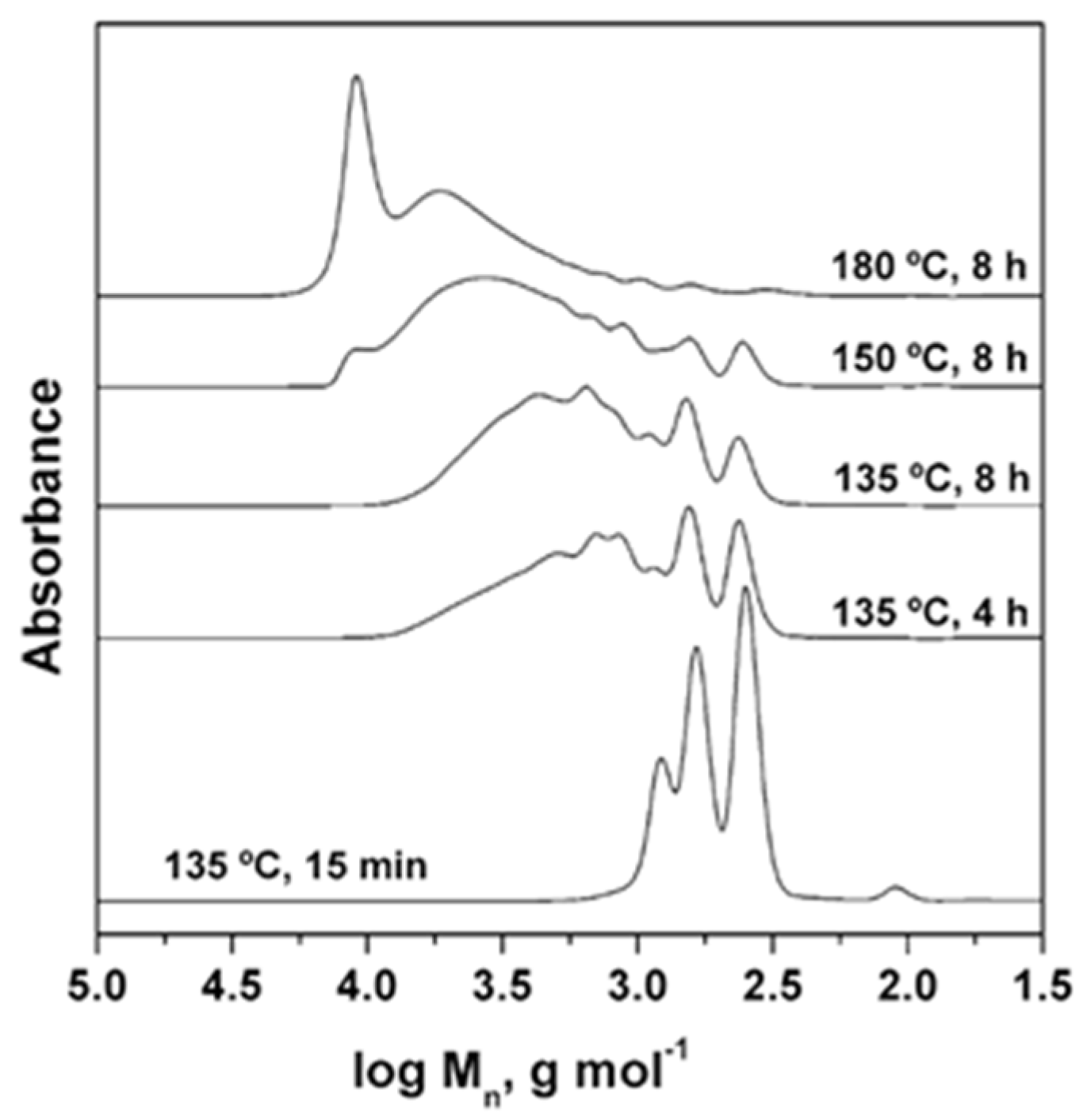
3.1. Resynthesis into PET
3.2. Resins and Paints
3.2.1. Polyester and Alkyd Resins
3.2.2. Epoxy Resins
3.3. Textile Industry Applications
3.4. Uses in Polyurethanes
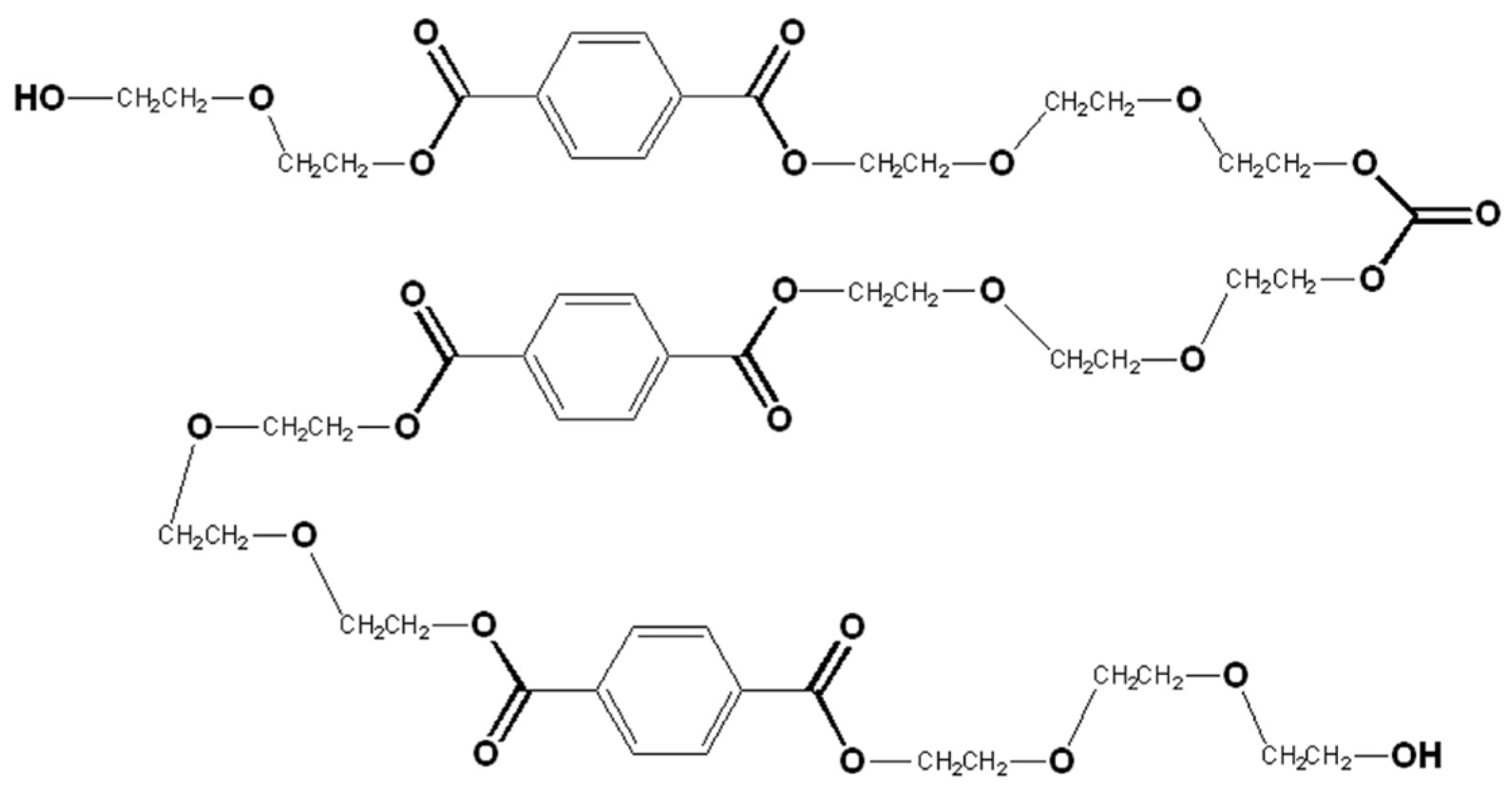
3.4.1. Oligomers for Polyurethanes
3.4.2. Chain Extenders
3.4.3. Foams
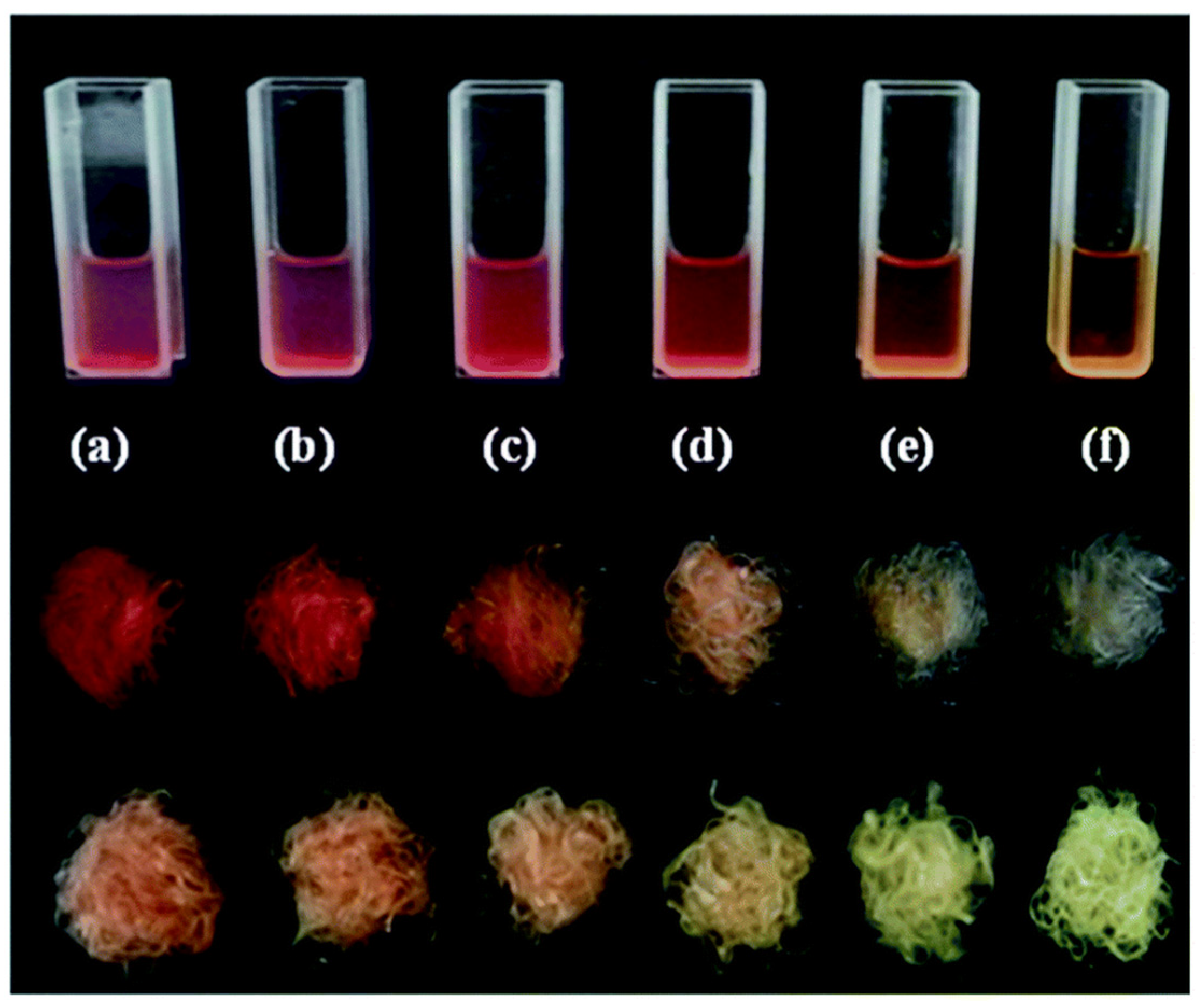
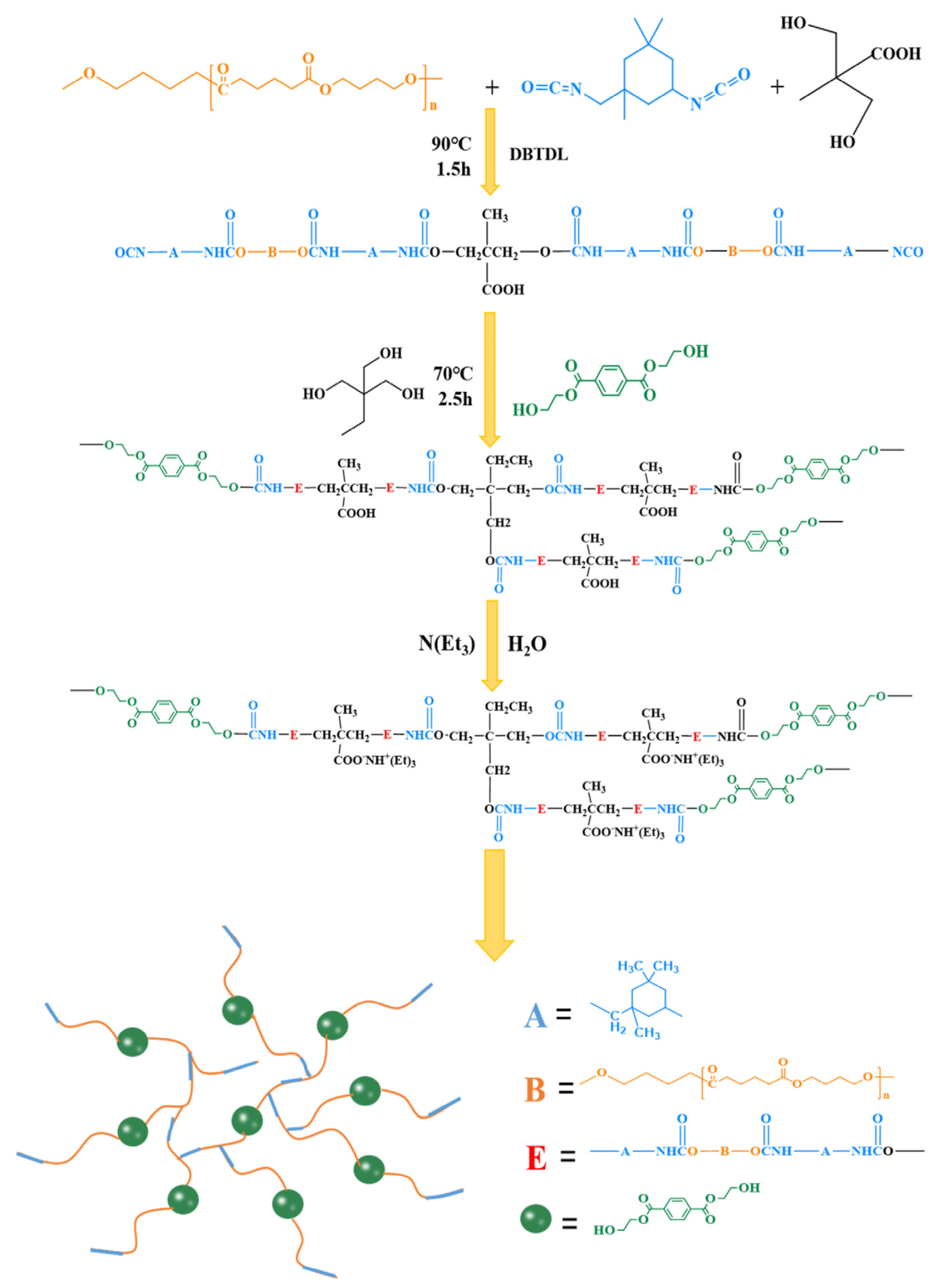
3.4.4. Biocompatible and Biodegradable Polyurethanes
3.4.5. Other Polyurethane Work
3.5. Biocompatible and Biodegradable Materials
3.6. Other Applications
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Polyethylene Terephthalate Global Market Report 2022; The Business Research Company: London, UK, 2022.
- Benyathiar, P.; Kumar, P.; Carpenter, G.; Brace, J.; Mishra, D.K. Polyethylene Terephthalate (PET) Bottle-to-Bottle Recycling for the Beverage Industry: A Review. Polymers 2022, 14, 2366. [Google Scholar] [CrossRef]
- 2021 Global Recycled PET Market Report; Market and Markets: Pune, India, 2021.
- Suhaimi, N.A.S.; Muhamad, F.; Abd Razak, N.A.; Zeimaran, E. Recycling of polyethylene terephthalate wastes: A review of technologies, routes, and applications. Polym. Eng. Sci. 2022, 62, 2355–2375. [Google Scholar] [CrossRef]
- Ragaert, K.; Delva, L.; Van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 2017, 69, 24–58. [Google Scholar] [CrossRef]
- Achilias, D.S.; Karayannidis, G.P. The Chemical Recycling of PET in the Framework of Sustainable Development. Water Air Soil Pollut. Focus 2004, 4, 385–396. [Google Scholar] [CrossRef]
- Hopewell, J.; Dvorak, R.; Kosior, E. Plastics recycling: Challenges and opportunities. Philos. Trans. R. Soc. B 2009, 364, 2115–2126. [Google Scholar] [CrossRef]
- Allen, R.D.; James, M.I. Chemical Recycling of PET. In ACS Symposium Series; Collias, D.I., James, M.I., Layman, J.M., Eds.; American Chemical Society: Washington, DC, USA, 2021; Volume 1391, pp. 61–80. [Google Scholar] [CrossRef]
- Schyns, Z.O.G.; Shaver, M.P. Mechanical Recycling of Packaging Plastics: A Review. Macromol. Rapid Commun. 2021, 42, 2000415. [Google Scholar] [CrossRef]
- Dios Caputto, M.D.; Navarro, R.; Valentín, J.L.; Marcos-Fernández, Á. Chemical upcycling of poly(ethylene terephthalate) waste: Moving to a circular model. J. Polym. Sci. 2022, 60, 3269–3283. [Google Scholar] [CrossRef]
- Shojaei, B.; Abtahi, M.; Najafi, M. Chemical recycling of PET: A stepping-stone toward sustainability. Polym. Adv. Technol. 2020, 31, 2912–2938. [Google Scholar] [CrossRef]
- Gracida-Alvarez, U.R.; Xu, H.; Benavides, P.T.; Wang, M.; Hawkins, T.R. Circular Economy Sustainability Analysis Framework for Plastics: Application for Poly(ethylene Terephthalate) (PET). ACS Sustain. Chem. Eng. 2023, 11, 514–524. [Google Scholar] [CrossRef]
- Kim, N.-K.; Lee, S.-H.; Park, H.-D. Current biotechnologies on depolymerization of polyethylene terephthalate (PET) and repolymerization of reclaimed monomers from PET for bio-upcycling: A critical review. Bioresour. Technol. 2022, 363, 127931. [Google Scholar] [CrossRef]
- Jafari, H. Investigating environmental and economic aspects of sustainability by recycling PET plastic bottles: A game-theoretic approach. Clean Technol. Environ. Policy 2022, 24, 829–842. [Google Scholar] [CrossRef]
- Ghasemi, M.H.; Neekzad, N.; Ajdari, F.B.; Kowsari, E.; Ramakrishna, S. Mechanistic aspects of poly(ethylene terephthalate) recycling–toward enabling high quality sustainability decisions in waste management. Environ. Sci. Pollut. Res. 2021, 28, 43074–43101. [Google Scholar] [CrossRef]
- Sarioğlu, E.; Kaynak, H.K. PET Bottle Recycling for Sustainable Textiles. In Polyester—Production, Characterization and Innovative Applications; Camlibel, N.O., Ed.; InTech: London, UK, 2018. [Google Scholar] [CrossRef]
- Sarda, P.; Hanan, J.C.; Lawrence, J.G.; Allahkarami, M. Sustainability performance of polyethylene terephthalate, clarifying challenges and opportunities. J. Polym. Sci. 2022, 60, 7–31. [Google Scholar] [CrossRef]
- Chaudhari, U.S.; Lin, Y.; Thompson, V.S.; Handler, R.M.; Pearce, J.M.; Caneba, G.; Muhuri, P.; Watkins, D.; Shonnard, D.R. Systems Analysis Approach to Polyethylene Terephthalate and Olefin Plastics Supply Chains in the Circular Economy: A Review of Data Sets and Models. ACS Sustain. Chem. Eng. 2021, 9, 7403–7421. [Google Scholar] [CrossRef]
- Tsironi, T.N.; Chatzidakis, S.M.; Stoforos, N.G. The future of polyethylene terephthalate bottles: Challenges and sustainability. Packag. Technol. Sci. 2022, 35, 317–325. [Google Scholar] [CrossRef]
- Singh, A.; Rorrer, N.A.; Nicholson, S.R.; Erickson, E.; DesVeaux, J.S.; Avelino, A.F.T.; Lamers, P.; Bhatt, A.; Zhang, Y.; Avery, G.; et al. Techno-economic, life-cycle, and socioeconomic impact analysis of enzymatic recycling of poly(ethylene terephthalate). Joule 2021, 5, 2479–2503. [Google Scholar] [CrossRef]
- Uekert, T.; Singh, A.; DesVeaux, J.S.; Ghosh, T.; Bhatt, A.; Yadav, G.; Afzal, S.; Walzberg, J.; Knauer, K.M.; Nicholson, S.R.; et al. Technical, Economic, and Environmental Comparison of Closed-Loop Recycling Technologies for Common Plastics. ACS Sustain. Chem. Eng. 2023, 11, 965–978. [Google Scholar] [CrossRef]
- Cao, F.; Wang, L.; Zheng, R.; Guo, L.; Chen, Y.; Qian, X. Research and progress of chemical depolymerization of waste PET and high-value application of its depolymerization products. RSC Adv. 2022, 12, 31564–31576. [Google Scholar] [CrossRef]
- Bhanderi, K.K.; Joshi, J.R.; Patel, J.V. Recycling of polyethylene terephthalate (PET Or PETE) plastics—An alternative to obtain value added products: A review. J. Indian Chem. Soc. 2023, 100, 100843. [Google Scholar] [CrossRef]
- Foti, D. Recycled waste PET for sustainable fiber-reinforced concrete. In Use of Recycled Plastics in Eco-Efficient Concrete; Elsevier: Amsterdam, The Netherlands, 2019; pp. 387–410. [Google Scholar] [CrossRef]
- Ferreira, J.W.d.S.; Marroquin, J.F.R.; Felix, J.F.; Farias, M.M.; Casagrande, M.D.T. The feasibility of recycled micro polyethylene terephthalate (PET) replacing natural sand in hot-mix asphalt. Constr. Build. Mater. 2022, 330, 127276. [Google Scholar] [CrossRef]
- Mohd Nasir, N.H.; Usman, F.; Saggaf, A.; Saloma. Development of composite material from Recycled Polyethylene Terephthalate and fly ash: Four decades progress review. Curr. Res. Green Sustain. Chem. 2022, 5, 100280. [Google Scholar] [CrossRef]
- Khan, T.; Acar, V.; Aydin, M.R.; Hülagü, B.; Akbulut, H.; Seydibeyoğlu, M.Ö. A review on recent advances in sandwich structures based on polyurethane foam cores. Polym. Compos. 2020, 41, 2355–2400. [Google Scholar] [CrossRef]
- Nikbin, I.M.; Rahimi, R.S.; Allahyari, H.; Fallah, F. Feasibility study of waste Poly Ethylene Terephthalate (PET) particles as aggregate replacement for acid erosion of sustainable structural normal and lightweight concrete. J. Clean. Prod. 2016, 126, 108–117. [Google Scholar] [CrossRef]
- Leng, Z.; Padhan, R.K.; Sreeram, A. Production of a sustainable paving material through chemical recycling of waste PET into crumb rubber modified asphalt. J. Clean. Prod. 2018, 180, 682–688. [Google Scholar] [CrossRef]
- Rahimi, R.S.; Nikbin, I.M.; Allahyari, H.; Habibi, T.S. Sustainable approach for recycling waste tire rubber and polyethylene terephthalate (PET) to produce green concrete with resistance against sulfuric acid attack. J. Clean. Prod. 2016, 126, 166–177. [Google Scholar] [CrossRef]
- Mirjalili, A.; Dong, B.; Pena, P.; Ozkan, C.S.; Ozkan, M. Upcycling of polyethylene terephthalate plastic waste to microporous carbon structure for energy storage. Energy Storage 2020, 2, e201. [Google Scholar] [CrossRef]
- Roy, S.; Maji, P.K.; Goh, K.-L. Sustainable design of flexible 3D aerogel from waste PET bottle for wastewater treatment to energy harvesting device. Chem. Eng. J. 2021, 413, 127409. [Google Scholar] [CrossRef]
- Tan, M.Y.; Goh, L.; Safanama, D.; Loh, W.W.; Ding, N.; Chien, S.W.; Goh, S.S.; Thitsartarn, W.; Lim, J.Y.C.; Fam, D.W.H. Upcycling waste poly(ethylene terephthalate) into polymer electrolytes. J. Mater. Chem. A 2022, 10, 24468–24474. [Google Scholar] [CrossRef]
- Qiu, J.; Ma, S.; Wang, S.; Tang, Z.; Li, Q.; Tian, A.; Xu, X.; Wang, B.; Lu, N.; Zhu, J. Upcycling of Polyethylene Terephthalate to Continuously Reprocessable Vitrimers through Reactive Extrusion. Macromolecules 2021, 54, 703–712. [Google Scholar] [CrossRef]
- Wu, S.-S.; Li, Y.-D.; Hu, Z.; Zeng, J.-B. Fast Upcycling of Poly(ethylene terephthalate) into Catalyst-Free Vitrimers. ACS Sustain. Chem. Eng. 2023, 11, 1974–1984. [Google Scholar] [CrossRef]
- Yuan, X.; Cho, M.-K.; Lee, J.G.; Choi, S.W.; Lee, K.B. Upcycling of waste polyethylene terephthalate plastic bottles into porous carbon for CF4 adsorption. Environ. Pollut. 2020, 265, 114868. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Ren, J.; Song, Y.; He, P.; Bai, H.; Fan, Z.; Niu, R.; Gong, J. Upcycling Waste Poly(ethylene terephthalate) into a Porous Carbon Cuboid through a MOF-Derived Carbonization Strategy for Interfacial Solar-Driven Water–Thermoelectricity Cogeneration. ACS Sustain. Chem. Eng. 2022, 10, 16427–16439. [Google Scholar] [CrossRef]
- Bonfim, D.P.F.; Cruz, F.G.S.; Bretas, R.E.S.; Guerra, V.G.; Aguiar, M.L. A Sustainable Recycling Alternative: Electrospun PET-Membranes for Air Nanofiltration. Polymers 2021, 13, 1166. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Wadgama, M.H.; Khan, A.L.; Yasin, M.; Akhtar, F.H. Upcycling Poly(ethylene terephthalate) by Fabricating Membranes for Desalination. ACS Sustain. Chem. Eng. 2023, 11, 726–732. [Google Scholar] [CrossRef]
- Topuz, F.; Oldal, D.G.; Szekely, G. Valorization of Polyethylene Terephthalate (PET) Plastic Wastes as Nanofibrous Membranes for Oil Removal: Sustainable Solution for Plastic Waste and Oil Pollution. Ind. Eng. Chem. Res. 2022, 61, 9077–9086. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, S.H. Poly (ethylene terephthalate) recycling for high value added textiles. Fash. Text. 2014, 1, 1. [Google Scholar] [CrossRef]
- Paszun, D.; Spychaj, T. Chemical Recycling of Poly(ethylene terephthalate). Ind. Eng. Chem. Res. 1997, 36, 1373–1383. [Google Scholar] [CrossRef]
- Raheem, A.B.; Noor, Z.Z.; Hassan, A.; Abd Hamid, M.K.; Samsudin, S.A.; Sabeen, A.H. Current developments in chemical recycling of post-consumer polyethylene terephthalate wastes for new materials production: A review. J. Clean. Prod. 2019, 225, 1052–1064. [Google Scholar] [CrossRef]
- Sheel, A.; Pant, D. Chemical Depolymerization of PET Bottles via Glycolysis. In Recycling of Polyethylene Terephthalate Bottles; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 61–84. [Google Scholar] [CrossRef]
- Payne, J.; Jones, M.D. The Chemical Recycling of Polyesters for a Circular Plastics Economy: Challenges and Emerging Opportunities. ChemSusChem 2021, 14, 4041–4070. [Google Scholar] [CrossRef]
- Damayanti; Wu, H.-S. Strategic Possibility Routes of Recycled PET. Polymers 2021, 13, 1475. [Google Scholar] [CrossRef]
- Barnard, E.; Rubio Arias, J.J.; Thielemans, W. Chemolytic depolymerisation of PET: A review. Green Chem. 2021, 23, 3765–3789. [Google Scholar] [CrossRef]
- Al-Sabagh, A.M.; Yehia, F.Z.; Eshaq, G.; Rabie, A.M.; ElMetwally, A.E. Greener routes for recycling of polyethylene terephthalate. Egypt. J. Pet. 2016, 25, 53–64. [Google Scholar] [CrossRef]
- Sinha, V.; Patel, M.R.; Patel, J.V. Pet Waste Management by Chemical Recycling: A Review. J. Polym. Environ. 2010, 18, 8–25. [Google Scholar] [CrossRef]
- Ellis, L.D.; Rorrer, N.A.; Sullivan, K.P.; Otto, M.; McGeehan, J.E.; Román-Leshkov, Y.; Wierckx, N.; Beckham, G.T. Chemical and biological catalysis for plastics recycling and upcycling. Nat. Catal. 2021, 4, 539–556. [Google Scholar] [CrossRef]
- Karayannidis, G.P.; Achilias, D.S. Chemical Recycling of Poly(ethylene terephthalate). Macromol. Mater. Eng. 2007, 292, 128–146. [Google Scholar] [CrossRef]
- George, N.; Kurian, T. Recent Developments in the Chemical Recycling of Postconsumer Poly(ethylene terephthalate) Waste. Ind. Eng. Chem. Res. 2014, 53, 14185–14198. [Google Scholar] [CrossRef]
- Xin, J.; Zhang, Q.; Huang, J.; Huang, R.; Jaffery, Q.Z.; Yan, D.; Zhou, Q.; Xu, J.; Lu, X. Progress in the catalytic glycolysis of polyethylene terephthalate. J. Environ. Manag. 2021, 296, 113267. [Google Scholar] [CrossRef]
- Jeya, G.; Dhanalakshmi, R.; Anbarasu, M.; Vinitha, V.; Sivamurugan, V. A short review on latest developments in catalytic depolymerization of Poly (ethylene terephathalate) wastes. J. Indian Chem. Soc. 2022, 99, 100291. [Google Scholar] [CrossRef]
- Tawfik, M.E.; Eskander, S.B. Polymer Concrete from Marble Wastes and Recycled Poly(ethylene terephthalate). J. Elastomers Plast. 2006, 38, 65–79. [Google Scholar] [CrossRef]
- Macijauskas, G.; Jankauskaitė, V. Epoxy Resin and Polyurethane Compositions from Glycolized Poly(ethylene terephthalate) Wastes. Mater. Sci. 2013, 19, 283–290. [Google Scholar] [CrossRef]
- Ghaderian, A.; Haghighi, A.H.; Taromi, F.A.; Abdeen, Z.; Boroomand, A.; Taheri, S.M.-R. Characterization of Rigid Polyurethane Foam Prepared from Recycling of PET Waste. Period. Polytech. Chem. Eng. 2015, 59, 296–305. [Google Scholar] [CrossRef]
- Cakić, S.M.; Ristić, I.S.; M-Cincović, M.; Nikolić, N.Č.; Ilić, O.Z.; Stojiljković, D.T.; B-Simendić, J.K. Glycolyzed products from PET waste and their application in synthesis of polyurethane dispersions. Prog. Org. Coat. 2012, 74, 115–124. [Google Scholar] [CrossRef]
- Vaidya, U.R.; Nadkarni, V.M. Unsaturated polyester resins from poly(ethylene terephthalate) waste. 1. Synthesis and characterization. Ind. Eng. Chem. Res. 1987, 26, 194–198. [Google Scholar] [CrossRef]
- Suh, D.J.; Park, O.O.; Yoon, K.H. The properties of unsaturated polyester based on the glycolyzed poly(ethylene terephthalate) with various glycol compositions. Polymer 2000, 41, 461–466. [Google Scholar] [CrossRef]
- Lu, M.; Kim, S. Unsaturated polyester resins based on recycled PET: Preparation and curing behavior. J. Appl. Polym. Sci. 2001, 80, 1052–1057. [Google Scholar] [CrossRef]
- Abdel-Azim, A.-A.A.; Atta, A.M. Recycled Flexible Resins in Concrete. Polym. J. 1997, 29, 21–24. [Google Scholar] [CrossRef]
- Abdel-Azim, A.; Attia, I.A. Making polymer concrete and polymer mortar using synthesized unsaturated polyester resins from poly(ethylene terephthalate) waste. Polym. Adv. Technol. 1995, 6, 688–692. [Google Scholar] [CrossRef]
- Katoch, S.; Sharma, V.; Kundu, P.P. Water sorption and diffusion through saturated polyester and their nanocomposites synthesized from glycolyzed PET waste with varied composition. Chem. Eng. Sci. 2010, 65, 4378–4387. [Google Scholar] [CrossRef]
- Saravari, O.; Vessabutr, B.; Pimpan, V. Synthesis of urethane oils from waste poly(ethylene terephthalate) bottles. J. Appl. Polym. Sci. 2004, 92, 3040–3045. [Google Scholar] [CrossRef]
- Essawy, H.A.; Tawfik, M.E.; Elsayed, N.H. Effect of addition of glycolysis products of poly(ethyleneterephthalate) wastes to urea-formaldehyde resin on its adhesion performance to wood substrates and formaldehyde emission. J. Appl. Polym. Sci. 2012, 123, 2377–2383. [Google Scholar] [CrossRef]
- Phuangngamphan, P.; Thongpin, C. Synthesize of Polydiacetylene Containing Copolyurethane Using Polyol Product Derived from Chemical Recycling of PET Bottles. Energy Procedia 2014, 56, 326–333. [Google Scholar] [CrossRef]
- Luo, X.; Li, Y. Synthesis and Characterization of Polyols and Polyurethane Foams from PET Waste and Crude Glycerol. J. Polym. Environ. 2014, 22, 318–328. [Google Scholar] [CrossRef]
- Kirpluks, M.; Cabulis, U.; Ivdre, A.; Kuranska, M.; Zieleniewska, M.; Auguscik, M. Mechanical and Thermal Properties of High-Density Rigid Polyurethane Foams from Renewable Resources. J. Renew. Mater. 2016, 4, 86–100. [Google Scholar] [CrossRef]
- Mecit, O.; Akar, A. Synthesis of Urethane Oil Varnishes from Waste Poly(ethylene terephthalate). Macromol. Mater. Eng. 2001, 286, 513–515. [Google Scholar] [CrossRef]
- Karayannidis, G.P.; Achilias, D.S.; Sideridou, I.D.; Bikiaris, D.N. Alkyd resins derived from glycolized waste poly(ethylene terephthalate). Eur. Polym. J. 2005, 41, 201–210. [Google Scholar] [CrossRef]
- Billiau-Loreau, M.; Durand, G.; Tersac, G. Structural effects of diacidic and glycolic moieties on physicochemical properties of aromatic polyesterdiols from glycolysis/esterification of poly(ethylene terephthalate) wastes. Polymer 2002, 43, 21–28. [Google Scholar] [CrossRef]
- Abdelaal, M.Y.; Sobahi, T.R.; Makki, M.S.I. Chemical transformation of pet waste through glycolysis. Constr. Build. Mater. 2011, 25, 3267–3271. [Google Scholar] [CrossRef]
- Torlakoğlu, A.; Güçlü, G. Alkyd–amino resins based on waste PET for coating applications. Waste Manag. 2009, 29, 350–354. [Google Scholar] [CrossRef]
- Scremin, D.M.; Miyazaki, D.Y.; Lunelli, C.E.; Silva, S.A.; Zawadzki, S.F. PET Recycling by Alcoholysis Using a New Heterogeneous Catalyst: Study and its Use in Polyurethane Adhesives Preparation. Macromol. Symp. 2019, 383, 1800027. [Google Scholar] [CrossRef]
- Atta, A.M.; El-Kafrawy, A.F.; Aly, M.H.; Abdel-Azim, A.-A.A. New epoxy resins based on recycled poly(ethylene terephthalate) as organic coatings. Prog. Org. Coat. 2007, 58, 13–22. [Google Scholar] [CrossRef]
- Kim, J.; Jeong, D.; Son, C.; Lee, Y.; Kim, E.; Moon, I. Synthesis and applications of unsaturated polyester resins based on PET waste. Korean J. Chem. Eng. 2007, 24, 1076–1083. [Google Scholar] [CrossRef]
- Halacheva, N.; Novakov, P. Preparation of oligoester diols by alcoholytic destruction of poly(ethylene terephthalate). Polymer 1995, 36, 867–874. [Google Scholar] [CrossRef]
- Farahat, M.S. Mechanical characteristics of modified unsaturated polyester resins derived from poly(ethylene terephthalate) waste. Polym. Int. 2002, 51, 183–189. [Google Scholar] [CrossRef]
- Farahat, M.S.; Nikles, D.E. On the UV Curability and Mechanical Properties of Novel Binder Systems Derived from Poly(ethylene terephthalate) (PET) Waste for Solventless Magnetic Tape Manufacturing, 1. Acrylated Oligoesters. Macromol. Mater. Eng. 2001, 286, 695–704. [Google Scholar] [CrossRef]
- Mahdi, F.; Abbas, H.; Khan, A.A. Strength characteristics of polymer mortar and concrete using different compositions of resins derived from post-consumer PET bottles. Constr. Build. Mater. 2010, 24, 25–36. [Google Scholar] [CrossRef]
- Hoang, C.N.; Pham, C.T.; Dang, T.M.; Hoang, D.; Lee, P.-C.; Kang, S.-J.; Kim, J. Novel Oligo-Ester-Ether-Diol Prepared by Waste Poly(ethylene terephthalate) Glycolysis and Its Use in Preparing Thermally Stable and Flame Retardant Polyurethane Foam. Polymers 2019, 11, 236. [Google Scholar] [CrossRef] [PubMed]
- Aslan, S.; Immirzi, B.; Laurienzo, P.; Malinconico, M.; Martuscelli, E.; Volpe, M.G.; Pelino, M.; Savini, L. Unsaturated polyester resins from glycolysed waste polyethyleneterephthalate: Synthesis and comparison of properties and performance with virgin resin. J. Mater. Sci. 1997, 32, 2329–2336. [Google Scholar] [CrossRef]
- Iturrondobeitia, M.; Alonso, L.; Lizundia, E. Prospective life cycle assessment of poly(ethylene terephthalate) upcycling via chemoselective depolymerization. Resour. Conserv. Recycl. 2023, 198, 107182. [Google Scholar] [CrossRef]
- Lang, W.T.; Mehta, S.A.; Thomas, M.M.; Openshaw, D.; Westgate, E.; Bagnato, G. Chemical recycling of polyethylene terephthalate, an industrial and sustainable opportunity for Northwest of England. J. Environ. Chem. Eng. 2023, 11, 110585. [Google Scholar] [CrossRef]
- Luo, Y.; Selvam, E.; Vlachos, D.G.; Ierapetritou, M. Economic and Environmental Benefits of Modular Microwave-Assisted Polyethylene Terephthalate Depolymerization. ACS Sustain. Chem. Eng. 2023, 11, 4209–4218. [Google Scholar] [CrossRef]
- Ghosh, T.; Avery, G.; Bhatt, A.; Uekert, T.; Walzberg, J.; Carpenter, A. Towards a circular economy for PET bottle resin using a system dynamics inspired material flow model. J. Clean. Prod. 2023, 383, 135208. [Google Scholar] [CrossRef]
- Davidson, M.G.; Furlong, R.A.; McManus, M.C. Developments in the life cycle assessment of chemical recycling of plastic waste—A review. J. Clean. Prod. 2021, 293, 126163. [Google Scholar] [CrossRef]
- Xi, G.; Lu, M.; Sun, C. Study on depolymerization of waste polyethylene terephthalate into monomer of bis(2-hydroxyethyl terephthalate). Polym. Degrad. Stab. 2005, 87, 117–120. [Google Scholar] [CrossRef]
- Aguado, A.; Martínez, L.; Becerra, L.; Arieta-araunabeña, M.; Arnaiz, S.; Asueta, A.; Robertson, I. Chemical depolymerisation of PET complex waste: Hydrolysis vs. glycolysis. J. Mater. Cycles Waste Manag. 2014, 16, 201–210. [Google Scholar] [CrossRef]
- Capeletti, M.R.; Passamonti, F.J. Optimization of reaction parameters in the conversion of PET to produce BHET. Polym. Eng. Sci. 2018, 58, 1500–1507. [Google Scholar] [CrossRef]
- Chen, C.-H.; Chen, C.-Y.; Lo, Y.-W.; Mao, C.-F.; Liao, W.-T. Studies of glycolysis of poly(ethylene terephthalate) recycled from postconsumer soft-drink bottles. I. Influences of glycolysis conditions. J. Appl. Polym. Sci. 2001, 80, 943–948. [Google Scholar] [CrossRef]
- Wang, Q.; Geng, Y.; Lu, X.; Zhang, S. First-Row Transition Metal-Containing Ionic Liquids as Highly Active Catalysts for the Glycolysis of Poly(ethylene terephthalate) (PET). ACS Sustain. Chem. Eng. 2015, 3, 340–348. [Google Scholar] [CrossRef]
- Pingale, N.D.; Palekar, V.S.; Shukla, S.R. Glycolysis of postconsumer polyethylene terephthalate waste. J. Appl. Polym. Sci. 2010, 115, 249–254. [Google Scholar] [CrossRef]
- Guo, Z.; Lindqvist, K.; de la Motte, H. An efficient recycling process of glycolysis of PET in the presence of a sustainable nanocatalyst. J. Appl. Polym. Sci. 2018, 135, 46285. [Google Scholar] [CrossRef]
- Shah, T.H.; Bhatty, J.I.; Gamlen, G.A.; Dollimore, D. Aspects of the chemistry of poly(ethylene terephthalate): 5. Polymerization of bis(hydroxyethyl)terephthalate by various metallic catalysts. Polymer 1984, 25, 1333–1336. [Google Scholar] [CrossRef]
- Lin, C.-C.; Baliga, S. A study on the polycondensation of bis-hydroxyethyl terephthalate. J. Appl. Polym. Sci. 1986, 31, 2483–2489. [Google Scholar] [CrossRef]
- Huang, J.; Yan, D.; Dong, H.; Li, F.; Lu, X.; Xin, J. Removal of trace amount impurities in glycolytic monomer of polyethylene terephthalate by recrystallization. J. Environ. Chem. Eng. 2021, 9, 106277. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Li, X.; Ge, M.; Li, Y. Removal of disperse dye from alcoholysis products of waste PET fabrics by nitric acid-modified activated carbon as an adsorbent: Kinetic and thermodynamic studies. Text. Res. J. 2020, 90, 2058–2069. [Google Scholar] [CrossRef]
- Koo, H.J.; Chang, G.S.; Kim, S.H.; Hahm, W.G.; Park, S.Y. Effects of recycling processes on physical, mechanical and degradation properties of PET yarns. Fibers Polym. 2013, 14, 2083–2087. [Google Scholar] [CrossRef]
- Jehanno, C.; Flores, I.; Dove, A.P.; Müller, A.J.; Ruipérez, F.; Sardon, H. Organocatalysed depolymerisation of PET in a fully sustainable cycle using thermally stable protic ionic salt. Green Chem. 2018, 20, 1205–1212. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Y.; Zhang, X.; Qian, J.; Xing, X.; Wang, X. Synthesis of poly(ethylene terephthalate) based on glycolysis of waste PET fiber. J. Macromol. Sci. Part A 2020, 57, 430–438. [Google Scholar] [CrossRef]
- Olewnik, E.; Czerwiński, W.; Nowaczyk, J.; Sepulchre, M.-O.; Tessier, M.; Salhi, S.; Fradet, A. Synthesis and structural study of copolymers of l-lactic acid and bis(2-hydroxyethyl terephthalate). Eur. Polym. J. 2007, 43, 1009–1019. [Google Scholar] [CrossRef]
- Tong, S.N.; Chen, D.S.; Chen, C.C.; Chung, L.Z. Unsaturated polyesters based on bis(2-hydroxyethyl)terephthalate. Polymer 1983, 24, 469–472. [Google Scholar] [CrossRef]
- Pimpan, V.; Sirisook, R.; Chuayjuljit, S. Synthesis of unsaturated polyester resin from postconsumer PET bottles: Effect of type of glycol on characteristics of unsaturated polyester resin. J. Appl. Polym. Sci. 2003, 88, 788–792. [Google Scholar] [CrossRef]
- Zahedi, A.R.; Rafizadeh, M.; Ghafarian, S.R. Unsaturated polyester resin via chemical recycling of off-grade poly(ethylene terephthalate). Polym. Int. 2009, 58, 1084–1091. [Google Scholar] [CrossRef]
- Duque-Ingunza, I.; López-Fonseca, R.; de Rivas, B.; Gutiérrez-Ortiz, J.I. Synthesis of unsaturated polyester resin from glycolysed postconsumer PET wastes. J. Mater. Cycles Waste Manag. 2013, 15, 256–263. [Google Scholar] [CrossRef]
- Öztürk, Y.; Güçlü, G. Unsaturated Polyester Resins Obtained from Glycolysis Products of Waste PET. Polym.-Plast. Technol. Eng. 2005, 43, 1539–1552. [Google Scholar] [CrossRef]
- Ertas, K.; Güçlü, G. Alkyd Resins Synthesized from Glycolysis Products of Waste PET. Polym.-Plast. Technol. Eng. 2005, 44, 783–794. [Google Scholar] [CrossRef]
- Mahdi, F.; Khan, A.A.; Abbas, H. Physiochemical properties of polymer mortar composites using resins derived from post-consumer PET bottles. Cem. Concr. Compos. 2007, 29, 241–248. [Google Scholar] [CrossRef]
- Nevrekar, N.B.; Naik, G.A.; Joshi, K.A. A hot melt adhesive from polyester waste. J. Adhes. Sci. Technol. 1987, 1, 201–207. [Google Scholar] [CrossRef]
- El Mejjatti, A.; Harit, T.; Riahi, A.; Khiari, R.; Bouabdallah, I.; Malek, F. Chemical recycling of poly(ethylene terephthalate). Application to the synthesis of multiblock copolyesters. Express Polym. Lett. 2014, 8, 544–553. [Google Scholar] [CrossRef]
- Gara, M.B.; Kammoun, W.; Delaite, C.; Abid, S.; Gharbi, R.E. Synthesis and Characterization of Aliphatic-Aromatic Copolyesters From Pet Waste and e-Caprolactone. J. Macromol. Sci. Part A 2015, 52, ebi. [Google Scholar] [CrossRef]
- Karanastasis, A.A.; Safin, V.; Pitet, L.M. Bio-Based Upcycling of Poly(ethylene terephthalate) Waste for the Preparation of High-Performance Thermoplastic Copolyesters. Macromolecules 2022, 55, 1042–1049. [Google Scholar] [CrossRef]
- Radenkov, P.; Radenkov, M.; Grancharov, G.; Troev, K. Direct usage of products of poly(ethylene terephthalate) glycolysis for manufacturing of glass-fibre-reinforced plastics. Eur. Polym. J. 2003, 39, 1223–1228. [Google Scholar] [CrossRef]
- Ou, J.; Yang, R.; Dai, Z.; Kong, Z.; Shu, H.; Huang, X. Synthesis, Characterization, Application of PET Waste Blended with Hybrid Fibers and Preparation of Fiber Derived Unsaturated Resin. Polym. Sci. Ser. A 2022, 64, 818–833. [Google Scholar] [CrossRef]
- Czub, P. Synthesis and modification of epoxy resins using recycled poly(ethylene terephthalate). Polym. Adv. Technol. 2009, 20, 183–193. [Google Scholar] [CrossRef]
- Çam, Ç.; Bal, A.; Güçlü, G. Synthesis and film properties of epoxy esters modified with amino resins from glycolysis products of postconsumer PET bottles: Synthesis and Film Properties of Epoxy Esters Modified with Amino Resins. Polym. Eng. Sci. 2015, 55, 2519–2525. [Google Scholar] [CrossRef]
- Bal, K.; Ünlü, K.C.; Acar, I.; Güçlü, G. Epoxy-based paints from glycolysis products of postconsumer PET bottles: Synthesis, wet paint properties and film properties. J. Coat. Technol. Res. 2017, 14, 747–753. [Google Scholar] [CrossRef]
- An, W.; Wang, X.-L.; Liu, X.; Wu, G.; Xu, S.; Wang, Y.-Z. Chemical recovery of thermosetting unsaturated polyester resins. Green Chem. 2022, 24, 701–712. [Google Scholar] [CrossRef]
- Barker, C.M.; Nayyar, G.; Chase, T.P.; Long, T.E. Solvent-free vat photopolymerization of unsaturated polyesters: Structure-property relationships for depolymerization. MRS Commun. 2023, 13, 841–847. [Google Scholar] [CrossRef]
- Shukla, S.R.; Harad, A.M.; Jawale, L.S. Recycling of waste PET into useful textile auxiliaries. Waste Manag. 2008, 28, 51–56. [Google Scholar] [CrossRef]
- Shukla, S.R.; Harad, A.M.; Jawale, L.S. Chemical recycling of PET waste into hydrophobic textile dyestuffs. Polym. Degrad. Stab. 2009, 94, 604–609. [Google Scholar] [CrossRef]
- Li, M.; Huang, Y.; Yu, T.; Chen, S.; Ju, A.; Ge, M. Chemical recycling of waste poly(ethylene terephthalate) fibers into azo disperse dyestuffs. RSC Adv. 2014, 4, 46476–46480. [Google Scholar] [CrossRef]
- Li, M.-J.; Huang, Y.-H.; Ju, A.-Q.; Yu, T.-S.; Ge, M.-Q. Synthesis and characterization of azo dyestuff based on bis(2-hydroxyethyl) terephthalate derived from depolymerized waste poly(ethylene terephthalate) fibers. Chin. Chem. Lett. 2014, 25, 1550–1554. [Google Scholar] [CrossRef]
- Guo, X.; Xin, J.; Lu, X.; Ren, B.; Zhang, S. Preparation of 1,4-cyclohexanedimethanol by selective hydrogenation of a waste PET monomer bis(2-hydroxyethylene terephthalate). RSC Adv. 2015, 5, 485–492. [Google Scholar] [CrossRef]
- Hou, D.; Xin, J.; Lu, X.; Guo, X.; Dong, H.; Ren, B.; Zhang, S. Conversion of bis(2-hydroxyethylene terephthalate) into 1,4-cyclohexanedimethanol by selective hydrogenation using RuPtSn/Al2O3. RSC Adv. 2016, 6, 48737–48744. [Google Scholar] [CrossRef]
- Lu, J.; Li, M.; Li, Y.; Li, X.; Gao, Q.; Ge, M. Synthesis and sizing performances of water-soluble polyester based on bis(2-hydroxyethyl) terephthalate derived from depolymerized waste poly(ethylene terephthalate) fabrics. Text. Res. J. 2019, 89, 572–579. [Google Scholar] [CrossRef]
- Chen, Z.; Sun, H.; Kong, W.; Chen, L.; Zuo, W. Closed-loop utilization of polyester in the textile industry. Green Chem. 2023, 25, 4429–4437. [Google Scholar] [CrossRef]
- Ngo, D.M.; Lee, K.; Ho, L.N.T.; Lee, J.; Jung, H.M. Direct conversion of waste polyesters to low molecular weight polyols for polyurethane production. Polym. Degrad. Stab. 2022, 205, 110147. [Google Scholar] [CrossRef]
- Lin, C.-C.; Yang, B.-Z. Rheological and mechanical properties of polyurethane modified by BHET. J. Appl. Polym. Sci. 1980, 25, 1875–1882. [Google Scholar] [CrossRef]
- Vaidya, U.R.; Nadkarni, V.M. Polyester polyols from glycolyzed PET waste: Effect of glycol type on kinetics of polyesterification. J. Appl. Polym. Sci. 1989, 38, 1179–1190. [Google Scholar] [CrossRef]
- Vaidya, U.R.; Nadkarni, V.M. Polyester polyols for polyurethanes from pet waste: Kinetics of polycondensation. J. Appl. Polym. Sci. 1988, 35, 775–785. [Google Scholar] [CrossRef]
- Espinoza García, K.; Navarro, R.; Ramírez-Hernández, A.; Marcos-Fernández, Á. New routes to difunctional macroglycols using ethylene carbonate: Reaction with bis-(2-hydroxyethyl) terephthalate and degradation of poly(ethylene terephthalate). Polym. Degrad. Stab. 2017, 144, 195–206. [Google Scholar] [CrossRef]
- Barot, A.A.; Patel, C.M.; Sinha, V.K. Synthesis of Flame Retardant Polyurethane Coating Using Bis(2-hydroxyethyl) terephthalate Derived from Chemical Scavenging of Waste Clothes. Arch. Appl. Sci. Res. 2016, 8, 12–22. [Google Scholar]
- Jamdar, V.; Kathalewar, M.; Dubey, K.A.; Sabnis, A. Recycling of PET wastes using Electron beam radiations and preparation of polyurethane coatings using recycled material. Prog. Org. Coat. 2017, 107, 54–63. [Google Scholar] [CrossRef]
- Chiu, W.-Y.; Chen, L.-W.; Lee, S.-H. Structural and Mechanical Properties of Polybutadiene-Urethane Elastomer. J. Chin. Chem. Soc. 1987, 34, 63–69. [Google Scholar] [CrossRef]
- Pu, M.; Zhou, X.; Liu, X.; Fang, C.; Wang, D. A facile, alternative and sustainable feedstock for transparent polyurethane elastomers from chemical recycling waste PET in high-efficient way. Waste Manag. 2023, 155, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Maafi, E.M.; Malek, F.; Tighzert, L. Synthesis and characterization of new polyurethane based on polycaprolactone. J. Appl. Polym. Sci. 2010, 115, 3651–3658. [Google Scholar] [CrossRef]
- Maafi, E.M.; Malek, F.; Tighzert, L.; Dony, P. Synthesis of Polyurethane and Characterization of its Composites Based on Alfa Cellulose Fibers. J. Polym. Environ. 2010, 18, 638–646. [Google Scholar] [CrossRef]
- Li, Q.; He, H.; Zhang, C.; Liang, X.; Shen, Y. Research on synthesis of polyurethane based on a new chain extender obtained from waste polyethylene terephthalate. J. Appl. Polym. Sci. 2022, 139, e52402. [Google Scholar] [CrossRef]
- Mendiburu-Valor, E.; Calvo-Correas, T.; Martin, L.; Harismendy, I.; Peña-Rodriguez, C.; Eceiza, A. Synthesis and characterization of sustainable polyurethanes from renewable and recycled feedstocks. J. Clean. Prod. 2023, 400, 136749. [Google Scholar] [CrossRef]
- Mendiburu-Valor, E.; Larraza, I.; Echeverria-Altuna, O.; Harismendy, I.; Peña-Rodriguez, C.; Eceiza, A. Thermoset polyurethanes from biobased and recycled components. J. Polym. Environ. 2023, 31, 4946–4959. [Google Scholar] [CrossRef]
- Li, M.; Luo, J.; Huang, Y.; Li, X.; Yu, T.; Ge, M. Recycling of Waste Poly(ethylene terephthalate) into Flame-Retardant Rigid Polyurethane Foams. J. Appl. Polym. Sci. 2014, 131, 40857. [Google Scholar] [CrossRef]
- Pham, C.T.; Nguyen, B.T.; Nguyen, M.T.; Nguyen, T.H.; Hoang, C.N.; Ngan Nguyen, N.; Lee, P.-C.; Kim, J.; Hoang, D. The advancement of bis(2-hydroxyethyl)terephthalate recovered from post-consumer poly(ethylene terephthalate) bottles compared to commercial polyol for preparation of high performance polyurethane. J. Ind. Eng. Chem. 2021, 93, 196–209. [Google Scholar] [CrossRef]
- Lee, P.S.; Kim, S.-C.; Tikue, E.T.; Jung, S.M. One-Pot Reaction of Waste PET to Flame Retardant Polyurethane Foam, via Deep Eutectic Solvents-Based Conversion Technology. J. Polym. Environ. 2022, 30, 333–343. [Google Scholar] [CrossRef]
- Lee, P.S.; Jung, S.M. Flame retardancy of polyurethane foams prepared from green polyols with flame retardants. J. Appl. Polym. Sci. 2022, 139, 52010. [Google Scholar] [CrossRef]
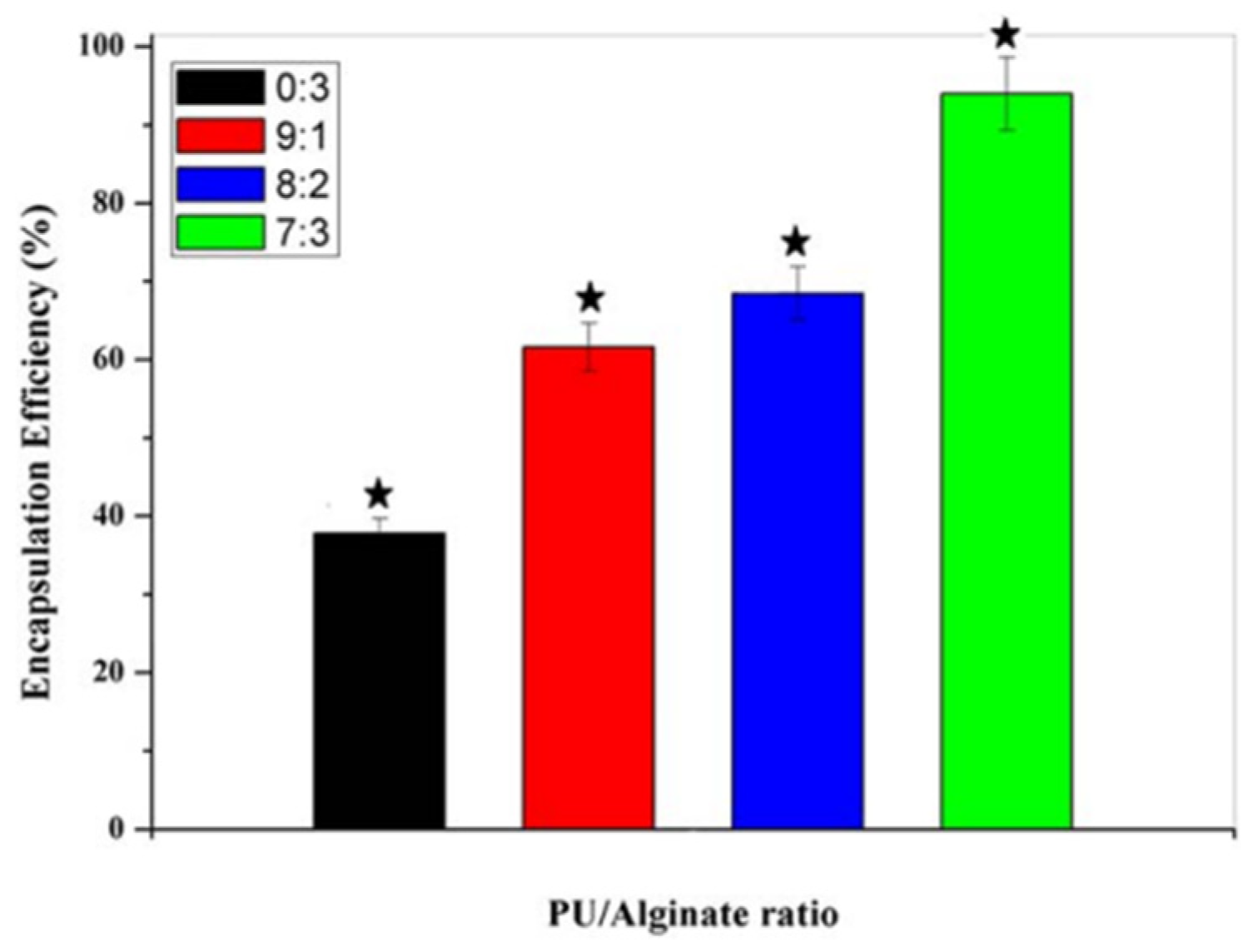
- Bhattacharyya, A.; Mukhopadhyay, P.; Kundu, P.P. Synthesis of a novel pH-sensitive polyurethane-alginate blend with poly(ethylene terephthalate) waste for the oral delivery of protein: Article. J. Appl. Polym. Sci. 2014, 131, 40650. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Mukherjee, D.; Mishra, R.; Kundu, P.P. Preparation of polyurethane–alginate/chitosan core shell nanoparticles for the purpose of oral insulin delivery. Eur. Polym. J. 2017, 92, 294–313. [Google Scholar] [CrossRef]
- Singh, A.; Banerjee, S.L.; Dhiman, V.; Bhadada, S.K.; Sarkar, P.; Khamrai, M.; Kumari, K.; Kundu, P.P. Fabrication of calcium hydroxyapatite incorporated polyurethane-graphene oxide nanocomposite porous scaffolds from poly(ethylene terephthalate) waste: A green route toward bone tissue engineering. Polymer 2020, 195, 122436. [Google Scholar] [CrossRef]
- Kamali, F.; Faghihi, K.; Mirhoseini, F. High antibacterial activity of new eco-friendly and biocompatible polyurethane nanocomposites based on Fe3O4/Ag and starch moieties. Polym. Eng. Sci. 2022, 62, 1444–1462. [Google Scholar] [CrossRef]
- Santra, S.; Ghosh, A.; Mondal, A.; Ali, S.M.; Das, D.; Sarkar, K.; Roy, L.; Molla, M.R. Stabilizing Entropically Driven Self-Assembly of Self-Immolative Polyurethanes in Water: A Strategy for Tunable Encapsulation Stability and Controlled Cargo Release. ACS Appl. Polym. Mater. 2022, 4, 7614–7625. [Google Scholar] [CrossRef]
- Santra, S.; Kolay, S.; Sk, S.; Ghosh, D.; Mishra, A.; Roy, L.; Sarkar, K.; Molla, M.R. Supramolecularly cross-linked nanoassemblies of self-immolative polyurethane from recycled plastic waste: High encapsulation stability and the triggered release of guest molecules. Polym. Chem. 2022, 13, 3294–3303. [Google Scholar] [CrossRef]
- Chung, Y.-C.; Kim, S.H.; Choi, J.W.; Chun, B.C. Characterization of Polyurethane Grafted with Bis(2-hydroxyethyl) Terephthalate. Polym.-Plast. Technol. Eng. 2018, 57, 1474–1484. [Google Scholar] [CrossRef]
- Cevher, D.; Sürdem, S. Polyurethane adhesive based on polyol monomers BHET and BHETA depolymerised from PET waste. Int. J. Adhes. Adhes. 2021, 105, 102799. [Google Scholar] [CrossRef]
- Gayathri, V.; Sheyara, R.T.B.; Devassy, N.; Samanta, D. Investigating the degradation of PET utilizing NHC-based catalysts and effective reuse of the degradation product as an additive with polyurethane adhesive material. J. Appl. Polym. Sci. 2022, 139, e52474. [Google Scholar] [CrossRef]
- He, Q.L.H.; Wang, S.; Shen, Y.; Zhang, C.; Liang, X. Bis(2-hydroxyethyl) terephthalate from depolymerized waste polyester modified graphene as a novel functional crosslinker for electrical and thermal conductive polyurethane composites. Compos. Commun. 2022, 35, 101343. [Google Scholar] [CrossRef]
- Zhang, C.; He, H.; Li, Q.; Liang, X. Synthesis and characterization of flame-retardant polyurethane based on new chain extenders. Polym. Int. 2022, 71, 1193–1200. [Google Scholar] [CrossRef]
- Li, Q.; He, H.; Wang, S.; Zhai, H.; Shen, Y.; Li, A.; Guan, F. Bis(2-hydroxyethyl) Terephthalate-Modified Ti3C2Tx/Graphene Nanohybrids as Three-Dimensional Functional Chain Extenders for Polyurethane Composite Films with Strain-Sensing and Conductive Properties. ACS Appl. Mater. Interfaces 2023, 15, 12403–12413. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, N.; Viada, G.; Galliano, S.; Menozzi, A.; Tammaro, F.; Gianelli, W.; Bonomo, M.; Barolo, C. Increasing circular and bio-based content of a thermosetting polyurethane for encapsulation of optoelectronic devices: A multivariate investigation. J. Clean. Prod. 2023, 408, 137161. [Google Scholar] [CrossRef]
- Kemona, A.; Piotrowska, M. Polyurethane Recycling and Disposal: Methods and Prospects. Polymers 2020, 12, 1752. [Google Scholar] [CrossRef]
- Sarkar, K.; Krishna Meka, S.R.; Bagchi, A.; Krishna, N.S.; Ramachandra, S.G.; Madras, G.; Chatterjee, K. Polyester derived from recycled poly(ethylene terephthalate) waste for regenerative medicine. RSC Adv. 2014, 4, 58805–58815. [Google Scholar] [CrossRef]
- Ramírez-Centeno, S.; Marcos-Fernández, A.; Aparicio-Saguilán, A.; Navarro-Crespo, R.; Báez-García, J.E.; Páramo-Calderón, D.E.; Ramírez-Hernández, A. Modified starch with bis(2-hydroxyethyl) terephthalate: Synthesis, characterization and elaboration of films. J. Polym. Res. 2020, 27, 270. [Google Scholar] [CrossRef]
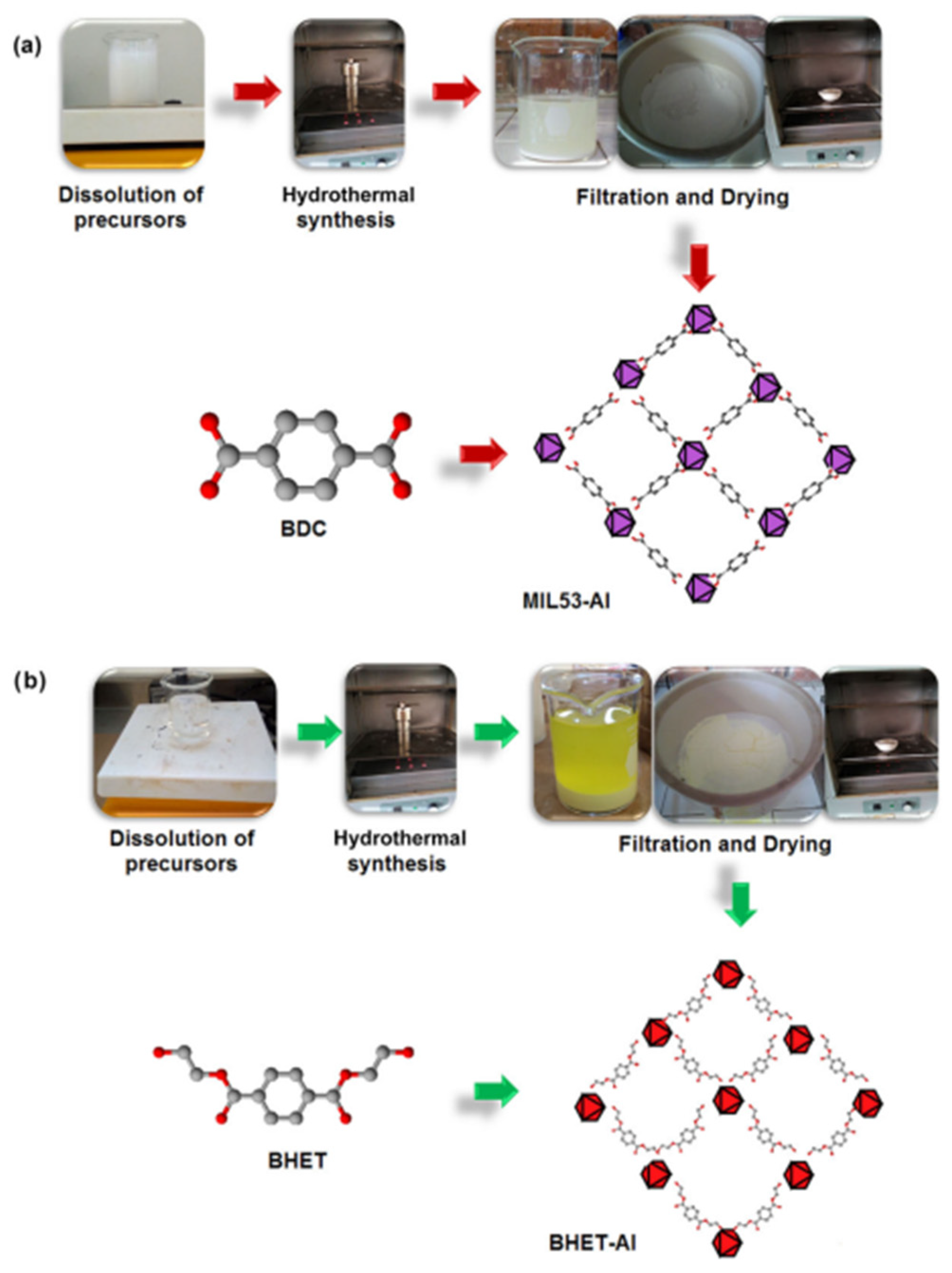
- Cabrera-Munguia, D.A.; León-Campos, M.I.; Claudio-Rizo, J.A.; Solís-Casados, D.A.; Flores-Guia, T.E.; Cano Salazar, L.F. Potential biomedical application of a new MOF based on a derived PET: Synthesis and characterization. Bull. Mater. Sci. 2021, 44, 245. [Google Scholar] [CrossRef]
- Cabrera-Munguia, D.A.; Claudio-Rizo, J.A.; Becerra-Rodríguez, J.J.; Flores-Guia, T.E.; Rico, J.L.; Vásquez-García, S.R. Enhanced biocompatibility and bactericidal properties of hydrogels based on collagen–polyurethane–aluminium MOFs for biomedical applications. Bull. Mater. Sci. 2023, 46, 100. [Google Scholar] [CrossRef]
- Viante, M.F.; Zanela, T.M.P.; Stoski, A.; Muniz, E.C.; Almeida, C.A.P. Magnetic microspheres composite from poly(ethylene terephthalate) (PET) waste: Synthesis and characterization. J. Clean. Prod. 2018, 198, 979–986. [Google Scholar] [CrossRef]
- Palhano Zanela, T.M.; Latczuk, I.F.; Muniz, E.C.; Almeida, C.A.P. Synthesis of bolaform surfactants from recycled poly(ethylene terephthalate) waste. J. Clean. Prod. 2021, 320, 128762. [Google Scholar] [CrossRef]
- Şimşek, B. Bis-hydroxyethyl terephthalate cementitious composites properties: A comparative study including hydrogen bonding mechanism with the dioctyl terephthalate. Constr. Build. Mater. 2020, 258, 119691. [Google Scholar] [CrossRef]
- Cruz-Aguilar, A.; Herrera-González, A.M.; Vázquez-García, R.A.; Navarro-Rodríguez, D.; Coreño, J. Synthesis of acrylic and allylic bifunctional cross-linking monomers derived from PET waste. IOP Conf. Ser. Mater. Sci. Eng. 2013, 45, 012007. [Google Scholar] [CrossRef]
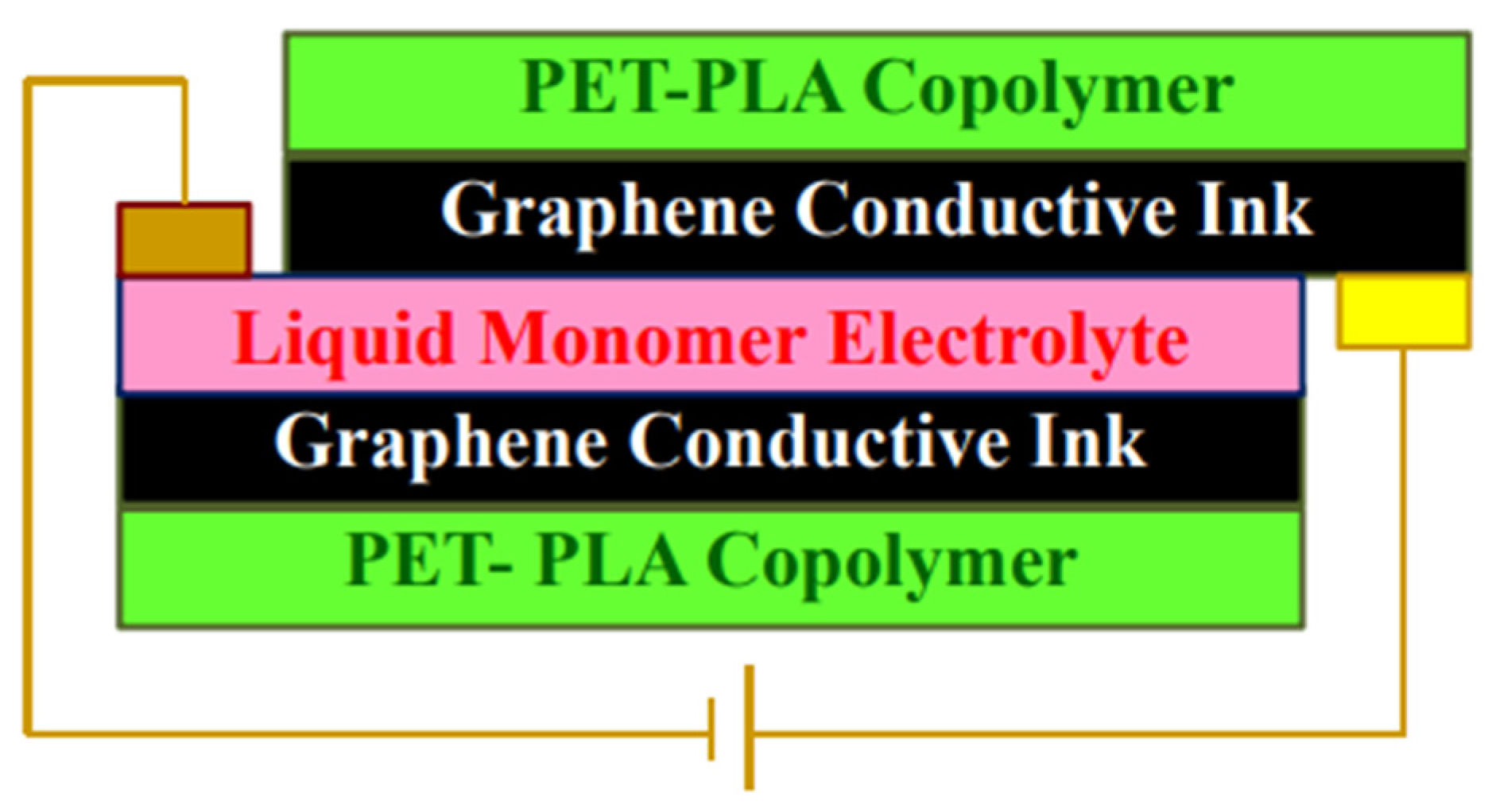
- Buasri, A.; Ongmali, D.; Sriboonpeng, P.; Prompanut, S.; Loryuenyong, V. Synthesis of PET-PLA copolymer from recycle plastic bottle and study of its applications in the electrochromic devices with graphene conductive ink. Mater. Today Proc. 2018, 5, 11060–11067. [Google Scholar] [CrossRef]
- Goujon, N.; Demarteau, J.; Lopez de Pariza, X.; Casado, N.; Sardon, H.; Mecerreyes, D. Chemical Upcycling of PET Waste towards Terephthalate Redox Nanoparticles for Energy Storage. Sustain. Chem. 2021, 2, 610–621. [Google Scholar] [CrossRef]
- Ranganathan, P.; Chen, Y.-H.; Rwei, S.-P.; Lee, Y.-H. Biomass upcycling of waste rPET to higher-value new-easy-recyclable microcellular thermoplastic (co)polyamide foams and hot-melt adhesives. Mater. Today Chem. 2022, 26, 101101. [Google Scholar] [CrossRef]
- Mikhaylov, P.A.; Zuev, K.V.; Filatova, M.P.; Strelets, B.K.; Kulichikhin, V.G. Synthesis and Properties of Thermotropic Copolyesters Based on Poly(ethylene terephthalate) and 4′-Acetoxy-4-biphenyl-carboxylic Acid. Polymers 2021, 13, 1720. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Westover, C.C.; Long, T.E. Envisioning a BHET Economy: Adding Value to PET Waste. Sustain. Chem. 2023, 4, 363-393. https://doi.org/10.3390/suschem4040025
Westover CC, Long TE. Envisioning a BHET Economy: Adding Value to PET Waste. Sustainable Chemistry. 2023; 4(4):363-393. https://doi.org/10.3390/suschem4040025
Chicago/Turabian StyleWestover, Clarissa C., and Timothy E. Long. 2023. "Envisioning a BHET Economy: Adding Value to PET Waste" Sustainable Chemistry 4, no. 4: 363-393. https://doi.org/10.3390/suschem4040025