Beta-Caryophyllene Induces Significant Changes in the Lipid Bilayer at Room and Physiological Temperatures: ATR-FTIR Spectroscopy Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Liposome Preparation
2.3. DLS Measurements
2.4. ATR-FTIR Spectroscopy
3. Results and Discussion
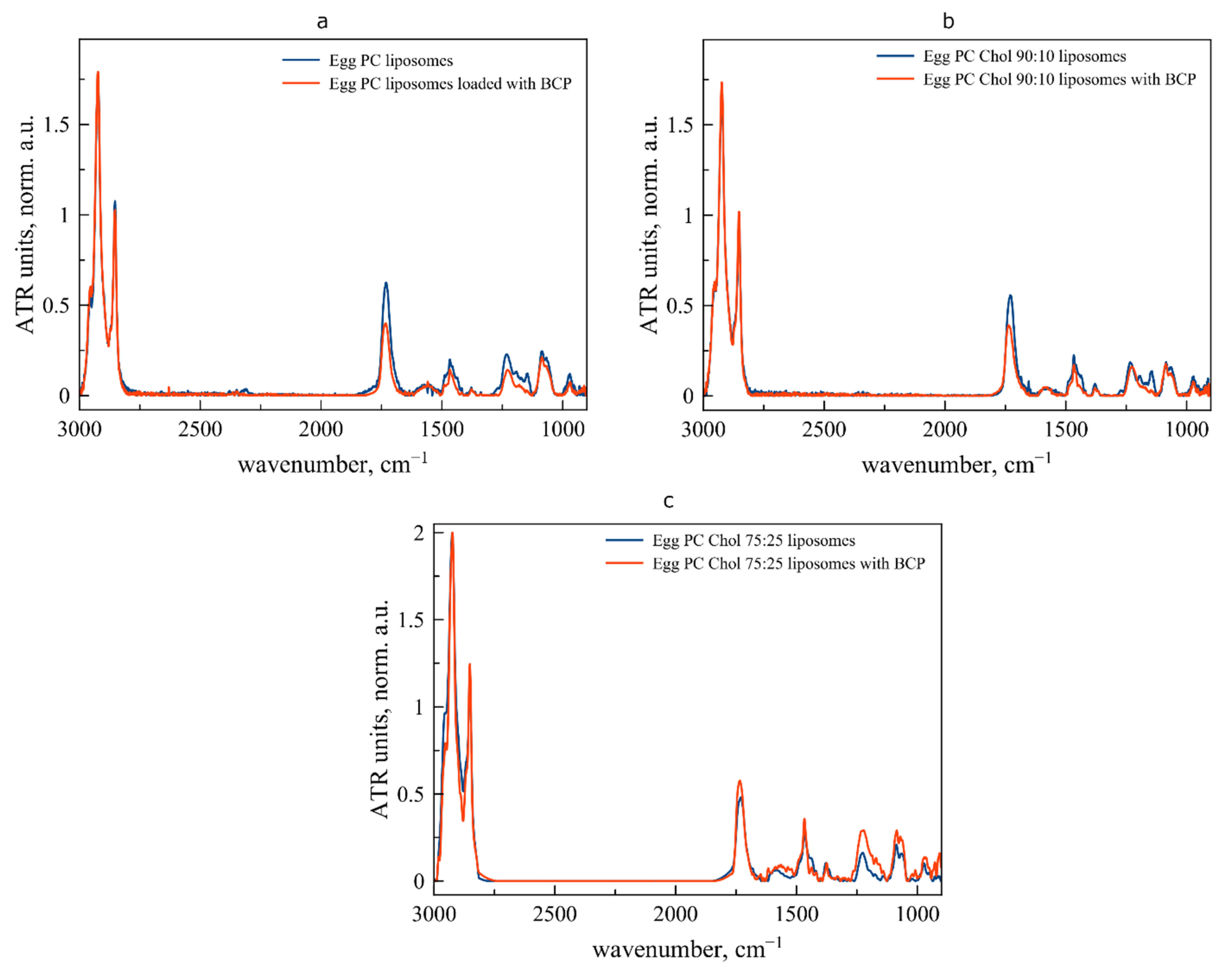
3.1. Interaction between Liposomal Membrane and BCP at Room Temperature
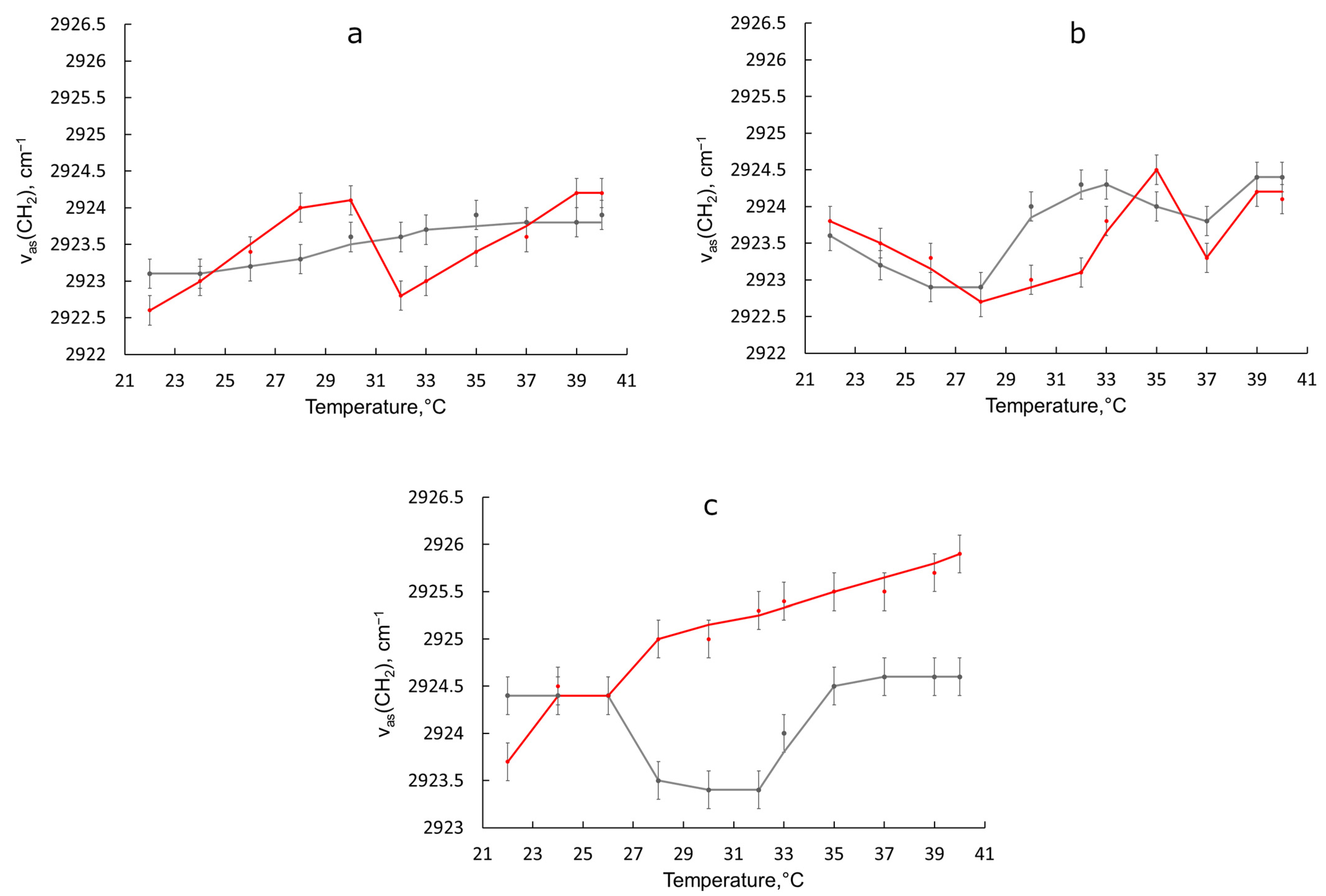
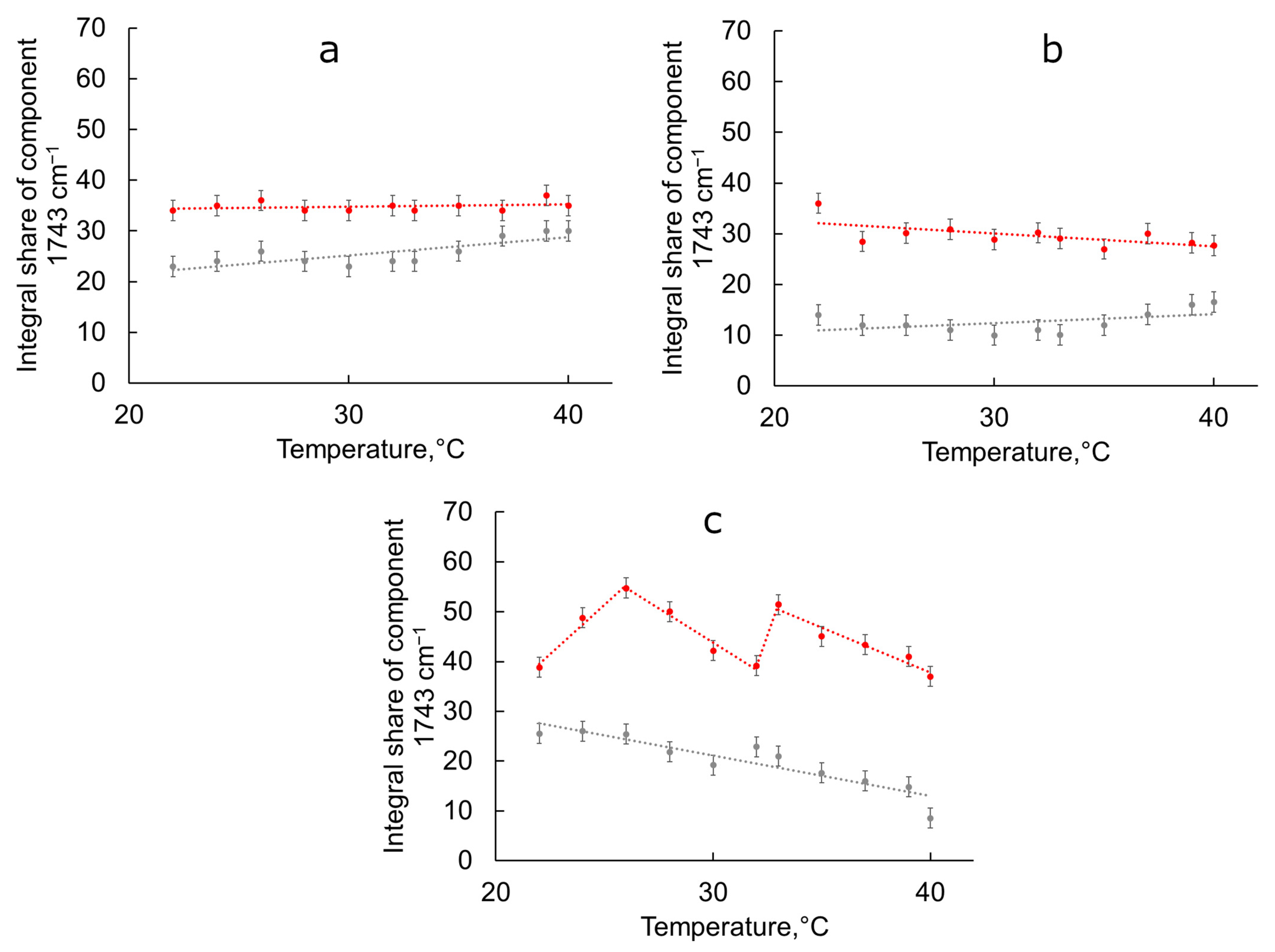
3.2. Behavior of Liposomal Forms of BCP during the Transition from Room to Physiological Temperature
3.3. The Effect of Lipid Composition on the Interaction of BCP with Liposomal Bilayers
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fidyt, K.; Fiedorowicz, A.; Strządała, L.; Szumny, A. β-caryophyllene and β-caryophyllene oxide—Natural compounds of anticancer and analgesic properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef]
- Di Giacomo, S.; Di Sotto, A.; Mazzanti, G.; Wink, M. Chemosensitizing properties of β-caryophyllene and β-caryophyllene oxide in combination with doxorubicin in human cancer cells. Anticancer Res. 2017, 37, 1191–1196. [Google Scholar] [PubMed]
- Orchard, A.; Van Vuuren, S. Commercial Essential Oils as Potential Antimicrobials to Treat Skin Diseases. Evid.-Based Complement. Altern. Med. 2017, 2017, 4517971. [Google Scholar] [CrossRef] [PubMed]
- Ojha, S.; Javed, H.; Azimullah, S.; Haque, M.E. β-Caryophyllene, a phytocannabinoid attenuates oxidative stress, neuroinflammation, glial activation, and salvages dopaminergic neurons in a rat model of Parkinson disease. Mol. Cell. Biochem. 2016, 418, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Alberti, T.B.; Barbosa, W.L.R.; Vieira, J.L.F.; Raposo, N.R.B.; Dutra, R.C. (−)-β-caryophyllene, a CB2 receptor-selective phytocannabinoid, suppresses motor paralysis and neuroinflammation in a murine model of multiple sclerosis. Int. J. Mol. Sci. 2017, 18, 691. [Google Scholar] [CrossRef] [PubMed]
- Baldissera, M.D.; Souza, C.F.; Grando, T.H.; Stefani, L.M.; Monteiro, S.G. β-caryophyllene reduces atherogenic index and coronary risk index in hypercholesterolemic rats: The involvement of cardiac oxidative damage. Chem. Biol. Interact. 2017, 270, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Mannino, F.; Pallio, G.; Corsaro, R.; Minutoli, L.; Altavilla, D.; Vermiglio, G.; Allegra, A.; Eid, A.H.; Bitto, A.; Squadrito, F.; et al. Beta-caryophyllene exhibits anti-proliferative effects through apoptosis induction and cell cycle modulation in multiple myeloma cells. Cancers 2021, 13, 5741. [Google Scholar] [CrossRef]
- Di Sotto, A.; Paolicelli, P.; Nardoni, M.; Abete, L.; Garzoli, S.; Di Giacomo, S.; Mazzanti, G.; Casadei, M.A.; Petralito, S. SPC liposomes as possible delivery systems for improving bioavailability of the natural sesquiterpene β-caryophyllene: Lamellarity and drug-loading as key features for a rational drug delivery design. Pharmaceutics 2018, 10, 274. [Google Scholar] [CrossRef]
- Ceccarelli, I.; Fiorenzani, P.; Pessina, F.; Pinassi, J.; Aglianò, M.; Miragliotta, V.; Aloisi, A.M. The CB2 Agonist β-Caryophyllene in Male and Female Rats Exposed to a Model of Persistent Inflammatory Pain. Front. Neurosci. 2020, 14, 850. [Google Scholar] [CrossRef]
- Kremkow, J.; Luck, M.; Huster, D.; Müller, P.; Scheidt, H.A. Membrane interaction of ibuprofen with cholesterol-containing lipid membranes. Biomolecules 2020, 10, 1384. [Google Scholar] [CrossRef]
- Arsov, Z.; Quaroni, L. Direct interaction between cholesterol and phosphatidylcholines in hydrated membranes revealed by ATR-FTIR spectroscopy. Chem. Phys. Lipids 2007, 150, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Le-Deygen, I.M.; Safronova, A.S.; Mamaeva, P.V.; Kolmogorov, I.M.; Skuredina, A.A.; Kudryashova, E.V. Drug–Membrane Interaction as Revealed by Spectroscopic Methods: The Role of Drug Structure in the Example of Rifampicin, Levofloxacin and Rapamycin. Biophysica 2022, 2, 353–365. [Google Scholar] [CrossRef]
- Le-Deygen, I.M.; Safronova, A.S.; Mamaeva, P.V.; Skuredina, A.A.; Kudryashova, E.V. Cholesterol Significantly Affects the Interactions between Pirfenidone and DPPC Liposomes: Spectroscopic Studies. Biophysica 2022, 2, 79–88. [Google Scholar] [CrossRef]
- Taylor, K.M.G.; Morris, R.M. Thermal analysis of phase transition behaviour in liposomes. Thermochim. Acta 1995, 248, 289–301. [Google Scholar] [CrossRef]
- Liu, D.Z.; Chen, W.Y.; Tasi, L.M.; Yang, S.P. Microcalorimetric and shear studies on the effects of cholesterol on the physical stability of lipid vesicles. Colloids Surf. A Physicochem. Eng. Asp. 2000, 172, 57–67. [Google Scholar] [CrossRef]
- Liang, X.; Mao, G.; Ng, K.Y.S. Mechanical properties and stability measurement of cholesterol-containing liposome on mica by atomic force microscopy. J. Colloid. Interface Sci. 2004, 278, 53–62. [Google Scholar] [CrossRef]
- Manrique-Moreno, M.; Moreno, M.M.; Garidel, P.; Suwalsky, M.; Howe, J.; Brandenburg, K. The membrane-activity of Ibuprofen, Diclofenac, and Naproxen: A physico-chemical study with lecithin phospholipids. Biochim. Biophys. Acta 2009, 1788, 1296–1303. [Google Scholar] [CrossRef]
- Disalvo, E.A.; Frias, M.A. Water state and carbonyl distribution populations in confined regions of lipid bilayers observed by FTIR spectroscopy. Langmuir 2013, 29, 6969–6974. [Google Scholar] [CrossRef]
- Lewis, R.N.; McElhaney, R.N.; Pohle, W.; Mantsch, H.H. Components of the carbonyl stretching band in the infrared spectra of hydrated 1,2-diacylglycerolipid bilayers: A reevaluation. Biophys. J. 1994, 67, 2367–2375. [Google Scholar] [CrossRef]
- Deygen, I.M.; Seidl, C.; Kolmel, D.K.; Bednarek, C.; Heissler, S.; Kudryashova, E.V.; Braese, S. Novel Prodrug of Doxorubicin Modified by Stearoylspermine encapsulated into PEG-Chitosan-Stabilized Liposomes. Langmuir 2016, 32, 10861–10869. [Google Scholar] [CrossRef]
- Coderch, L.; Fonollosa, J.; De Pera, M.; Estelrich, J.; De, A.; Maza, L.; Parra, J.L. Influence of cholesterol on liposome fluidity by EPR Relationship with percutaneous absorption. J. Control. Release 2000, 68, 85–95. [Google Scholar] [CrossRef]
- Barba-Bon, A.; Nilam, M.; Hennig, A. Supramolecular Chemistry in the Biomembrane. ChemBioChem 2020, 21, 886–910. [Google Scholar] [CrossRef]
- Korlach, J.; Schwille, P.; Webb, W.W.; Feigenson, G.W. Characterization of lipid bilayer phases by confocal microscopy and fluorescence correlation spectroscopy. Proc. Natl. Acad. Sci. USA 1999, 96, 8461–8466. [Google Scholar] [CrossRef] [PubMed]
- Grandois, J.L.E.; Marchioni, E.; Minjie Zhao, F.G.; Ennahar, S.; Bindler, F. investigation of natural phosphatidylcholine sources: Separation and identification by liquid chromatography-electrospray lonization-tandem mass spectrometry (lc-esi-ms2) of molecular species. J. Agric. Food Chem. 2009, 57, 6014–6020. [Google Scholar] [CrossRef]
- Le-Deygen, I.M.; Vlasova, K.Y.; Kutsenok, E.O.; Usvaliev, A.D.; Efremova, M.V.; Zhigachev, A.O.; Rudakovskaya, P.G.; Golovin, D.Y.; Gribanovsky, S.L.; Kudryashova, E.V.; et al. Magnetic nanorods for remote disruption of lipid membranes by non-heating low frequency magnetic field. Nanomedicine 2019, 21, 102065. [Google Scholar] [CrossRef] [PubMed]
- Yaroslavov, A.A.; Sybachin, A.V.; Zaborova, O.V.; Orlov, V.N.; Ballauff, M.; Talmon, Y.; Menger, F.M. Lipid segregation in membranes of anionic liposomes adsorbed onto polycationic brushes. Chem.-A Eur. J. 2013, 19, 13674–13678. [Google Scholar] [CrossRef] [PubMed]
- Khajeh, A.; Modarress, H. The influence of cholesterol on interactions and dynamics of ibuprofen in a lipid bilayer. Biochim. Biophys. Acta Biomembr. 2014, 1838, 2431–2438. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Li, Q.; Wu, Y.; Wang, D.; Xu, L.; Zhang, Y.; Wang, S.; Wang, T.; Liu, F.; et al. Cholesterol content in cell membrane maintains surface levels of ErbB2 and confers a therapeutic vulnerability in ErbB2-positive breast cancer. Cell Commun. Signal. 2019, 17, 15. [Google Scholar] [CrossRef]

| Lipid Composition | Dh, nm (Z-Average) | ζ-Potential, mV |
|---|---|---|
| Egg PC control | 106 ± 4 | 2.2 ± 1.2 |
| Egg PC + BCP | 108 ± 8 | 3.7 ± 1.0 |
| Egg PC:Chol 90:10 liposomes | 112 ± 3 | 6.6 ± 2.4 |
| Egg PC:Chol 90:10 liposomes + BCP | 110 ± 6 | 2.0 ± 0.6 |
| Egg PC:Chol 75:25 liposomes | 114 ± 4 | 3.3 ± 1.4 |
| Egg PC:Chol 75:25 liposomes + BCP | 116 ± 5 | 4.1 ± 2.0 |
| Sample | νCH2 as | νCH2 s | νCO | νPO2− as |
|---|---|---|---|---|
| Egg PC liposomes | 2923.1 ± 0.2 | 2852.6 ± 0.5 | 1731.3 ± 0.2 | 1231.9 ± 0.2 |
| Egg PC liposomes + BCP | 2922.6 ± 0.2 | 2853.0 ± 0.5 | 1732.1 ± 0.2 1736.0 shoulder | 1228.7 ± 0.2 |
| Egg PC:Chol 90:10 liposomes | 2923.6 ± 0.2 | 2852.6 ± 0.5 | 1730.0 ± 0.2 | 1233.8 ± 0.2 |
| Egg PC:Chol 90:10 liposomes + BCP | 2922.8 ± 0.2 | 2852.4 ± 0.5 | 1736.5 ± 0.2 1732.0 shoulder | 1227.1 ± 0.2 |
| Egg PC:Chol 75:25 liposomes | 2924.4 ± 0.2 | 2851.8 ± 0.5 | 1729.9 ± 0.2 1740.0 shoulder | 1226.0 ± 0.2 |
| Egg PC:Chol 75:25 liposomes + BCP | 2923.7 ± 0.2 | 2851.8 ± 0.5 | 1734.7 ± 0.2 | 1225.5 ± 0.2 |
| Sample | 1728 cm−1 (High Hydrated), Integral Share, % | 1743 cm−1 (Low Hydrated), Integral Share, % |
|---|---|---|
| Egg PC liposomes | 77 ± 2 | 23 ± 2 |
| Egg PC liposomes + BCP | 66± 2 | 34 ± 2 |
| Egg PC:Chol 90:10 liposomes | 86 ± 2 | 14 ± 2 |
| Egg PC:Chol 90:10 liposomes + BCP | 64 ± 2 | 36 ± 2 |
| Egg PC:Chol 75:25 liposomes | 74 ± 2 | 26 ± 2 |
| Egg PC:Chol 75:25 liposomes + BCP | 61 ± 2 | 39 ± 2 |
| Lipid Composition | Main Features of Interaction, RT | Main Features of Interaction upon Heating |
|---|---|---|
| Egg PC | Strong interaction with hydrophobic area + weak interaction with circumpolar area | Almost unchanged pattern of interaction |
| Egg PC:Chol 90:10 | Strong interaction with hydrophobic and circumpolar area | Almost unchanged pattern of interaction |
| Egg PC:Chol 75:25 liposomes | Strong interaction with hydrophobic and circumpolar area | Probably: formation of defects in bilayer in the temperature range 28–32 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yakimov, I.D.; Kolmogorov, I.M.; Le-Deygen, I.M. Beta-Caryophyllene Induces Significant Changes in the Lipid Bilayer at Room and Physiological Temperatures: ATR-FTIR Spectroscopy Studies. Biophysica 2023, 3, 501-512. https://doi.org/10.3390/biophysica3030033
Yakimov ID, Kolmogorov IM, Le-Deygen IM. Beta-Caryophyllene Induces Significant Changes in the Lipid Bilayer at Room and Physiological Temperatures: ATR-FTIR Spectroscopy Studies. Biophysica. 2023; 3(3):501-512. https://doi.org/10.3390/biophysica3030033
Chicago/Turabian StyleYakimov, Ivan D., Ilya M. Kolmogorov, and Irina M. Le-Deygen. 2023. "Beta-Caryophyllene Induces Significant Changes in the Lipid Bilayer at Room and Physiological Temperatures: ATR-FTIR Spectroscopy Studies" Biophysica 3, no. 3: 501-512. https://doi.org/10.3390/biophysica3030033






