Exosome Shedding Is Concordant with Objective Treatment Response Rate and Stratifies Time to Progression in Treatment Naïve, Non-Resectable Hepatocellular Carcinoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Cohort
2.2. Primary Outcome
2.3. Blood Collection
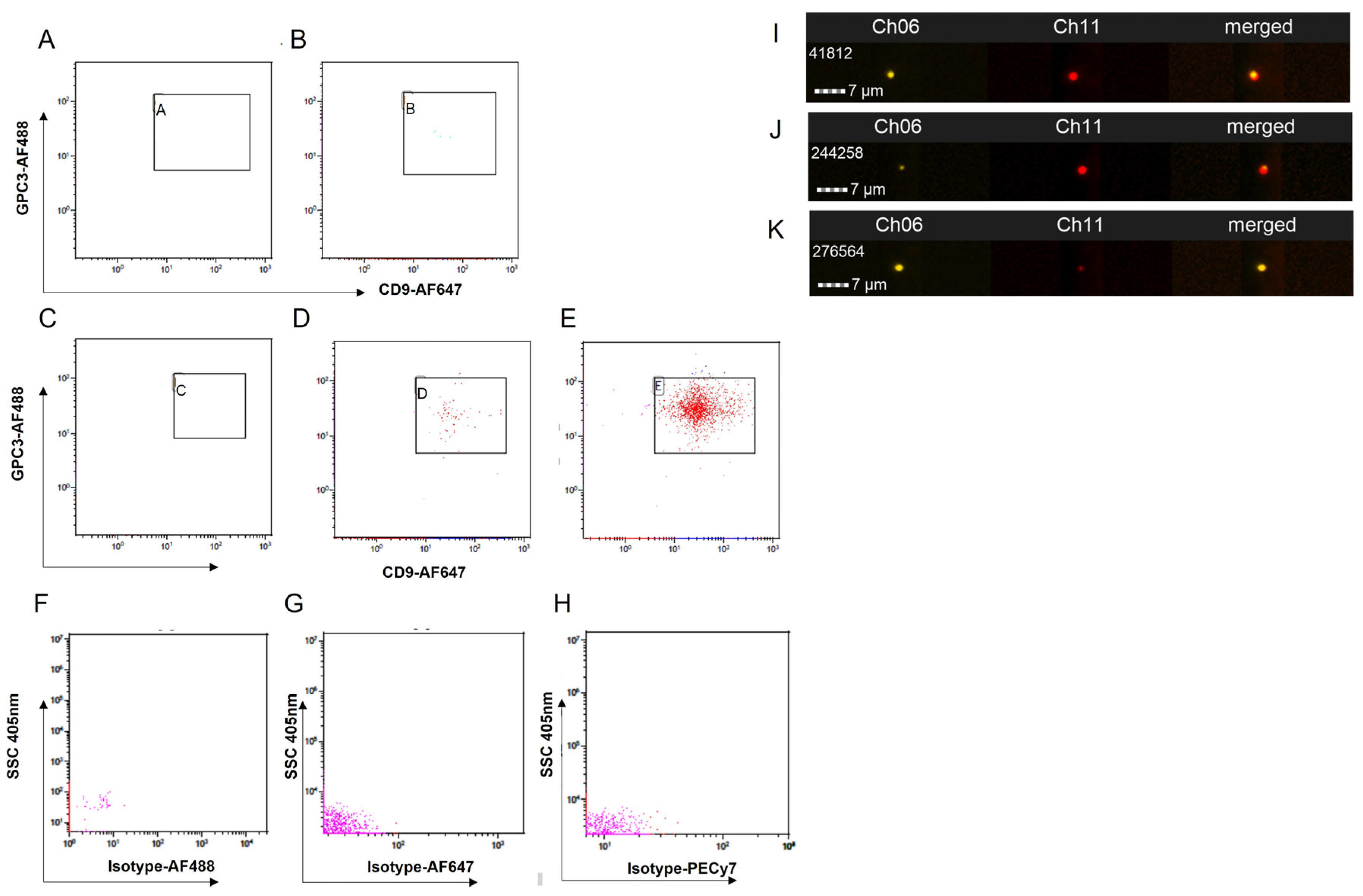
2.4. Exosome Isolation and Identification
2.5. Imaging Flow Cytometry Parameters
2.6. Statistical Analysis
3. Results
3.1. Protocol Setup for Detection of EXs Using Imaging Flow Cytometry
3.2. Patient Demographics
3.3. EX Shedding Following LDT
3.4. Prognostic Factors Associated with EX Shedding Following LDT
3.5. Post-LDT EX Shedding and TTP
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Villanueva, A. Hepatocellular Carcinoma. N. Engl. J. Med. 2019, 380, 1450–1462. [Google Scholar] [CrossRef]
- Lee, D.D.; Samoylova, M.; Mehta, N.; Musto, K.R.; Roberts, J.P.; Yao, F.Y.; Harnois, D.M. The mRECIST Classification Provides Insight into Tumor Biology for Patients with Hepatocellular Carcinoma Awaiting Liver Transplantation. Liver Transpl. 2019, 25, 228–241. [Google Scholar] [CrossRef]
- Sandow, T.A.; Arndt, S.E.; Albar, A.A.; DeVun, D.A.; Kirsch, D.S.; Gimenez, J.M.; Bohorquez, H.E.; Gilbert, P.J.; Thevenot, P.T.; Nunez, K.G.; et al. Assessment of Response to Transcatheter Arterial Chemoembolization with Doxorubicin-eluting Microspheres: Tumor Biology and Hepatocellular Carcinoma Recurrence in a 5-year Transplant Cohort. Radiology 2018, 286, 1072–1083. [Google Scholar] [CrossRef]
- Liu, Y.J.; Wang, C. A review of the regulatory mechanisms of extracellular vesicles-mediated intercellular communication. Cell Commun. Signal. 2023, 21, 77. [Google Scholar] [CrossRef]
- Zhou, B.; Xu, K.; Zheng, X.; Chen, T.; Wang, J.; Song, Y.; Shao, Y.; Zheng, S. Application of exosomes as liquid biopsy in clinical diagnosis. Signal. Transduct. Target. Ther. 2020, 5, 144. [Google Scholar] [CrossRef]
- Mathew, M.; Zade, M.; Mezghani, N.; Patel, R.; Wang, Y.; Momen-Heravi, F. Extracellular Vesicles as Biomarkers in Cancer Immunotherapy. Cancers 2020, 12, 2825. [Google Scholar] [CrossRef]
- Sun, Z.; Shi, K.; Yang, S.; Liu, J.; Zhou, Q.; Wang, G.; Song, J.; Li, Z.; Zhang, Z.; Yuan, W. Effect of exosomal miRNA on cancer biology and clinical applications. Mol. Cancer 2018, 17, 147. [Google Scholar] [CrossRef]
- Li, X.; Li, C.; Zhang, L.; Wu, M.; Cao, K.; Jiang, F.; Chen, D.; Li, N.; Li, W. The significance of exosomes in the development and treatment of hepatocellular carcinoma. Mol. Cancer 2020, 19, 1. [Google Scholar] [CrossRef]
- Li, S.; Chen, L. Exosomes in Pathogenesis, Diagnosis, and Treatment of Hepatocellular Carcinoma. Front. Oncol. 2022, 12, 793432. [Google Scholar] [CrossRef]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun. Signal. 2021, 19, 47. [Google Scholar] [CrossRef] [PubMed]
- Campos-Silva, C.; Suarez, H.; Jara-Acevedo, R.; Linares-Espinos, E.; Martinez-Pineiro, L.; Yanez-Mo, M.; Vales-Gomez, M. High sensitivity detection of extracellular vesicles immune-captured from urine by conventional flow cytometry. Sci. Rep. 2019, 9, 2042. [Google Scholar] [CrossRef]
- Vincenzi, B.; Di Maio, M.; Silletta, M.; D’Onofrio, L.; Spoto, C.; Piccirillo, M.C.; Daniele, G.; Comito, F.; Maci, E.; Bronte, G.; et al. Prognostic Relevance of Objective Response According to EASL Criteria and mRECIST Criteria in Hepatocellular Carcinoma Patients Treated with Loco-Regional Therapies: A Literature-Based Meta-Analysis. PLoS ONE 2015, 10, e0133488. [Google Scholar] [CrossRef]
- Llovet, J.M.; Lencioni, R. mRECIST for HCC: Performance and novel refinements. J. Hepatol. 2020, 72, 288–306. [Google Scholar] [CrossRef]
- Coughlan, C.; Bruce, K.D.; Burgy, O.; Boyd, T.D.; Michel, C.R.; Garcia-Perez, J.E.; Adame, V.; Anton, P.; Bettcher, B.M.; Chial, H.J.; et al. Exosome Isolation by Ultracentrifugation and Precipitation and Techniques for Downstream Analyses. Curr. Protoc. Cell Biol. 2020, 88, e110. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Lu, W.; Xu, P.; Shi, H.; Chen, D.; Chen, Y.; Shi, H.; Ma, Y. Hepatocyte-derived exosome may be as a biomarker of liver regeneration and prognostic valuation in patients with acute-on-chronic liver failure. Hepatol. Int. 2021, 15, 957–969. [Google Scholar] [CrossRef] [PubMed]
- Gorgens, A.; Bremer, M.; Ferrer-Tur, R.; Murke, F.; Tertel, T.; Horn, P.A.; Thalmann, S.; Welsh, J.A.; Probst, C.; Guerin, C.; et al. Optimisation of imaging flow cytometry for the analysis of single extracellular vesicles by using fluorescence-tagged vesicles as biological reference material. J. Extracell. Vesicles 2019, 8, 1587567. [Google Scholar] [CrossRef]
- Qu, Z.; Feng, J.; Pan, H.; Jiang, Y.; Duan, Y.; Fa, Z. Exosomes derived from HCC cells with different invasion characteristics mediated EMT through TGF-beta/Smad signaling pathway. Onco Targets Ther. 2019, 12, 6897–6905. [Google Scholar] [CrossRef]
- Chen, L.; Guo, P.; He, Y.; Chen, Z.; Chen, L.; Luo, Y.; Qi, L.; Liu, Y.; Wu, Q.; Cui, Y.; et al. HCC-derived exosomes elicit HCC progression and recurrence by epithelial-mesenchymal transition through MAPK/ERK signalling pathway. Cell Death Dis. 2018, 9, 513. [Google Scholar] [CrossRef]
- Wu, Q.; Zhou, L.; Lv, D.; Zhu, X.; Tang, H. Exosome-mediated communication in the tumor microenvironment contributes to hepatocellular carcinoma development and progression. J. Hematol. Oncol. 2019, 12, 53. [Google Scholar] [CrossRef]
- He, R.; Wang, Z.; Shi, W.; Yu, L.; Xia, H.; Huang, Z.; Liu, S.; Zhao, X.; Xu, Y.; Yam, J.W.P.; et al. Exosomes in hepatocellular carcinoma microenvironment and their potential clinical application value. Biomed. Pharmacother. 2021, 138, 111529. [Google Scholar] [CrossRef]
- Sun, J.F.; Zhang, D.; Gao, C.J.; Zhang, Y.W.; Dai, Q.S. Exosome-Mediated MiR-155 Transfer Contributes to Hepatocellular Carcinoma Cell Proliferation by Targeting PTEN. Med. Sci. Monit. Basic Res. 2019, 25, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.R.; Kim, G.; Tak, W.Y.; Jang, S.Y.; Kweon, Y.O.; Park, J.G.; Lee, H.W.; Han, Y.S.; Chun, J.M.; Park, S.Y.; et al. Circulating exosomal noncoding RNAs as prognostic biomarkers in human hepatocellular carcinoma. Int. J. Cancer 2019, 144, 1444–1452. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Sun, L.; Wen, S.; Deng, D.; Wan, F.; He, X.; Tian, L.; Liang, L.; Wei, C.; Gao, K.; et al. RNA sequencing of plasma exosomes revealed novel functional long noncoding RNAs in hepatocellular carcinoma. Cancer Sci. 2020, 111, 3338–3349. [Google Scholar] [CrossRef] [PubMed]
- Bobrie, A.; Colombo, M.; Krumeich, S.; Raposo, G.; Thery, C. Diverse subpopulations of vesicles secreted by different intracellular mechanisms are present in exosome preparations obtained by differential ultracentrifugation. J. Extracell. Vesicles 2012, 1, 18397. [Google Scholar] [CrossRef]
- Mathieu, M.; Nevo, N.; Jouve, M.; Valenzuela, J.I.; Maurin, M.; Verweij, F.J.; Palmulli, R.; Lankar, D.; Dingli, F.; Loew, D.; et al. Specificities of exosome versus small ectosome secretion revealed by live intracellular tracking of CD63 and CD9. Nat. Commun. 2021, 12, 4389. [Google Scholar] [CrossRef]
- Khushman, M.; Bhardwaj, A.; Patel, G.K.; Laurini, J.A.; Roveda, K.; Tan, M.C.; Patton, M.C.; Singh, S.; Taylor, W.; Singh, A.P. Exosomal Markers (CD63 and CD9) Expression Pattern Using Immunohistochemistry in Resected Malignant and Nonmalignant Pancreatic Specimens. Pancreas 2017, 46, 782–788. [Google Scholar] [CrossRef]
- Chen, W.; Mao, Y.; Liu, C.; Wu, H.; Chen, S. Exosome in Hepatocellular Carcinoma: An update. J. Cancer 2021, 12, 2526–2536. [Google Scholar] [CrossRef]
- Suehiro, T.; Miyaaki, H.; Kanda, Y.; Shibata, H.; Honda, T.; Ozawa, E.; Miuma, S.; Taura, N.; Nakao, K. Serum exosomal microRNA-122 and microRNA-21 as predictive biomarkers in transarterial chemoembolization-treated hepatocellular carcinoma patients. Oncol. Lett. 2018, 16, 3267–3273. [Google Scholar] [CrossRef]
- Shuen, T.W.H.; Alunni-Fabbroni, M.; Ocal, E.; Malfertheiner, P.; Wildgruber, M.; Schinner, R.; Pech, M.; Benckert, J.; Sangro, B.; Kuhl, C.; et al. Extracellular Vesicles May Predict Response to Radioembolization and Sorafenib Treatment in Advanced Hepatocellular Carcinoma: An Exploratory Analysis from the SORAMIC Trial. Clin. Cancer Res. 2022, 28, 3890–3901. [Google Scholar] [CrossRef]



| Demographic | Cohort |
|---|---|
| Patients, n (%) | 43 (100) |
| Age at diagnosis, years (IQR) | 61 (58–68) |
| Sex, male (%) | 33 (76) |
| Race, n (%) | |
| Caucasian | 25 (58) |
| African American | 12 (28) |
| Other | 6 (14) |
| Cirrhotic Etiology, n (%) | |
| HCV | 20 (47) |
| NASH | 10 (23) |
| Other | 13 (30) |
| Cirrhosis Status at Diagnosis, n (%) | |
| Compensated | 35 (81) |
| Decompensated | 8 (19) |
| Scores and Staging | |
| ECOG Performance Status of 0, n (%) | 33 (77) |
| Child–Pugh of A, n (%) | 39 (91) |
| BCLC Stage A, n (%) | 39 (91) |
| Clinical Hepatology Labs | |
| Sodium, mM (IQR) | 138 (137–141) |
| Creatinine, mg/dL (IQR) | 0.9 (0.8–1.1) |
| Bilirubin, mg/dL (IQR) | 0.9 (0.5–1.2) |
| Albumin, g/dL (IQR) | 3.5 (3.1–3.8) |
| INR, ratio (IQR) | 1.1 (1.0–1.2) |
| MELD-Na, score (IQR) | 8 (7–10) |
| Tumour Burden and Biomarkers | |
| Largest lesion, cm (IQR) | 2.7 (2.2–4.2) |
| Cumulative lesion, cm (IQR) | 3.4 (2.4–4.7) |
| Milan, within criteria (%) | 38 (88) |
| AFP, ng/mL (IQR) | 15 (6.6–44) |
| First-Line Liver-Directed Therapy | |
| DEE-TACE, (%) | 4 (9) |
| 90Y, n (%) | 23 (53) |
| MWA, n (%) | 16 (37) |
| Treatment Response to First-Line LDT | |
| ORR | 34 (79) |
| Non-ORR | 9 (21) |
| Study Endpoint | |
| Active, n (%) | 21 (49) |
| Tumor progression, n (%) | 8 (19) |
| Transplanted, n (%) | 14 (32) |
| EXosome Shedding | Baseline | Post-LDT | p Value |
|---|---|---|---|
| EXs by marker, median (IQR) | |||
| CD9+ | 7.2 × 106 (3.8 × 106–17.4 × 106) | 10.7 × 106 (4.3 × 106–20.7 × 106) | 0.545 |
| CD63+ | 0.5 × 106 (0.3 × 106–1.1 × 106) | 0.7 × 106 (0.4 × 106–0.9 × 106) | 0.734 |
| Double positive, CD9+ CD63+ | 0.08 × 106 (0.03 × 106–0.2 × 106) | 0.1 × 106 (0.05 × 106–0.4 × 106) | 0.632 |
| Exosome Shedding Group | |||
| CD9+ EXs, n (%) | 0.042 | ||
| High | 16 (41) | 20 (50) | |
| Low | 23 (59) | 20 (50) | |
| CD63+ EXs, n (%) | 0.004 | ||
| High | 17 (44) | 20 (50) | |
| Low | 22 (56) | 20 (50) | |
| Double positive, CD9+ CD63+ EXs, n (%) | 0.015 | ||
| High | 17 (44) | 20 (50) | |
| Low | 22 (56) | 20 (50) |
| CD9+ EXs | |||
|---|---|---|---|
| Demographic | High | Low | p Value |
| Age at diagnosis, median (IQR) | 61 (58–67) | 62 (55–68) | 0.926 |
| Sex, male, n (%) | 17 (85) | 14 (70) | 0.252 |
| Race, n (%) | 0.180 | ||
| Caucasian | 13 (65) | 10 (50) | |
| African American | 3 (15) | 8 (40) | |
| Other | 4 (20) | 2 (10 | |
| Cirrhotic etiology, n (%) | 0.066 | ||
| HCV | 11 (55) | 8 (40) | |
| NASH | 3 (15) | 7 (35) | |
| Other | 6 (30) | 5 (25) | |
| Cirrhosis status at diagnosis, n (%) | 0.427 | ||
| Compensated | 15 (75) | 17 (85) | |
| Decompensated | 5 (25) | 3 (15) | |
| Scores and Staging | |||
| ECOG performance status 0, n (%) | 15 (75) | 15 (75) | 1.0 |
| Child–Pugh A, n (%) | 17 (85) | 19 (95) | 0.282 |
| BCLC HCC stage A, n (%) | 20 (100) | 17 (85) | 0.036 |
| Clinical Hepatology Labs | |||
| Sodium | 138 (137–141) | 139 (136–140) | 0.866 |
| Creatinine | 1.0 (0.8–1.1) | 0.9 (0.8–1.2) | 0.219 |
| Bilirubin | 0.7 (0.5–1.0) | 1.1 (0.6–1.3) | 0.181 |
| Albumin | 3.8 (3.1–4.1) | 3.4 (3.1–3.6) | 0.901 |
| INR | 1.1 (1.0–1.3) | 1.1 (1.0–1.2) | 0.184 |
| MELD-Na | 8 (7–10) | 9 (7–11) | 0.527 |
| Tumor Burden and Biomarkers | |||
| Largest lesion | 2.7 (2.3–3.6) | 3.1 (2.2–4.8) | 0.187 |
| Cumulative lesion | 3.0 (2.3–4.7) | 3.8 (2.6–4.8) | 0.233 |
| Milan criteria | 19 (95) | 17 (85) | 0.282 |
| AFP | 7.8 (4.0–38) | 21 (8.2–75) | 0.067 |
| First-Line Liver-Directed Therapy | 0.066 | ||
| DEE-TACE, n (%) | 2 (10) | 0 (0) | |
| 90Y, n (%) | 8 (40) | 14 (70) | |
| MWA, n (%) | 10 (50) | 6 (30) | |
| Treatment Response to First-Cycle LDT | 0.030 | ||
| ORR, n (%) | 19 (95) | 14 (70) | |
| Non-ORR, n (%) | 1 (5) | 6 (30) | |
| Study Endpoint | <0.001 | ||
| Tumor progression, n (%) | 0 (0) | 7 (78) | |
| Transplanted, n (%) | 10 (100) | 2 (22) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Núñez, K.G.; Wyczechowska, D.; Hibino, M.; Sandow, T.; Gimenez, J.; Koksal, A.R.; Aydin, Y.; Dash, S.; Cohen, A.J.; Thevenot, P.T. Exosome Shedding Is Concordant with Objective Treatment Response Rate and Stratifies Time to Progression in Treatment Naïve, Non-Resectable Hepatocellular Carcinoma. Livers 2023, 3, 727-738. https://doi.org/10.3390/livers3040047
Núñez KG, Wyczechowska D, Hibino M, Sandow T, Gimenez J, Koksal AR, Aydin Y, Dash S, Cohen AJ, Thevenot PT. Exosome Shedding Is Concordant with Objective Treatment Response Rate and Stratifies Time to Progression in Treatment Naïve, Non-Resectable Hepatocellular Carcinoma. Livers. 2023; 3(4):727-738. https://doi.org/10.3390/livers3040047
Chicago/Turabian StyleNúñez, Kelley G., Dorota Wyczechowska, Mina Hibino, Tyler Sandow, Juan Gimenez, Ali R. Koksal, Yucel Aydin, Srikanta Dash, Ari J. Cohen, and Paul T. Thevenot. 2023. "Exosome Shedding Is Concordant with Objective Treatment Response Rate and Stratifies Time to Progression in Treatment Naïve, Non-Resectable Hepatocellular Carcinoma" Livers 3, no. 4: 727-738. https://doi.org/10.3390/livers3040047
APA StyleNúñez, K. G., Wyczechowska, D., Hibino, M., Sandow, T., Gimenez, J., Koksal, A. R., Aydin, Y., Dash, S., Cohen, A. J., & Thevenot, P. T. (2023). Exosome Shedding Is Concordant with Objective Treatment Response Rate and Stratifies Time to Progression in Treatment Naïve, Non-Resectable Hepatocellular Carcinoma. Livers, 3(4), 727-738. https://doi.org/10.3390/livers3040047






