The Effect of Barley Bran Polyphenol-Rich Extracts on the Development of Nonalcoholic Steatohepatitis in Sprague–Dawley Rats Fed a High-Fat and High-Cholesterol Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of BP
2.2. Animals and Experimental Design
2.3. Serum Biochemical and Hepatic Lipid Analyses
2.4. Histopathological Assessment of the Liver
2.5. Quantification of mRNA Using Real-Time Reverse Transcription Polymerase Chain Reaction (PCR)
2.6. Statistical Analysis
3. Results
3.1. The Final Body Weight, Body Weight Gain, Cumulative Energy Intake, Food Efficacy, and Relative Organ Weights
3.2. Serum Biochemical Parameters and Hepatic Lipid Concentrations
3.3. Histopathological Findings of the Liver
3.4. Hepatic mRNA Expression
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Henry, L.; Paik, J.; Younossi, Z.M. Review article: The epidemiologic burden of non-alcoholic fatty liver disease across the world. Aliment. Pharmacol. Ther. 2022, 56, 942–956. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol. 2023, 79, 1542–1556. [Google Scholar] [CrossRef]
- Powell, E.E.; Wong, V.W.-S.; Rinella, M. Non-alcoholic fatty liver disease. Lancet 2019, 397, 2212–2224. [Google Scholar] [CrossRef] [PubMed]
- Day, C.P.; James, O.F.W. Steatohepatitis: A tale of two “hits”? Gastroenterology 1998, 114, 842–845. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Moschen, A.R. Evolution of inflammation in nonalcoholic fatty liver disease: The multiple parallel hits hypothesis. Hepatology 2010, 52, 1836–1846. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Allen, A.M.; Dubourg, J.; Noureddin, M.; Alkhouri, N. Challenges and opportunities in NASH drug development. Nat. Med. 2023, 29, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Badr, A.; Muller, K.; Schafer-Pregl, R.; El Rabey, H.; Effgen, S.; Ibrahim, H.H.; Pozzi, C.; Rohde, W.; Salamini, F. On the origin and domestication history of barley (Hordeum vulgare). Mol. Biol. Evol. 2000, 17, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Byun, A.R.; Chun, H.; Lee, J.; Lee, S.W.; Lee, H.S.; Shim, K.W. Effects of a dietary supplement with barley sprout extract on blood cholesterol metabolism. Evid. Based Complement. Alternat. Med. 2015, 2015, 473056. [Google Scholar] [CrossRef]
- El-Sayyad, H.I.H.; El-Shershaby, E.M.F.; El-Mansi, A.A.; El-Ashry, N. Anti-hypercholesterolemic impacts of barley and date palm fruits on the ovary of Wistar albino rats and their offspring. Reprod. Biol. 2018, 18, 236–251. [Google Scholar] [CrossRef]
- Ishiyama, S.; Kimura, M.; Umihira, N.; Matsumoto, S.; Takahashi, A.; Nakagawa, T.; Wakayama, T.; Kishigami, S.; Mochizuki, K. Consumption of barley ameliorates the diabetic steatohepatitis and reduces the high transforming growth factor β expression in mice grown in α-minimum essential medium in vitro as embryos. Biochem. Biophys. Rep. 2021, 27, 101029. [Google Scholar] [CrossRef]
- Zhu, C.; Guan, Q.; Song, C.; Zhong, L.; Ding, X.; Zeng, H.; Nie, P.; Song, L. Regulatory effects of Lactobacillus fermented black barley on intestinal microbiota of NAFLD rats. Food Res. Int. 2021, 147, 110467. [Google Scholar] [CrossRef]
- Zhao, C.-Z.; Jiang, W.; Zhu, Y.-Y.; Wang, C.-Z.; Zhong, W.-H.; Wu, G.; Chen, J.; Zhu, M.-N.; Wu, Q.-L.; Du, X.-L.; et al. Highland barley Monascus purpureus Went extract ameliorates high-fat, high-fructose, high-cholesterol diet induced nonalcoholic fatty liver disease by regulating lipid metabolism in golden hamsters. J. Ethnopharmacol. 2022, 286, 114922. [Google Scholar] [CrossRef]
- Liu, H.; Sun, Y.; Nie, C.; Xie, X.; Yuan, X.; Ma, Q.; Zhang, M.; Chen, Z.; Hu, X.; Li, J. Highland barley β-glucan alleviated western diet-induced non-alcoholic fatty liver disease via increasing energy expenditure and regulating bile acid metabolism in mice. Food Funct. 2022, 13, 11664–11675. [Google Scholar] [CrossRef] [PubMed]
- Esfandi, R.; Walters, M.E.; Tsopmo, A. Antioxidant properties and potential mechanisms of hydrolyzed proteins and peptides from cereals. Heliyon 2019, 5, e01538. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-S.; Tong, X.-M.; Wu, X.-M.; Bai, J.; Fan, S.-T.; Zhu, Y.; Zhang, J.-Y.; Xiao, X. Metabolomics reveal the regulatory effect of polysaccharides from fermented barley bran extract on lipid accumulation in HepG2 cells. Metabolites 2023, 13, 223. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, X.; Zhou, W.; Tu, Z.; Yang, X.; Hao, J.; Liang, F.; Chen, Z.; Du, Y. Impacts of proanthocyanidin binding on conformational and functional properties of decolorized highland barley protein. Foods 2023, 12, 481. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, M.; Kawase, M.; Masuzumi, M.; Sakaki, M.; Nagata, Y.; Tanaka, K.; Suruga, K.; Tamaru, S.; Kato, S.; Tsuneyama, K.; et al. High-fat and high-cholesterol diet rapidly induces non-alcoholic steatohepatitis with advanced fibrosis in Sprague-Dawley rats. Hepatol. Res. 2015, 45, 458–469. [Google Scholar] [CrossRef]
- Omagari, K.; Maruta, A.; Yayama, N.; Yoshida, Y.; Okamoto, K.; Shirouchi, B.; Takeuchi, S.; Suruga, K.; Koba, K.; Ichimura-Shimizu, M.; et al. The effects of overnight fasting duration on glucose and lipid metabolism in a Sprague-Dawley rat model of nonalcoholic steatohepatitis with advanced fibrosis. J. Nutr. Sci. Vitaminol. 2023, 69, 357–369. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.-C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Holmgren, A. The thioredoxin antioxidant system. Free Radic. Biol. Med. 2014, 66, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.; Juan, B.; Li, M.S.; Zhang, J.Y.; Sun, X.J.; Dong, Y. Supplementation of fermented barley extracts with Lactobacillus Plantarum dy-1 inhibits obesity via a UCP1-dependent mechanism. Biomed. Environ. Sci. 2019, 32, 578–591. [Google Scholar]
- Kohyama, N.; Ono, H.; Yanagisawa, T. Changes in anthocyanins in the grains of purple waxy hull-less barley during seed maturation and after harvest. J. Agric. Food Chem. 2008, 56, 5770–5774. [Google Scholar] [CrossRef] [PubMed]
- McMurrough, I.; Loughrey, M.J.; Hennigan, G.P. Content of (+)-catechin and proanthocyanidins in barley and malt grain. J. Sci. Food Agric. 1983, 34, 62–72. [Google Scholar] [CrossRef]
- Guan, Q.; Ding, X.-W.; Zhong, L.-Y.; Zhu, C.; Nie, P.; Song, L.-H. Beneficial effects of Lactobacillus-fermented black barley on high fat diet-induced fatty liver in rats. Food Funct. 2021, 12, 6526–6539. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Ul-Haq, I.; Patel, S.; Pan, X.; Naz, S.; Silva, A.S.; Saeed, F.; Suleria, H.A.R. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef] [PubMed]
- Baba, S.; Osakabe, N.; Kato, Y.; Natsume, M.; Yasuda, A.; Kido, T.; Fukuda, K.; Muto, Y.; Kondo, K. Continuous intake of polyphenolic compounds containing cocoa powder reduces LDL oxidative susceptibility and has beneficial effects on plasma HDL-cholesterol concentrations in humans. Am. J. Clin. Nutr. 2007, 85, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Wang, L.; Nanba, F.; Ito, C.; Toda, T.; Ashida, H. Procyanidin promotes translocation of glucose transporter 4 in muscle of mice through activation of insulin and AMPK signaling pathways. PLoS ONE 2016, 11, e0161704. [Google Scholar] [CrossRef]
- Nie, C.; Wang, B.; Fan, M.; Wang, Y.; Sun, Y.; Qian, H.; Li, Y.; Wang, L. Highland barley tea polyphenols extract alleviates skeletal muscle fibrosis in mice by reducing oxidative stress, inflammation, and cell senescence. J. Agric. Food Chem. 2023, 71, 739–748. [Google Scholar] [CrossRef]
- Yamashita, Y.; Okabe, M.; Natsume, M.; Ashida, H. Prevention mechanisms of glucose intolerance and obesity by cacao liquor procyanidin extract in high-fat diet-fed C57BL/6 mice. Arch. Biochem. Biophys. 2012, 527, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhang, H.; Cheng, L.; Wang, L.; Qian, H.; Qi, X. In vitro and in vivo antioxidant activity of polyphenols extracted from black highland barley. Food Chem. 2016, 194, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Nie, C.; Li, T.; Zhao, J.; Fan, M.; Li, Y.; Qian, H.; Wang, L. Effect of boiling and roasting on phenolic components and their bioaccessibilities of highland barley. Food Res. Int. 2022, 162 Pt B, 112137. [Google Scholar] [CrossRef]
- Kang, J.; Postigo-Fernandez, J.; Kim, K.; Zhu, C.; Yu, J.; Meroni, M.; Mayfield, B.; Bartolome, A.; Dapito, D.H.; Ferrante, A.W.J.; et al. Notch-mediated hepatocyte MCP-1 secretion causes liver fibrosis. JCI Insight 2023, 8, e165369. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Roh, Y.S.; Song, J.; Zhang, B.; Liu, C.; Loomba, R.; Seki, E. TGF-β signaling in hepatocytes participates in steatohepatitis through regulation of cell death and lipid metabolism. Hepatology 2014, 59, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Gaddam, N.; Chandler, V.; Chakraborty, S. Oxidative stress-induced liver damage and remodeling of the liver vasculature. Am. J. Pathol. 2023, 193, 1400–1414. [Google Scholar] [CrossRef]
- Krautbauer, S.; Eisinger, K.; Lupke, M.; Wanninger, J.; Ruemmele, P.; Hader, Y.; Weiss, T.S.; Buechler, C. Manganese superoxide dismutase is reduced in the liver of male but not female humans and rodents with non-alcoholic fatty liver disease. Exp. Mol. Pathol. 2013, 95, 330–335. [Google Scholar] [CrossRef]
- Yang, B.-Y.; Zhang, X.-Y.; Guan, S.-W.; Hua, Z.-C. Protective effect of procyanidin B2 against CCl4-induced acute liver injury in mice. Molecules 2015, 20, 12250–12265. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Y.; Li, D.; Ho, C.-T.; Li, J.; Wan, X. The absorption, distribution, metabolism and excretion of procyanidins. Food Funct. 2016, 7, 1273–1281. [Google Scholar] [CrossRef]
- Wren-Dail, M.A.; Dauchy, R.T.; Blask, D.E.; Hill, S.M.; Ooms, T.G.; Dupepe, L.M.; Bohm Jr, R.P. Effect of isoflurane anesthesia on circadian metabolism and physiology in rats. Comp. Med. 2017, 67, 138–146. [Google Scholar]
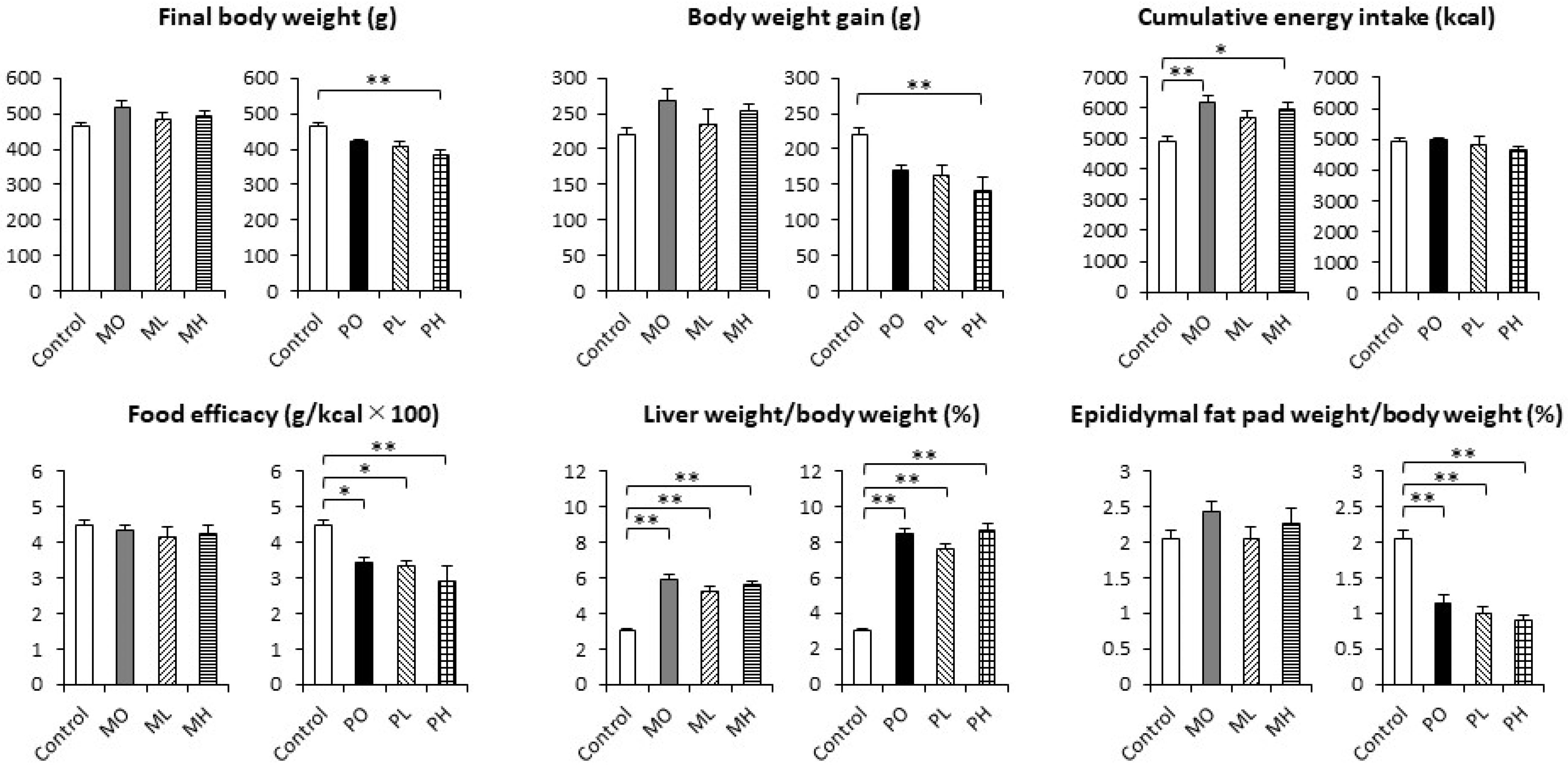

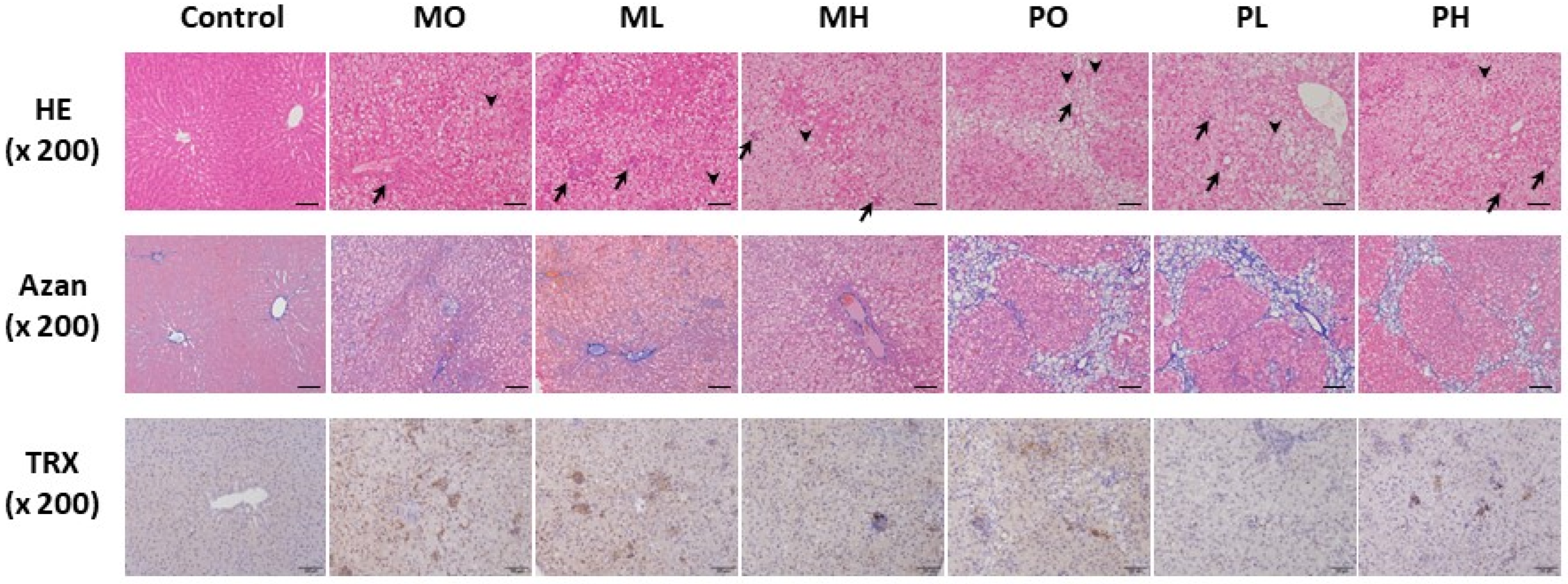
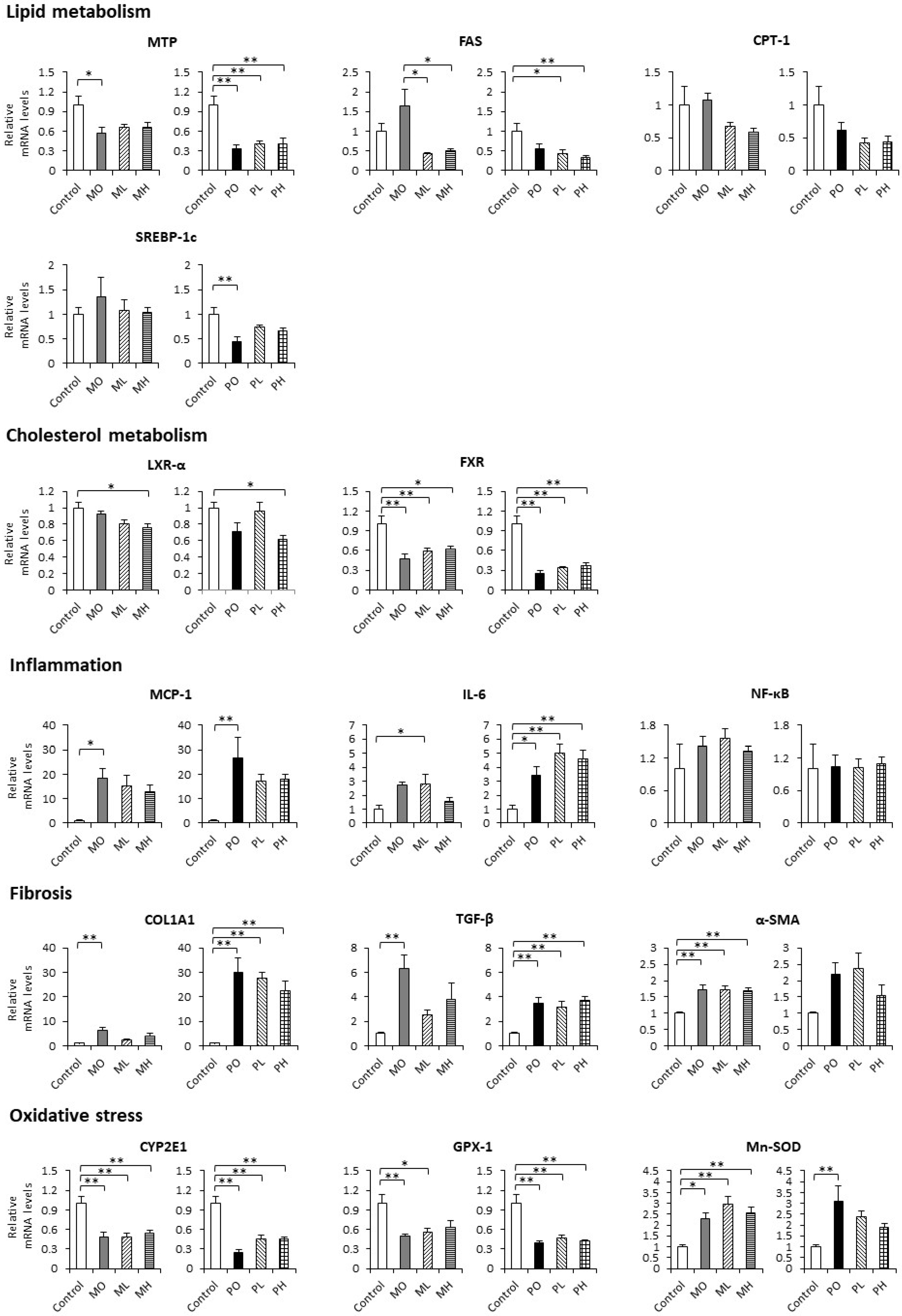
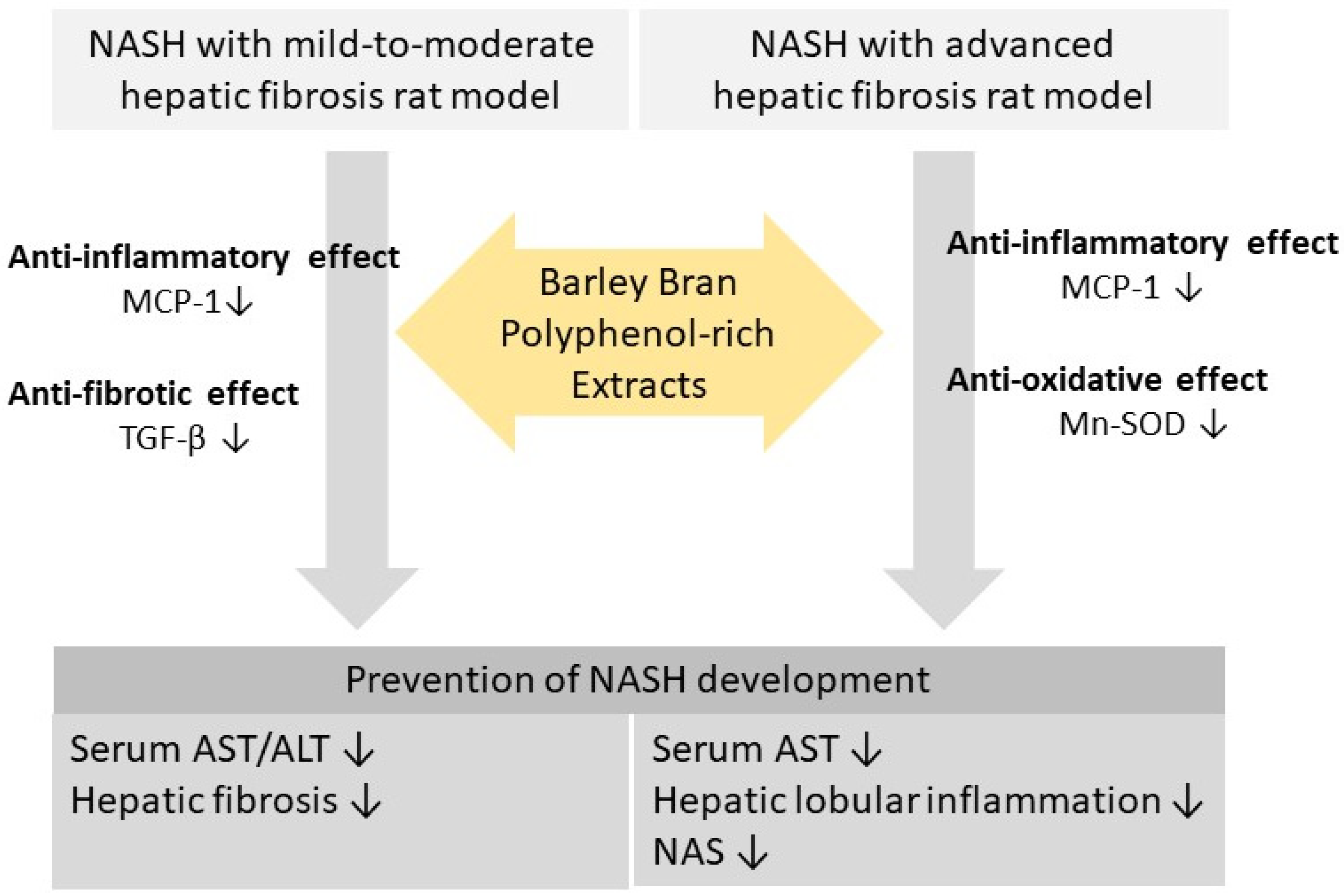
| Constituents/Group | Control | MO | ML | MH | PO | PL | PH |
|---|---|---|---|---|---|---|---|
| Water (g) | 7.90 | 5.49 | 5.49 | 5.48 | 5.37 | 5.37 | 5.37 |
| Crude protein (g) | 23.10 | 16.05 | 16.02 | 15.97 | 15.71 | 15.69 | 15.64 |
| Crude lipid (g) | 5.10 | 3.54 | 3.53 | 3.52 | 3.47 | 3.46 | 3.45 |
| Crude ash (g) | 5.80 | 4.03 | 4.03 | 4.03 | 3.94 | 3.94 | 3.94 |
| Crude fiber (g) | 2.80 | 1.95 | 1.95 | 1.95 | 1.90 | 1.90 | 1.90 |
| Nitrogen-free extract (g) | 55.30 | 38.43 | 38.68 | 39.15 | 37.60 | 37.86 | 38.33 |
| Palm oil (g) | 0.00 | 28.75 | 28.55 | 28.18 | 27.50 | 27.31 | 26.95 |
| Cholesterol (g) | 0.00 | 1.25 | 1.24 | 1.23 | 2.50 | 2.48 | 2.45 |
| Sodium cholate (g) | 0.00 | 0.50 | 0.50 | 0.49 | 2.00 | 1.99 | 1.96 |
| Total (g) | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Protein energy ratio (%) | 25.70 | 12.62 | 12.62 | 12.63 | 12.77 | 12.78 | 12.82 |
| Lipid energy ratio (%) | 12.77 | 57.15 | 56.87 | 56.40 | 56.66 | 56.39 | 55.89 |
| Carbohydrate energy ratio (%) | 61.53 | 30.23 | 30.51 | 30.97 | 30.57 | 30.83 | 31.29 |
| Energy (kcal/100 g) | 359.50 | 508.60 | 507.64 | 505.85 | 491.96 | 491.11 | 489.54 |
| Primer | Sequence (5′ to 3′) | |
|---|---|---|
| Mttp | Forward: | CAAGCTCAAGGCAGTGGTTG |
| Reverse: | AGCAGGTACATCGTGGTGTC | |
| Fasn | Forward: | CAACATTGACGCCAGTTCC |
| Reverse: | TTCGAGCCAGTGTCTTCCAC | |
| Cpt1a | Forward: | AACCTCGGACCCAAATTGC |
| Reverse: | GGCCCCGCAGGTAGATATATT | |
| Srebf1 | Forward: | CATGGACGAGCTACCCTTCG |
| Reverse: | GAAGCATGTCTTCGATGTCGG | |
| Nr1h3 | Forward: | CAGGACCAGCTCCAAGTAGA |
| Reverse: | GAACATCAGTCGGTCGTGG | |
| Nr1h4 | Forward: | TGGGAATGTTGGCTGAATGTTTG |
| Reverse: | TGCTAGCTTGGTCGTGGAG | |
| Ccl2 | Forward: | TCTGTCACGCTTCTGGGCCTGT |
| Reverse: | GGGGCATTAACTGCATCTGGCTGAG | |
| Il6 | Forward: | GATACCACCCACAACAGACCAGTA |
| Reverse: | TGCACAACTCTTTTCTCATTTCCA | |
| Nfkb1 | Forward: | TGACATCATCAACATGAGAACGA |
| Reverse: | CCCCAACCCTCAGCAAGTC | |
| Col1a1 | Forward: | GCGTAGCCTACATGGACCAA |
| Reverse: | AAGTTCCGGTGTGACTCGTG | |
| Tgfb1 | Forward: | CTTTGTACAACAGCACCCGC |
| Reverse: | TAGATTGCGTTGTTGCGGTC | |
| Acta2 | Forward: | GCCAAGAAGACATCCCTGAAGT |
| Reverse: | TGTGGCAGATACAGATCAAGCAT | |
| Cyp2e1 | Forward: | CCCATCCTTGGGAACATTTTT |
| Reverse: | GCCAAGGTGCAGTGTGAACA | |
| Gpx1 | Forward: | GCTGCTCATTGAGAATGTCG |
| Reverse: | GAATCTCTTCATTCTTGCCATT | |
| Sod2 | Forward: | GACCTGCCTTACGACTATG |
| Reverse: | TACTTCTCCTCGGTGACG | |
| Rplp0 | Forward: | ATTGCGGACACCCTCTAGGA |
| Reverse: | GGTGTTTGACAATGGCAGCAT | |
| Item/Group | Score | Control (n = 5) | MO (n = 5) | ML (n = 5) | MH (n = 6) | PO (n = 5) | PL (n = 5) | PH (n = 6) |
|---|---|---|---|---|---|---|---|---|
| Steatosis * | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | |
| 3 | 0 | 5 | 4 | 6 | 5 | 5 | 6 | |
| Lobular inflammation * | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 0 | 0 | 0 | 0 | 0 | 1 | 4 | |
| 2 | 0 | 1 | 3 | 2 | 2 | 2 | 0 | |
| 3 | 0 | 4 | 2 | 4 | 3 | 2 | 2 | |
| Hepatocyte ballooning * | 0 | 5 | 2 | 2 | 5 | 3 | 2 | 5 |
| 1 | 0 | 3 | 3 | 1 | 0 | 3 | 0 | |
| 2 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | |
| NAFLD activity score * (NAS) | 0–2 | 5 | 0 | 0 | 0 | 0 | 0 | 0 |
| 3–4 | 0 | 0 | 0 | 0 | 0 | 1 | 4 | |
| 5–8 | 0 | 5 | 5 | 6 | 5 | 4 | 2 | |
| Fibrosis * | 0 | 5 | 0 | 2 | 3 | 0 | 0 | 1 |
| 1 | 0 | 3 | 3 | 2 | 1 | 2 | 1 | |
| 2 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | |
| 3 | 0 | 0 | 0 | 0 | 1 | 2 | 3 | |
| 4 | 0 | 0 | 0 | 0 | 3 | 1 | 1 | |
| TRX staining ** | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 2 | 0 | 3 | 3 | 6 | 1 | 2 | 1 | |
| 3 | 0 | 1 | 1 | 0 | 3 | 3 | 5 | |
| 4 | 0 | 1 | 1 | 0 | 1 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omagari, K.; Ishida, J.; Murata, K.; Araki, R.; Yogo, M.; Shirouchi, B.; Suruga, K.; Sera, N.; Koba, K.; Ichimura-Shimizu, M.; et al. The Effect of Barley Bran Polyphenol-Rich Extracts on the Development of Nonalcoholic Steatohepatitis in Sprague–Dawley Rats Fed a High-Fat and High-Cholesterol Diet. Livers 2024, 4, 193-208. https://doi.org/10.3390/livers4020015
Omagari K, Ishida J, Murata K, Araki R, Yogo M, Shirouchi B, Suruga K, Sera N, Koba K, Ichimura-Shimizu M, et al. The Effect of Barley Bran Polyphenol-Rich Extracts on the Development of Nonalcoholic Steatohepatitis in Sprague–Dawley Rats Fed a High-Fat and High-Cholesterol Diet. Livers. 2024; 4(2):193-208. https://doi.org/10.3390/livers4020015
Chicago/Turabian StyleOmagari, Katsuhisa, Juna Ishida, Konomi Murata, Ryoko Araki, Mizuki Yogo, Bungo Shirouchi, Kazuhito Suruga, Nobuko Sera, Kazunori Koba, Mayuko Ichimura-Shimizu, and et al. 2024. "The Effect of Barley Bran Polyphenol-Rich Extracts on the Development of Nonalcoholic Steatohepatitis in Sprague–Dawley Rats Fed a High-Fat and High-Cholesterol Diet" Livers 4, no. 2: 193-208. https://doi.org/10.3390/livers4020015






