Investigation of Pazopanib and Human Serum Albumin Interaction Using Spectroscopic and Molecular Docking Approaches
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Materials and Methods
2.2. Fluorescence Spectroscopy Measurements
2.3. CD Measurements
2.4. Molecular Docking Studies
2.5. UV–Visible Spectroscopy Measurements
3. Results and Discussion
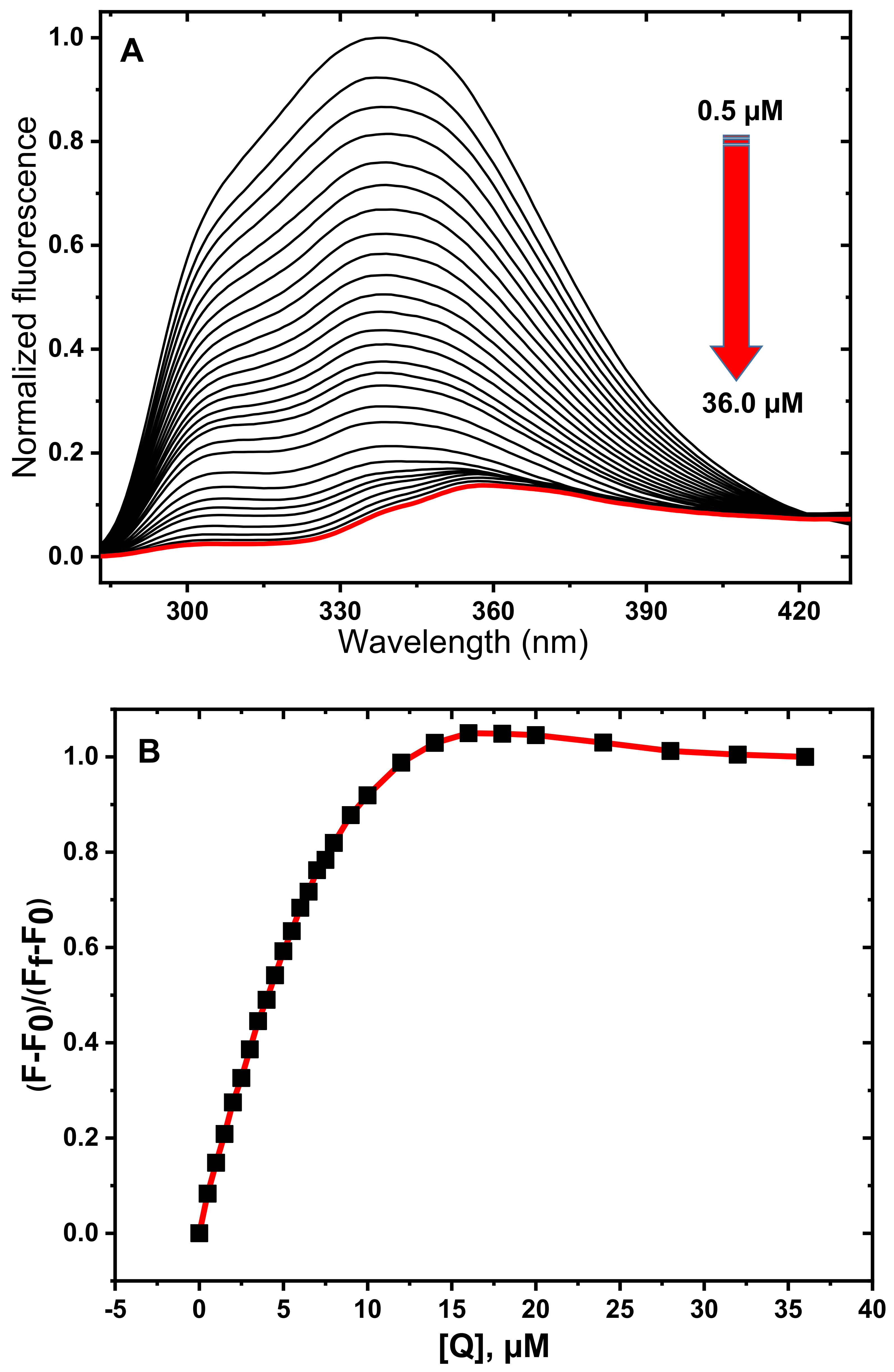
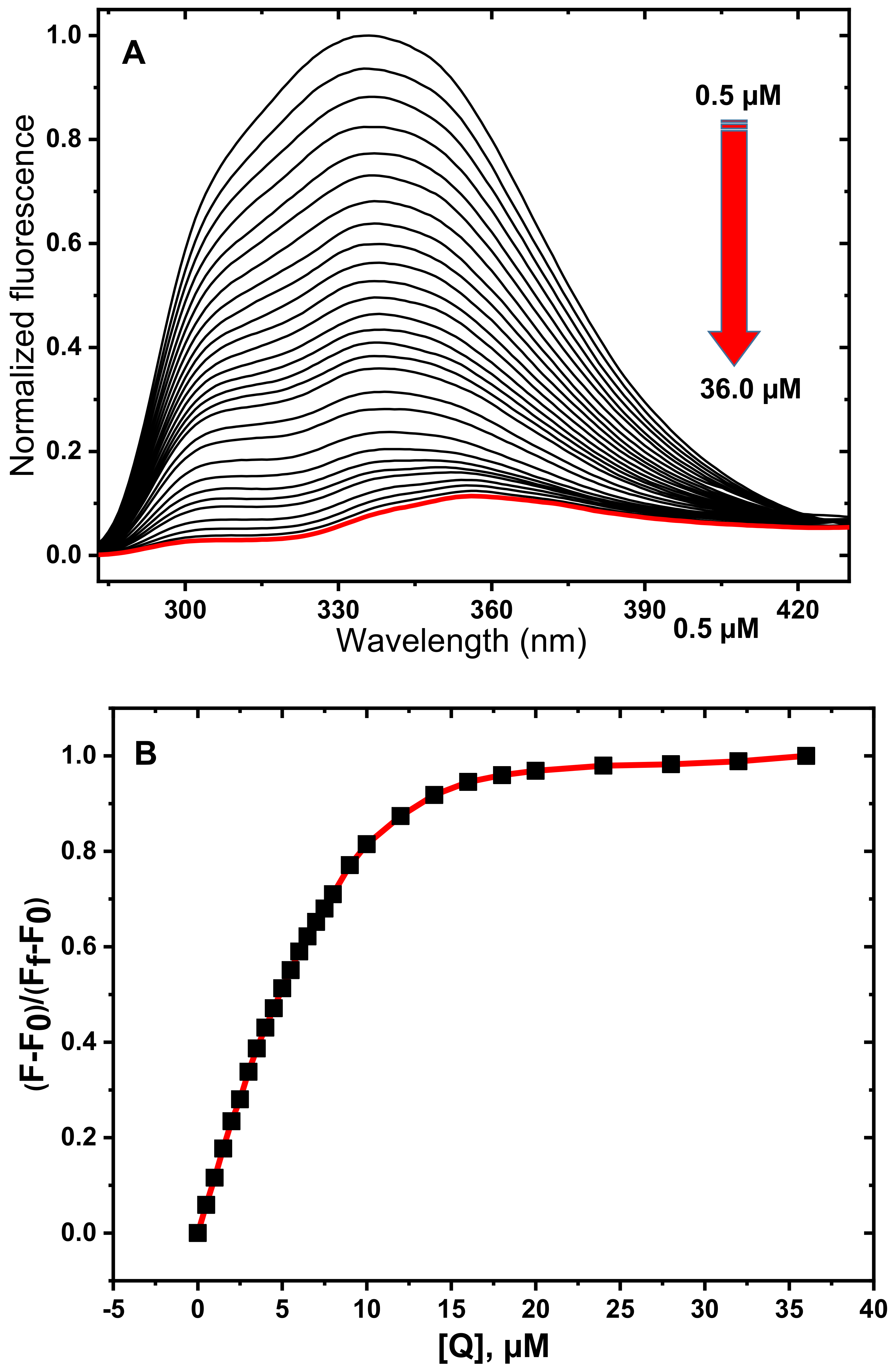
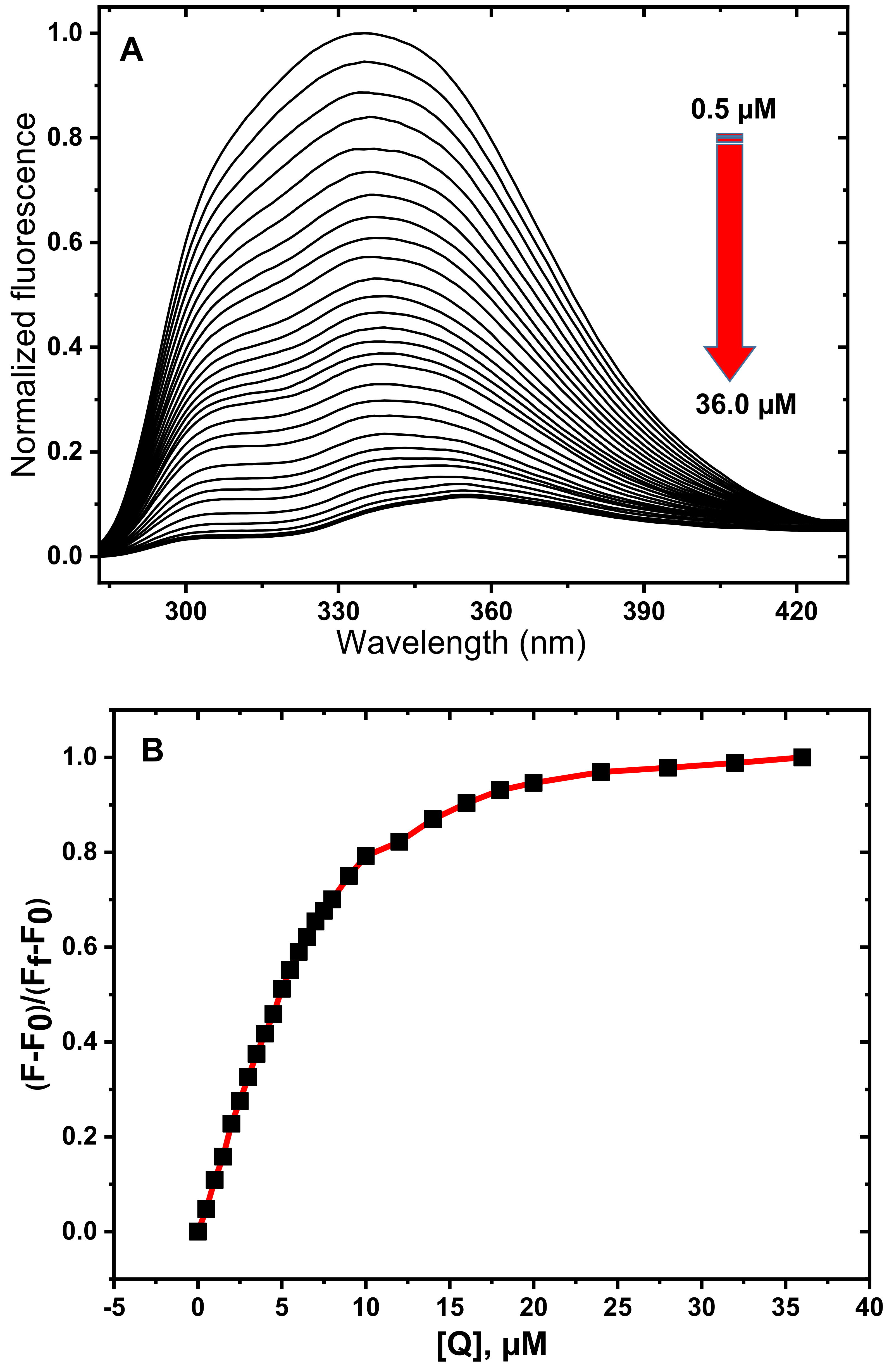
3.1. Fluorescence Spectroscopy Studies
3.2. Determination of Quenching Mechanism
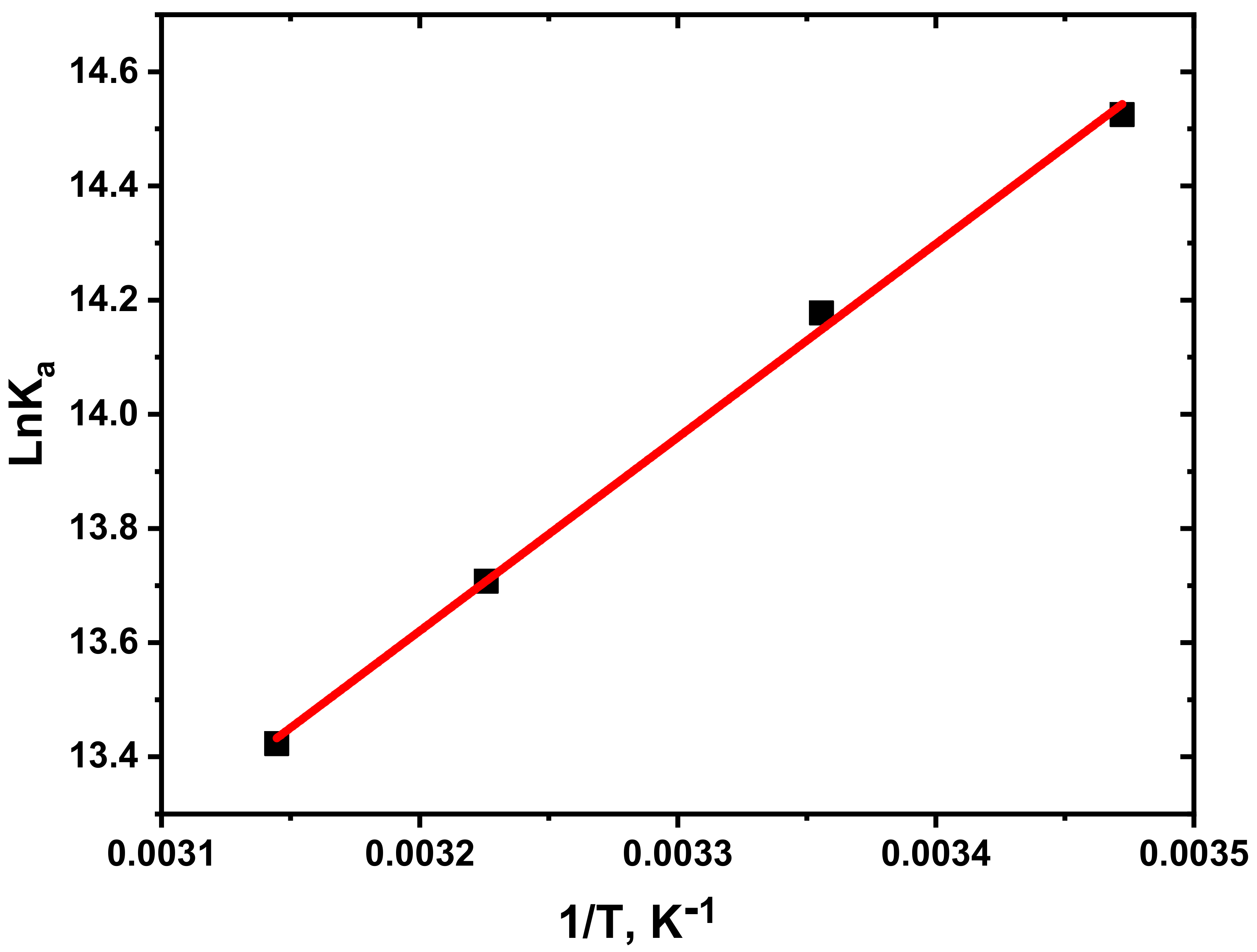
3.3. Determination of Thermodynamic Parameters and Binding Constants
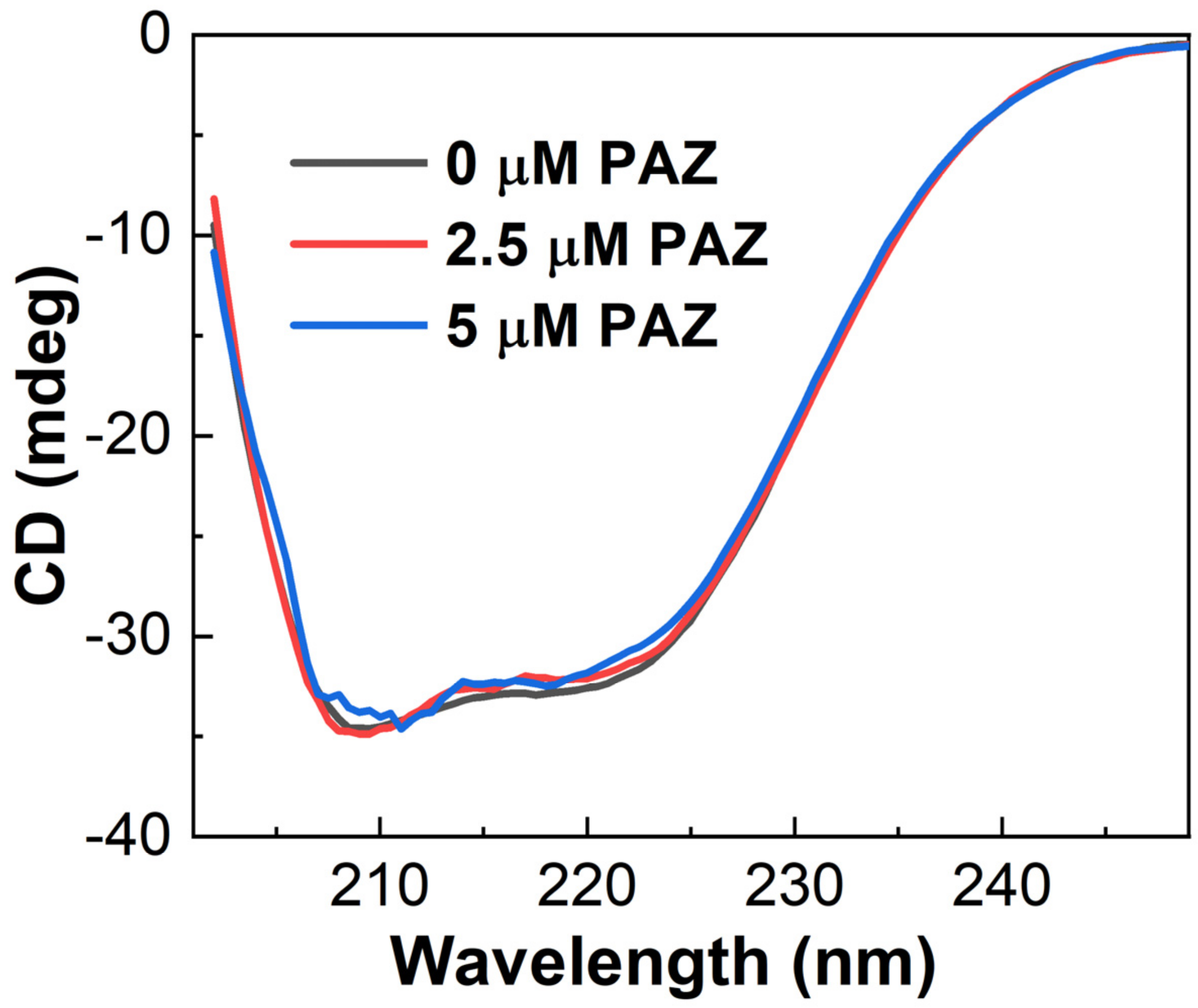
3.4. CD Spectroscopy
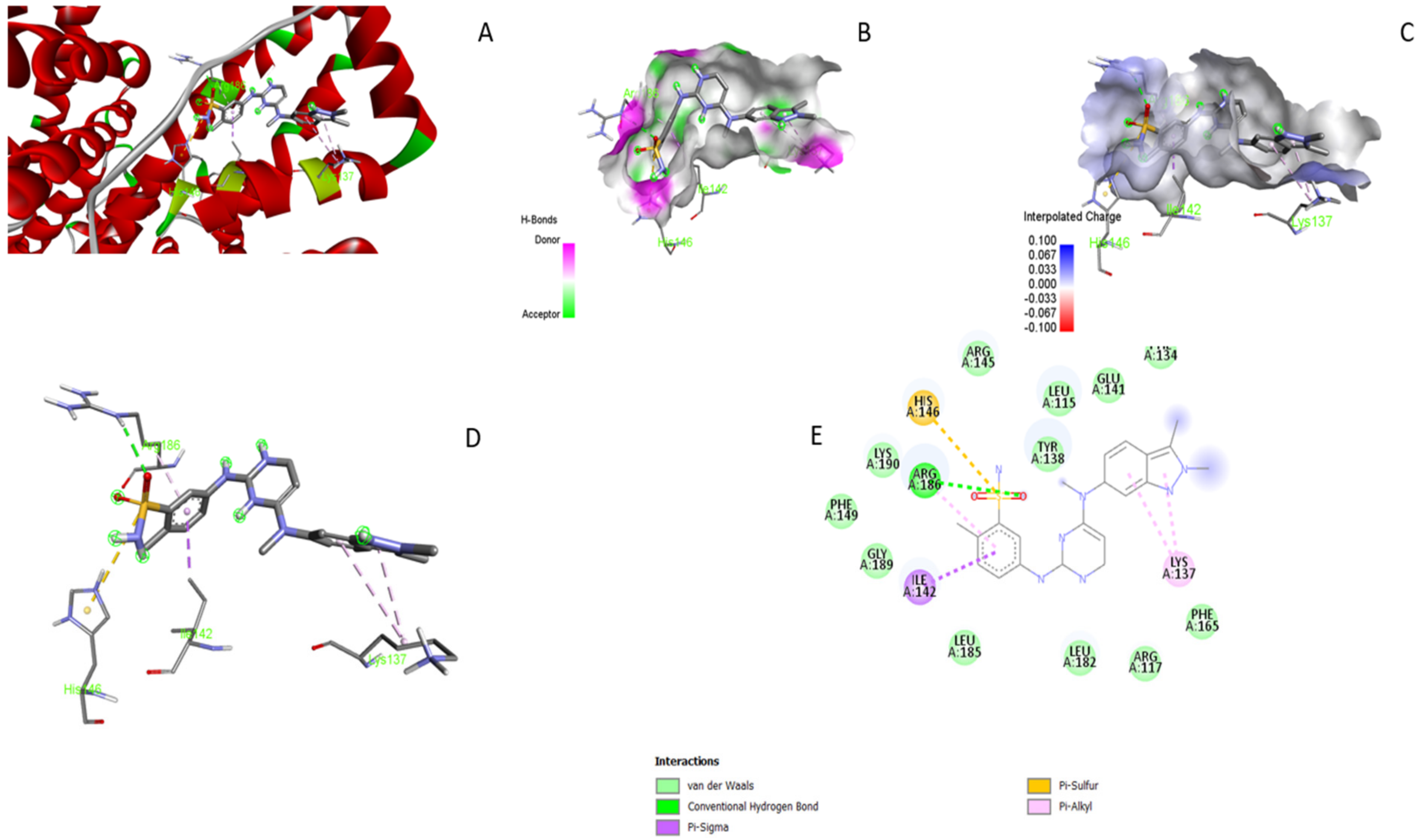
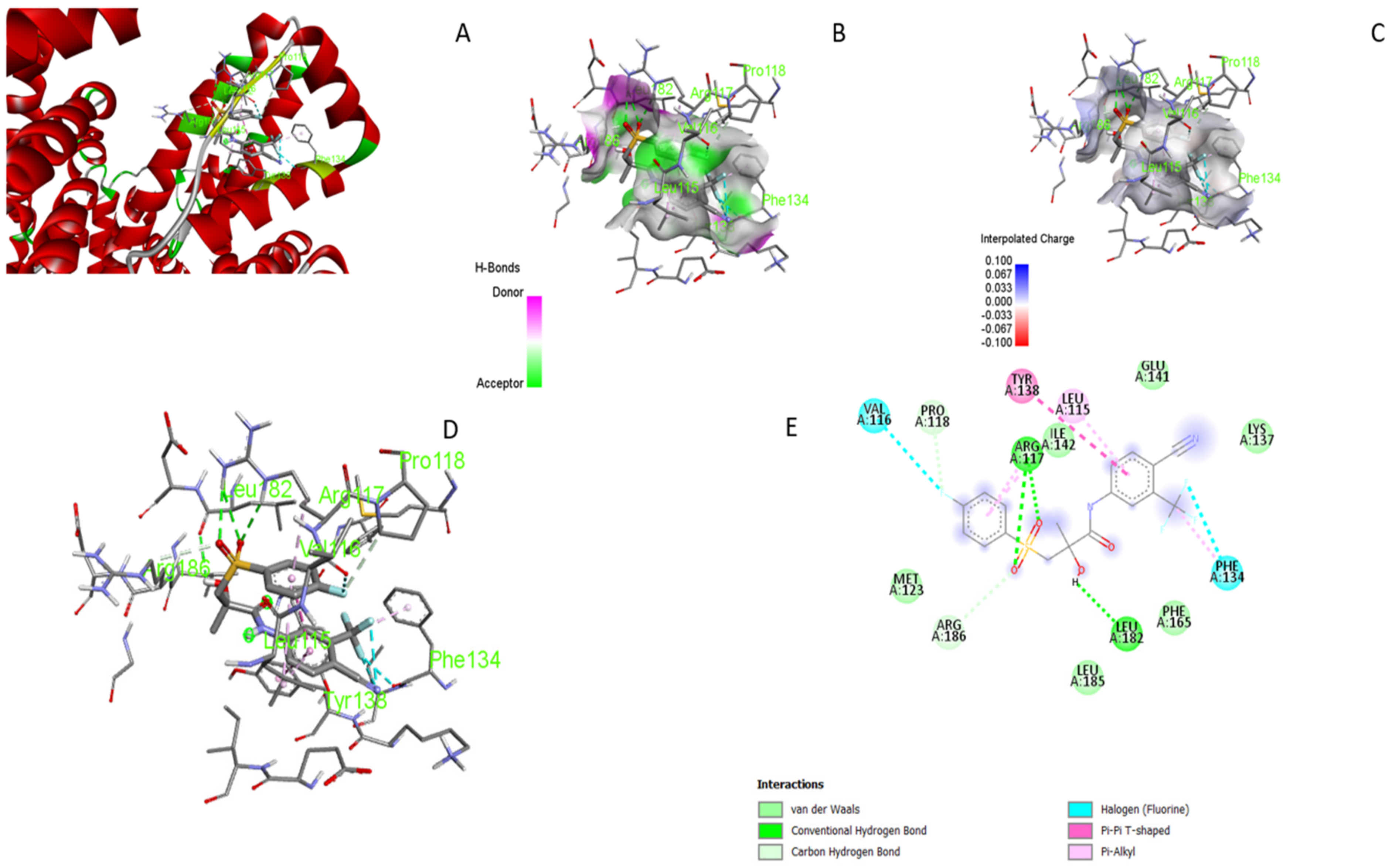
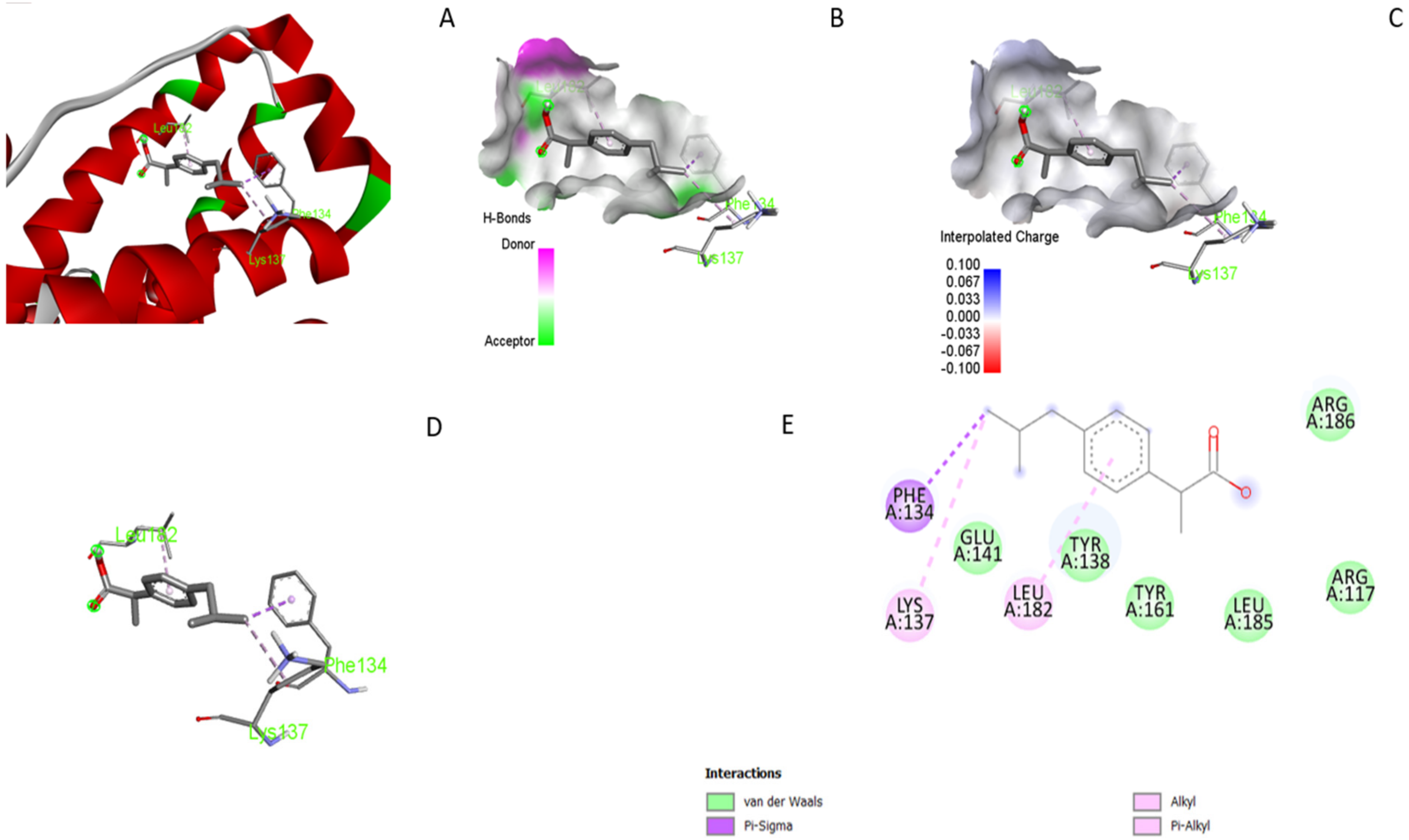
3.5. Molecular Docking
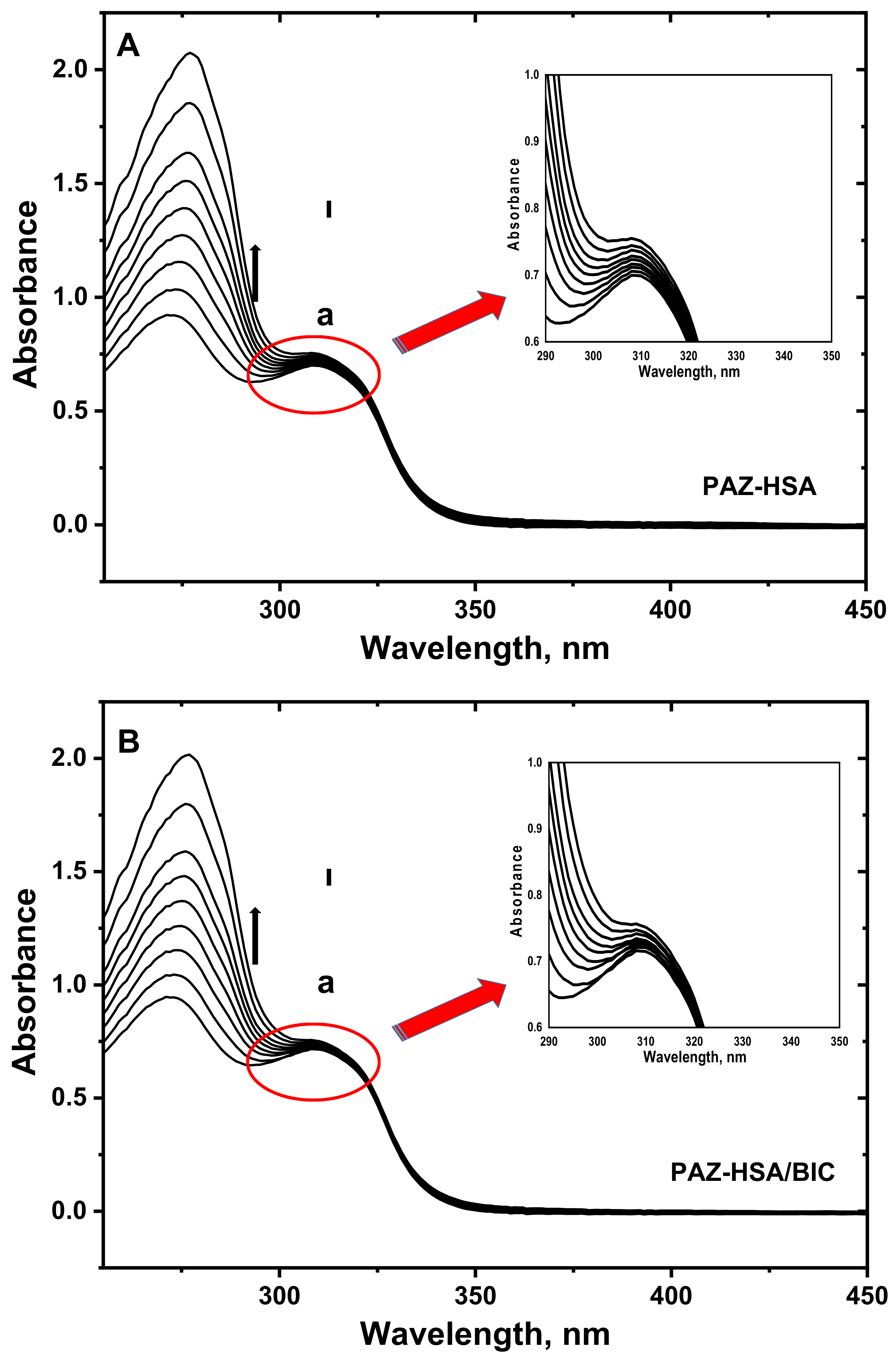
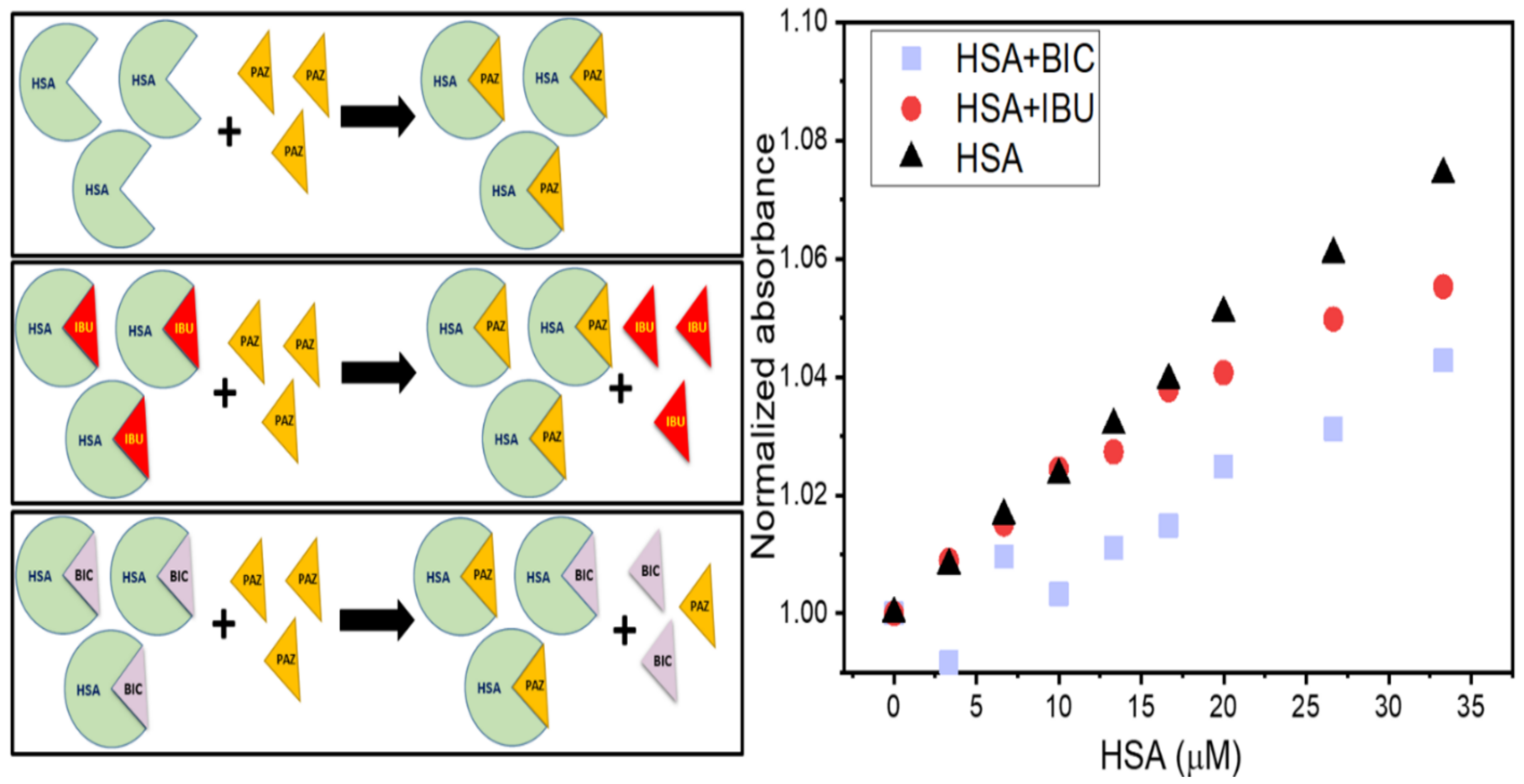
3.6. UV–Visible Absorption Spectroscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beljanski, V. Pazopanib. In xPharm: The Comprehensive Pharmacology Reference; Elsevier BV: Amsterdam, The Netherlands, 2010; pp. 1–5. [Google Scholar]
- Pazopanib. In Meyler’s Side Effects of Drugs; Elsevier BV: Amsterdam, The Netherlands, 2016; pp. 537–540.
- Zhang, L.; Liu, Y.; Wang, Y. Interaction between an (−)-epigallocatechin-3-gallate-copper complex and bovine serum albumin: Fluorescence, circular dichroism, HPLC, and docking studies. Food Chem. 2019, 301, 125294. [Google Scholar] [CrossRef] [PubMed]
- Radha, A.; Singh, A.; Sharma, L.; Thakur, K.K. Molecular interactions of acebutolol hydrochloride to human serum albumin: A combined calorimetric, spectroscopic and molecular modelling approach. Mater. Today Proc. 2021, 44, 1700–1706. [Google Scholar] [CrossRef]
- Singh, I.; Luxami, V.; Paul, K. Spectroscopy and molecular docking approach for investigation on the binding of nocodazole to human serum albumin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 235, 118289. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Singh, B.; Sharma, A.; Saraswat, J.; Dohare, N.; Parray, M.U.D.; Siddiquee, A.; Alanazi, A.M.; Khan, A.A. Interaction and esterase activity of albumin serums with orphenadrine: A spectroscopic and computational approach. J. Mol. Struct. 2021, 1239, 130522. [Google Scholar] [CrossRef]
- Sonpavde, G.; Hutson, T.E. Pazopanib: A novel multitargeted tyrosine kinase inhibitor. Curr. Oncol. Rep. 2007, 9, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Tunç, S.; Çetinkaya, A.; Duman, O. Spectroscopic investigations of the interactions of tramadol hydrochloride and 5-azacytidine drugs with human serum albumin and human hemoglobin proteins. J. Photochem. Photobiol. B Biol. 2013, 120, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Ariga, G.G.; Naik, P.N.; Chimatadar, S.A.; Nandibewoor, S.T. Interactions between epinastine and human serum albumin: Investigation by fluorescence, UV–vis, FT–IR, CD, lifetime measurement and molecular docking. J. Mol. Struct. 2017, 1137, 485–494. [Google Scholar] [CrossRef]
- Rajdev, P.; Mondol, T.; Makhal, A.; Pal, S.K. Simultaneous binding of anti-tuberculosis and anti-thrombosis drugs to a human transporter protein: A FRET study. J. Photochem. Photobiol. B Biol. 2011, 103, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; He, J.; Yang, H.; Tang, P.; Tang, B.; Sun, Q.; Li, H. Investigation on the interaction of antibacterial drug moxifloxacin hydrochloride with human serum albumin using multi-spectroscopic approaches, molecular docking and dynamical simulation. RSC Adv. 2017, 7, 48942–48951. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Thyparambil, A.; Latour, R.A. Protein helical structure determination using CD spectroscopy for solutions with strong background absorbance from 190 to 230 nm. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2014, 1844, 2331–2337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez, L.; Andrade, R.; Birgin, E.G.; Martínez, J.M. Software News and Update Packmol: A Package for Building Initial Configurations for Molecular Dynamics Simulations. J. Comput. Chem. 2012, 30, 2157–2164. [Google Scholar] [CrossRef] [PubMed]
- Eftink, M.R.; Ghiron, C.A. Fluorescence quenching studies with proteins. Anal. Biochem. 1981, 114, 199–227. [Google Scholar] [CrossRef]
- Castellan, G.W. Physical Chemistry, 3rd ed.; Addison-Wesley Publishing Company: San Francisco, CA, USA, 1983; pp. 103–138. [Google Scholar]
- Menezes, T.M.; Neto, A.M.D.S.; Gubert, P.; Neves, J.L. Effects of human serum albumin glycation on the interaction with the tyrosine kinase inhibitor pazopanib unveiled by multi-spectroscopic and bioinformatic tools. J. Mol. Liq. 2021, 340, 116843. [Google Scholar] [CrossRef]



| T (K) | Ksv (M−1) | R | kq (M−1·s−1) |
|---|---|---|---|
| 288.15 | 1.377 × 105 | 0.995 | 1.377 × 1013 |
| 298.15 | 1.267 × 105 | 0.993 | 1.267 × 1013 |
| 310.15 | 1.261 × 105 | 0.994 | 1.261 × 1013 |
| 318.15 | 1.198 × 105 | 0.993 | 1.198 × 1013 |
| T (K) | Ka (M−1) | n | R | ΔG° (kJ mol−1) | ΔH° (kJ mol−1) | ΔS° (J mol−1 K−1) |
|---|---|---|---|---|---|---|
| 288.15 | 2.032 × 106 | 1.064 | 0.995 | −34.823 | −28.187 | 23.043 |
| 298.15 | 1.436 × 106 | 1.041 | 0.999 | −35.054 | ||
| 310.15 | 0.898 × 106 | 1.047 | 0.999 | −35.330 | ||
| 318.15 | 0.675 × 106 | 1.048 | 0.999 | −35.515 |
| Drug Substance | Docking Score (kcal/mol) |
|---|---|
| Pazopanib | −10.4 |
| Bicalutamide | −9.1 |
| Ibuprofen | −7.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cetinkaya, A.; Caglayan, M.G.; Unal, M.A.; Beyazkilic, P.; Elbuken, C.; Atici, E.B.; Ozkan, S.A. Investigation of Pazopanib and Human Serum Albumin Interaction Using Spectroscopic and Molecular Docking Approaches. Analytica 2022, 3, 144-160. https://doi.org/10.3390/analytica3010011
Cetinkaya A, Caglayan MG, Unal MA, Beyazkilic P, Elbuken C, Atici EB, Ozkan SA. Investigation of Pazopanib and Human Serum Albumin Interaction Using Spectroscopic and Molecular Docking Approaches. Analytica. 2022; 3(1):144-160. https://doi.org/10.3390/analytica3010011
Chicago/Turabian StyleCetinkaya, Ahmet, Mehmet Gokhan Caglayan, Mehmet Altay Unal, Pinar Beyazkilic, Caglar Elbuken, Esen Bellur Atici, and Sibel A. Ozkan. 2022. "Investigation of Pazopanib and Human Serum Albumin Interaction Using Spectroscopic and Molecular Docking Approaches" Analytica 3, no. 1: 144-160. https://doi.org/10.3390/analytica3010011









