Comprehensive Examination of Cu, Pb, Zn, Fe, Mn and Cd in Lackawanna County Waters, Northeastern Pennsylvania: A Brief Report
Abstract
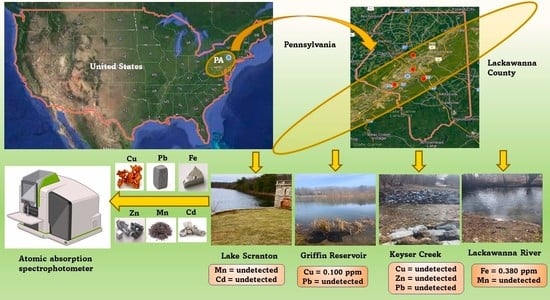
:1. Introduction
2. Materials and Methods
3. Results
3.1. Development of Calibration Curves
3.2. Analysis of Water Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The Lackawanna River Conservation Association (LRCA). Available online: https://lrca.org/the-lackawannawatershed (accessed on 29 May 2022).
- McMorran, C.P. Lackawanna River Priority Water Body Survey Report: Water Quality Standards Review; Publication No. 124; Susquehanna River Basin Commission: Harrisburg, PA, USA, 1989.
- Lackawanna River Information-Abington Regional Wastewater. Authority. Available online: https://abingtonwastewater.org/management/lackawanna-river-information/ (accessed on 30 May 2022).
- Access NEPA—A Round of Applause for the Lackawanna River. Available online: https://accessnepa.com/quills/a-round-of-applause-for-the-lackawanna-river#:~:text=A%20statewide%20competition%20that%20began,2020%20%E2%80%9CRiver%20of%20the%20Year! (accessed on 30 May 2022).
- Horvath, J. The Times Tribune: Microplastic Pollution Identified in Lackawanna River, 52 Other Pennsylvania Waterways. Available online: https://www.yahoo.com/now/microplastic-pollution-identified-lackawanna-river-050100567.html (accessed on 29 May 2022).
- Reinhard, J. Report: Microplastics Found in Susquehanna and Lackawanna Rivers. Fox 56 Wolf News. Available online: https://fox56.com/news/local/report-microplastics-found-in-susquehanna-and-lackawanna-rivers (accessed on 29 May 2022).
- Lackawanna River Conservation Association. Leggetts Watershed 2020 Final Water Report. Available online: https://img1.wsimg.com/blobby/go/35928d34-4fb2-4795-b916-4e3efac60feb/downloads/Leggetts%20Watershed%20Report_FINAL_November%202020.pdf?ver=1652157387938 (accessed on 29 May 2022).
- Fitzgerald, D.J. Safety Guidelines for Copper in Water. Am. J. Clin. Nutr. 1998, 67, 1098S–1102S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medical News Today: Copper Toxicity, Symptoms and Treatment. Available online: https://www.medicalnewstoday.com/articles/copper-toxicity (accessed on 7 June 2022).
- Rodrigues, E.G.; Bellinger, D.C.; Valeri, L.; Hasan, M.O.S.I.; Quamruzzaman, Q.; Golam, M.; Kile, M.L.; Christiani, D.C.; Wright, R.O.; Mazumdar, M. Neurodevelopmental Outcomes among 2- to 3-Year-Old Children in Bangladesh with Elevated Blood Lead and Exposure to Arsenic and Manganese in Drinking Water. Environ. Health 2016, 15, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, M.J.; Margolis, S. Lead in Drinking Water and Human Blood Lead Levels in the United States. Morb. Mortal. Wkly. Rep. 2012, 61, 1–9. [Google Scholar]
- Steenland, K.; Boffetta, P. Lead and Cancer in Humans: Where Are We Now? Am. J. Ind. Med. 2000, 38, 295–299. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry. Public Health Statement. Available online: https://www.atsdr.cdc.gov/ToxProfiles/tp60-c1-b.pdf (accessed on 30 May 2022).
- Minnesota Department of Public Health. Manganese in Drinking Water. Available online: https://www.health.state.mn.us/communities/environment/water/contaminants/manganese.html#HealthEffects (accessed on 30 May 2022).
- Peninsula Water Conditioning, Inc. What Are the Harmful Effects of Iron in Drinking Water? Available online: https://www.peninsulawater.com/is-iron-in-drinking-water-harmful/#:~:text=Effects%20on%20Your%20Health,liver%2C%20pancreas%2C%20and%20heart. (accessed on 30 May 2022).
- Water Quality Association. Cadmium. Available online: https://www.wqa.org/Learn-About-Water/Common-Contaminants/Cadmium (accessed on 30 May 2022).
- Nucor Skyline. Lake Scranton Dam Rehabilitation. Available online: https://www.nucorskyline.com/file%20library/document%20library/english/case%20studies/cs_lake-scranton-dam_en.pdf (accessed on 30 May 2022).
- Commonwealth of Pennsylvania. Pennsylvania Code & Bulletin. Available online: http://www.pacodeandbulletin.gov/ (accessed on 5 June 2022).
- Pennsylvania Fish and Boat Commission. Trout. Available online: https://www.fishandboat.com:443/Fish/PennsylvaniaFishes/Trout/Pages/default.aspx (accessed on 5 June 2022).
- Hook and Bullet. Rocky Glen Dam Fishing near Moosic, Pennsylvania. Available online: https://www.hookandbullet.com/fishing-rocky-glen-dam-moosic-pa/ (accessed on 5 June 2022).
- The Lackawanna River Corridor Association. Lackawanna River Watershed Conservation Plan; The Lackawanna River Conservation Association: Scranton, PA, USA, 2001. [Google Scholar]
- The US Environmental Protection Agency. What Is Superfund? Available online: https://www.epa.gov/superfund/what-superfund (accessed on 10 May 2022).
- The US Environmental Protection Agency. Lackawanna Refuse Site Profile. Available online: https://cumulis.epa.gov/supercpad/SiteProfiles/index.cfm?fuseaction=second.Cleanup&id=0301220#bkground (accessed on 22 May 2022).
- The US Environmental Protection Agency. Aladdin Plating Site Profile. Available online: https://cumulis.epa.gov/supercpad/SiteProfiles/index.cfm?fuseaction=second.cleanup&id=0301099#bkground (accessed on 29 May 2022).
- Environmental Working Group’s Tap Water Database: What’s in Your Drinking Water? Available online: https://www.ewg.org/tapwater/system.php?pws=PA2359008?pws=PA2359008 (accessed on 3 May 2022).
- Pennsylvania Department of Environmental Protection. Lackawanna River Watershed TMDL: Lackawanna, Luzerne, Susquehanna, and Wayne Counties. Available online: https://www.dep.state.pa.us/dep/deputate/watermgt/wqp/wqstandards/tmdl/LackawannaTMDL_FINAL.pdf (accessed on 3 May 2022).
- Austin, J.; Sinha, A.; Stone, N.; Reed, W.; Daniels, M.; Haggard, B.E. How to Collect Your Water Sample and Interpret the Results for the Fish Pond; Arkansas Water Resource Center: Fayetteville, AR, USA, 2016; pp. 1–12. [Google Scholar]
- The US Environmental Protection Agency. Method 3005A: Acid Digestion of Waters for Total Recoverable or Dissolved Metals for Analysis by FLAA or ICP Spectroscopy. Available online: https://www.epa.gov/sites/default/files/2015-12/documents/3005a.pdf (accessed on 3 May 2022).
- National Academies. Allowable Levels of Copper in Drinking Water Should Not Be Increased Until Studies Are Done|National Academies. Available online: https://www.nationalacademies.org/news/2000/03/allowable-levels-of-copper-in-drinking-water-should-not-be-increased-until-studies-are-done (accessed on 5 May 2022).
- The US Environmental Protection Agency. Drinking Water Regulations and Contaminants. Available online: https://www.epa.gov/sdwa/drinking-water-regulations-and-contaminants (accessed on 17 July 2022).
- The Wisconsin Department of Natural Resources. Iron in Drinking Water. Available online: https://dnr.wi.gov/files/pdf/pubs/dg/dg0035.pdf (accessed on 5 May 2022).
- Byman, D.H.; Townsend, D.S.; Calabro, J.C.; Ciampichini, R.M.; Griguts, M.; Neureuter, J.; Pratt, A.R.; Spott, G. Effects of Three Sewage Treatment Plants on Water Chemistry of the Lackawanna River (Lackawanna County, Pennsylvania). J. Pa. Acad. Sci. 2004, 78, 29–33. [Google Scholar]
- The US Environmental Protection Agency. Deleted National Priorities List (NPL) Sites—By State. Available online: https://www.epa.gov/superfund/deleted-national-priorities-list-npl-sites-state (accessed on 30 May 2022).
- LennTech. Copper (Cu)—Chemical Properties, Health and Environmental Effects. Available online: https://www.lenntech.com/periodic/elements/cu.htm (accessed on 5 May 2022).
- LennTech. Lead (Pb) and Water. Available online: https://www.lenntech.com/periodic/water/lead/lead-and-water.htm (accessed on 5 May 2022).
- LennTech. Lead (Pb)—Chemical Properties, Health and Environmental Effects. Available online: https://www.lenntech.com/periodic/elements/pb.htm (accessed on 5 May 2022).
- Chowdhury, S.; Mazumder, M.A.J.; Al-Attas, O.; Husain, T. Heavy Metals in Drinking Water: Occurrences, Implications, and Future Needs in Developing Countries. Sci. Total Environ. 2016, 569–570, 476–488. [Google Scholar] [CrossRef] [PubMed]
- LennTech. Iron (Fe) and Water. Available online: https://www.lenntech.com/periodic/water/iron/iron-and-water.htm (accessed on 10 May 2022).
- Fanning, C. The Happenings Magazine: The Lackawanna River A Vital Resource. Available online: https://www.happeningsmagazinepa.com/2022/03/28/68613/ (accessed on 18 July 2022).
- Liu, X.; Yang, H.; Yan, X.; Xu, S.; Fan, Y.; Xu, H.; Ma, Y.; Hou, W.; Javed, R.; Zhang, Y. Co-Exposure of Polystyrene Microplastics and Iron Aggravates Cognitive Decline in Aging Mice via Ferroptosis Induction. Ecotoxicol. Environ. Saf. 2022, 233, 113342. [Google Scholar] [CrossRef] [PubMed]
- Pittura, L.; Avio, C.G.; Giuliani, M.E.; d’Errico, G.; Keiter, S.H.; Cormier, B.; Gorbi, S.; Regoli, F. Microplastics as Vehicles of Environmental PAHs to Marine Organisms: Combined Chemical and Physical Hazards to the Mediterranean Mussels, Mytilus Galloprovincialis. Front. Mar. Sci. 2018, 5, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Selvi, A.; Rajasekar, A.; Theerthagiri, J.; Ananthaselvam, A.; Sathishkumar, K.; Madhavan, J.; Rahman, P.K.S.M. Integrated Remediation Processes Toward Heavy Metal Removal/Recovery From Various Environments-A Review. Front. Environ. Sci. 2019, 7, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Bruggers, J. Coal Powered the Industrial Revolution:It Left behind an ‘Absolutely Massive’ Environmental Catastrophe. Available online: https://www.alleghenyfront.org/coal-powered-the-industrial-revolution-it-left-behind-an-absolutely-massive-environmental-catastrophe/ (accessed on 20 July 2022).



| Collection Site | Geographical Coordinates | Elevation (Feet) |
|---|---|---|
| Lake Scranton | 41°23′50″ N 75°37′32″ W | 1280 |
| Griffin Reservoir | 41°29′48″ N 75°39′48″ W | 1350 |
| Keyser Creek | 41°23′34″ N 75°42′10″ W | 680 |
| Lackawanna River | 41°24′57″ N 75°39′50″ W | 690 |
| Sample | [Cu] (ppm) | [Fe] (ppm) |
|---|---|---|
| Griffin Reservoir | 0.100 | NA |
| Lackawanna River | NA | 0.380 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dumancas, G.G.; Butka, J.R.; Mehall, A.M. Comprehensive Examination of Cu, Pb, Zn, Fe, Mn and Cd in Lackawanna County Waters, Northeastern Pennsylvania: A Brief Report. Analytica 2022, 3, 302-311. https://doi.org/10.3390/analytica3030021
Dumancas GG, Butka JR, Mehall AM. Comprehensive Examination of Cu, Pb, Zn, Fe, Mn and Cd in Lackawanna County Waters, Northeastern Pennsylvania: A Brief Report. Analytica. 2022; 3(3):302-311. https://doi.org/10.3390/analytica3030021
Chicago/Turabian StyleDumancas, Gerard G., Jake R. Butka, and Adam M. Mehall. 2022. "Comprehensive Examination of Cu, Pb, Zn, Fe, Mn and Cd in Lackawanna County Waters, Northeastern Pennsylvania: A Brief Report" Analytica 3, no. 3: 302-311. https://doi.org/10.3390/analytica3030021