A New Synthesis of Polyheterocyclic Compounds Containing Nitrogen and Boron Atoms †
Abstract
:1. Introduction
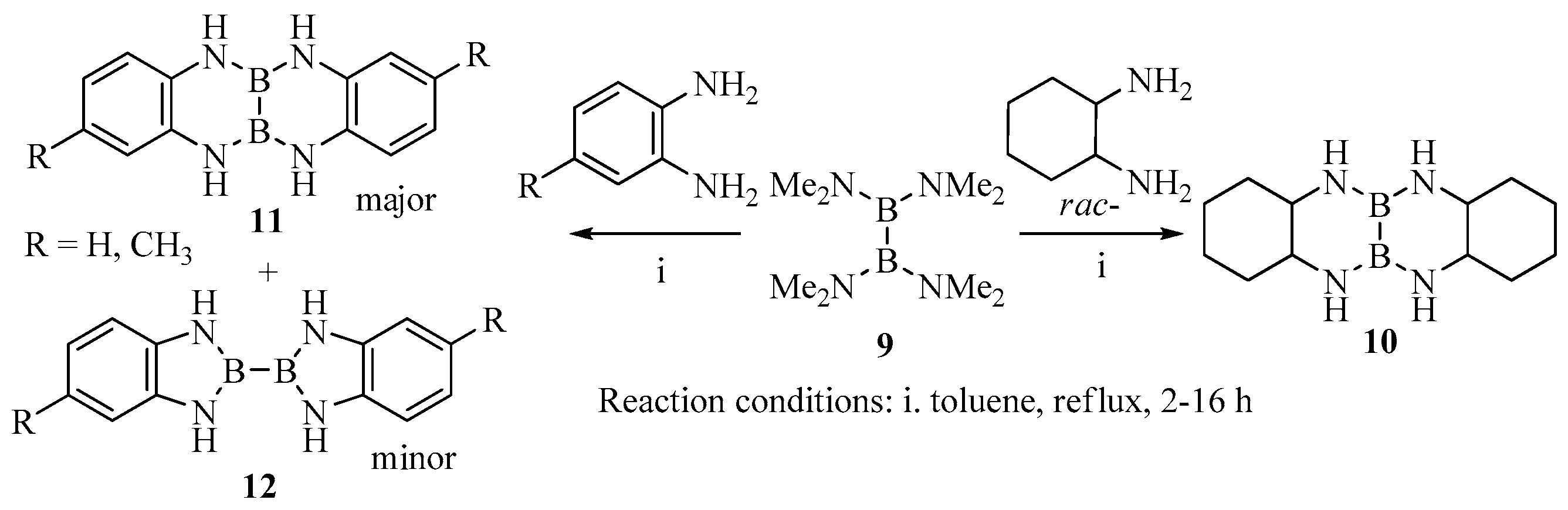
2. Results and Discussion
3. Conclusions
4. Experimental Part
- Heterocyclization of polyamines with tetrakis(dimethylamino)diborane (general procedure). A round-bottom flask mounted on a magnetic stirrer was charged with a solution of corresponding polyamine (2.00 mmol) in 5 mL of ethanol and was cooled in an ice bath at 0 °C, then tetrakis(dimethylamino)diborane (2.00 mmol) in 5 mL of ethanol was added. The mixture was stirred at 0 °C for 2 h and was left in the cold for 12 h. The resulting precipitate was filtered off. Pure compounds 13, 15, 17 were thus isolated as white powders.
- Hexahydro-3H,6H-2a,5,6,8a-tetraaza-5a,8b-diboraacenaphthylene (13): Yield 35%. 1H NMR: δ 2.53–2.59 (m, 8H), 2.62–2.65 (m, 4H). 13C NMR spectrum: δ 41.0, 49.0, 51.1. 11B{1H} NMR: δ 1.40 (br. s).
- Decahydro-2a,6,7,10a-tetraaza-6a,10b-diboracyclopenta[ef]heptalene (15): Yield 39%. 1H NMR: δ 2.43–2.47 (m, 4H), 2.54–2.59 (m, 8H), 2.64–2.69 (m, 4H). 13C NMR spectrum: δ 35.7, 46.5, 46.9, 50.2.
- Decahydro-1H-3a,7,8,11a-tetraaza-7a,11b-diborabenzo[ef]heptalene (17): Yield 42%. 1H NMR: δ 2.15–2.19 (m, 2H), 2.47–2.51 (m, 4H), 2.57–2.63 (m, 8H), 2.66–2.69 (m, 4H). 13C NMR spectrum: δ 28.2, 32.4, 45.2, 45.7, 49.5.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kerru, N.; Gummidi, L.; Maddila, S.; Gangu, K.K.; Jonnalagadda, S.B. A review on recent advances in nitrogen-containing molecules and their biological applications. Molecules 2020, 25, 1909. [Google Scholar] [CrossRef]
- Argese, M.; Ripa, G.; Scala, A.; Valle, V. Process for the Preparation of Tetraazamacrocycles. Patent № US6114521 A, 5 September 2000. [Google Scholar]
- Argese, M.; Brocchetta, M.; Manfredi, G.; Rebasti, F.; Ripa, G. A Process for the Preparation of Decahydro-2a,4a,6a,8a-tetraazacyclopent[f,g]acenaphthylene and Functionalized Derivatives. Patent № EP1272493 B1, 25 October 2001. [Google Scholar]
- Sandnes, R.; Vasilevskis, J.; Undheim, K.; Gacek, M. Process for Tetraazacycloalkane Preparation. Patent № EP0815090 B1, 19 September 1996. [Google Scholar]
- Herve, G.; Bernard, H.; Le Bris, N.; Le Baccon, M.; Yaquanc, J.-J.; Handel, H. Condensation of glyoxal with triethylenetetraamine. Stereochemistry, cyclization and deprotection. Tetrahedron Lett. 1999, 40, 2517–2520. [Google Scholar] [CrossRef]
- Herve, G.; Bernard, H.; Toupet, L.; Handel, H. Condensation of Glyoxal with Triethylenetetraamine; Isomerization and Cyclization. Eur. J. Org. Chem. 2000, 2000, 33–35. [Google Scholar] [CrossRef]
- Desogere, P.; Bernhard, C.; Goze, C.; Penouilh, M.-J.; Rousselin, Y.; Denat, F. Selectively Functionalized Constrained Polyazamacrocycles: Building Blocks for Multifunctional Chelating Agents. Eur. J. Org. Chem. 2013, 2013, 1538–1545. [Google Scholar] [CrossRef]
- Prokhorov, A.; Le Bris, N.; Bernard, H.; Claudon, G.; Handel, H. Cyclohexanedione Bisaminals as Intermediates for Cyclen, Homocyclen, and Cyclam Synthesis. Synth. Commun. 2006, 36, 3271–3282. [Google Scholar] [CrossRef]
- Xie, X.; Haddow, M.F.; Mansell, S.M.; Norman, N.C.; Russell, C.A. Diborane(4)compounds with bidentate diaminogroups. Dalton Trans. 2012, 41, 2140–2147. [Google Scholar] [CrossRef] [PubMed]
- Alibadi, M.A.M.; Batsanov, A.S.; Bramham, G.; Charmant, J.P.H.; Haddow, M.F.; MacKay, L.; Mansell, S.M.; Mc Grady, J.E.; Norman, N.C.; Roffey, A.; et al. 1,1- and 1,2-isomers of the diborane(4)compound B2{1,2-(NH)2C6H4}2 and a TCNQCo-crystal of the 1,1-isomer. Dalton Trans. 2009, 5348–5354. [Google Scholar] [CrossRef] [PubMed]
- Rakhimova, E.B.; Kirsanov, V.Y.; Mescheryakova, E.S.; Khalilov, L.M.; Ibragimov, A.G.; Dzhemileva, L.U.; D’yakonov, V.A.; Dzhemilev, U.M. First example of catalytic synthesis of difurazanohexahydrohexaazapyrenes and in vitro study of their antitumor activity. ACS Med. Chem. Lett. 2019, 10, 378–382. [Google Scholar] [CrossRef]
- Rakhimova, E.B.; Kirsanov, V.Y.; Tret’yakova, E.V.; Khalilov, L.M.; Ibragimov, A.G.; Dzhemileva, L.U.; D’yakonov, V.A.; Dzhemilev, U.M. Synthesis, structure, and antitumor activity of 2,9-disubstituted perhydro2,3a,7b,9,10a,14b-hexaazadibenzotetracenes. RSC Adv. 2020, 10, 21039–21048. [Google Scholar] [CrossRef] [PubMed]
- Rakhimova, E.B.; Kirsanov, V.Y.; Kuzmina, U.S.; Galyautdinov, I.V.; Vakhitova, Y.V. Synthesis and cytotoxic activity of new hexaazadibenzotetracenes derived from trans-1,2-diaminocyclohexane. Mendeleev Commun. 2023, 33, 112–114. [Google Scholar] [CrossRef]

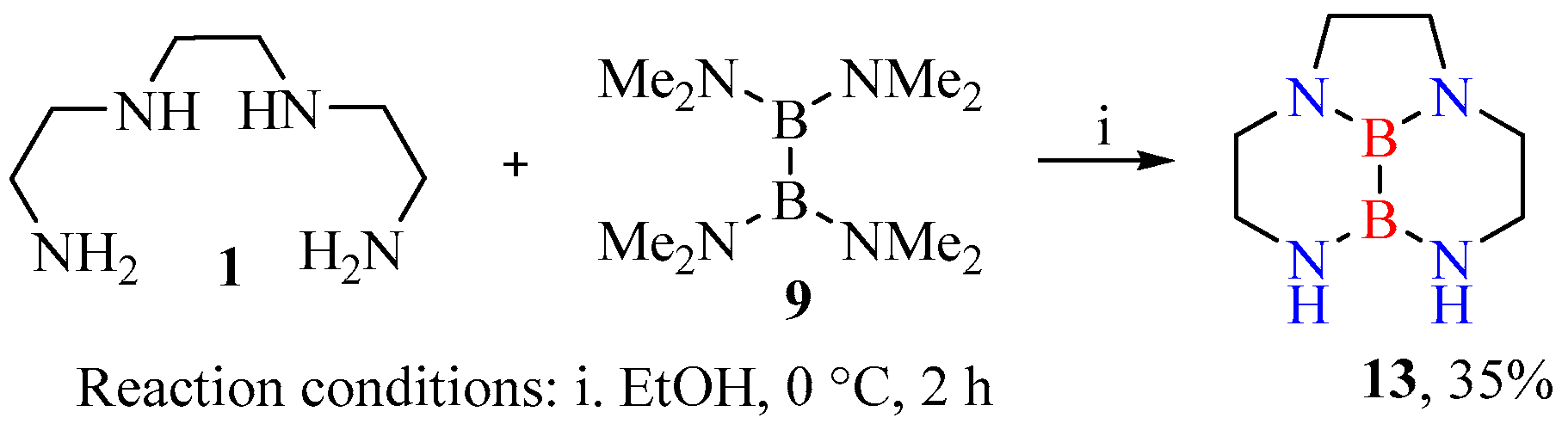

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirsanov, V.Y.; Rakhimova, E.B. A New Synthesis of Polyheterocyclic Compounds Containing Nitrogen and Boron Atoms. Chem. Proc. 2023, 14, 31. https://doi.org/10.3390/ecsoc-27-16103
Kirsanov VY, Rakhimova EB. A New Synthesis of Polyheterocyclic Compounds Containing Nitrogen and Boron Atoms. Chemistry Proceedings. 2023; 14(1):31. https://doi.org/10.3390/ecsoc-27-16103
Chicago/Turabian StyleKirsanov, Victor Yu, and Elena B. Rakhimova. 2023. "A New Synthesis of Polyheterocyclic Compounds Containing Nitrogen and Boron Atoms" Chemistry Proceedings 14, no. 1: 31. https://doi.org/10.3390/ecsoc-27-16103
APA StyleKirsanov, V. Y., & Rakhimova, E. B. (2023). A New Synthesis of Polyheterocyclic Compounds Containing Nitrogen and Boron Atoms. Chemistry Proceedings, 14(1), 31. https://doi.org/10.3390/ecsoc-27-16103