Optimization, First-Order Hyperpolarizability Studies of o, m, and p-Cl Benzaldehydes Using DFT Studies †
Abstract
:1. Introduction
2. Computational Details
3. Results and Discussion
3.1. Molecular Geometry
3.2. NLO
3.3. Molecular Electrostatic Potential Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Datta, A.; Pati, S.K. Effects of dipole orientations on nonlinear optical properties of oxo-bridged dinitroaniline systems. J. Phys. Chem. A 2004, 108, 320. [Google Scholar] [CrossRef]
- Petrosyan, A.M. Salts of l-histidine as nonlinear optical materials: A review. J. Cryst. Phys. Chem. 2010, 1, 33–56. [Google Scholar]
- Karakas, A.; Elmali, A.; Unver, H.; Svoboda, I. Nonlinear optical properties of some derivatives of salicylaldimine-based ligands. J. Mol. Struct. 2004, 702, 103. [Google Scholar] [CrossRef]
- Mendes, P.J.; Ramalho, J.P.P.; Candeias, A.J.E.; Robalo, M.P.; Garcia, M.H. Density functional theory calculations on η5-monocyclopentadienylnitrilecobalt complexes concerning their second-order nonlinear optical properties. J. Mol. Struct. (Theochem.) 2005, 729, 109. [Google Scholar]
- Ahmed, A.B.; Elleuch, N.; Feki, H.; Abid, Y.; Minot, C. Vibrational spectra and non-linear optical proprieties of l-histidine oxalate: DFT studies. Spectrochim. Acta A 2011, 79, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, C.; Joe, I.H. Electronic absorption and vibrational spectra and nonlinear optical properties of 4-methoxy-2-nitroaniline. Phys. Chem. Chem. Phys. 2010, 12, 9452–9460. [Google Scholar]
- Borbone, F.; Carella, A.; Roviello, A.; Casalboni, M.; De Matteis, F.; Stracci, G.; della Rovere, F.; Evangelisti, A.; Dispenza, M. Outstanding poling stability of a new cross-linked nonlinear optical (NLO) material from a low molecular weight chromophore. J. Phys. Chem. B 2011, 115, 11993–12000. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, B.B.; Spiteller, M. Physical optical properties and crystal structures of organic 5-sulfosalicylates–Theoretical and experimental study. J. Mol. Struct. 2011, 1003, 1–9. [Google Scholar] [CrossRef]
- Linet, J.M.; Das, S.J. Optical, mechanical and transport properties of unidirectional grown l-tartaric acid bulk single crystal for non-linear optical application. Mater. Chem. Phys. 2011, 126, 886–890. [Google Scholar]
- Sajan, D.; Ravindra, H.J.; Misra, N.; Joe, I.H. Intramolecular charge transfer and hydrogen bonding interactions of nonlinear optical material N-benzoyl glycine: Vibrational spectral study. Vib. Spectrosc. 2010, 54, 72–80. [Google Scholar] [CrossRef]
- Chis, V.; Oltean, M.; Pirnau, A.; Miclaus, V.; Filip, S. Spectral and theoretical studies of 2-naphthalenol: An organic nonlinear optical crystalline material. J. Optoelectron. Adv. Mater. 2006, 8, 1143–1147. [Google Scholar]
- Atkins, P.; Jones, L. Chemistry: Molecules Matter and Change; W.H. Freeman and Co.: New York, NY, USA, 1997. [Google Scholar]
- Available online: http://www.intermesh.net/Benzald.htm (accessed on 1 November 2023).
- Available online: https://en.wikipedia.org/wiki/Benzaldehyde (accessed on 1 November 2023).
- Bednarek, P.; Bally, T.; Gebicki, J. Characterization of Rotameric Mixtures in o- and m-Substituted Benzaldehydes by Matrix Isolation IR Spectroscopy. J. Org. Chem. 2002, 67, 1319. [Google Scholar] [PubMed]
- Ribeiro-Claro, P.J.A.; de Carvalho, L.A.E.B.; Amado, A.M. Evidence of dimerization through C—H···O interactions in liquid 4-methoxybenzaldehyde from Raman spectra and ab initio calculations. J. Raman Spectrosc. 1997, 28, 867. [Google Scholar] [CrossRef]
- Karger, N.; da Costa, A.M.A.; Ribeiro-Claro, P.J.A. C−H---O bonded dimers in liquid 4-methoxybenzaldehyde: A study by NMR, vibrational spectroscopy, and ab initio calculations. J. Phys. Chem. A 1999, 103, 8672. [Google Scholar] [CrossRef]
- Ribeiro-Claro, P.J.A.; Drew, M.G.B.; Felix, V. C–H⋯ O bonded dimers in 2-methoxy-benzaldehyde studied by X-ray crystallography, vibrational spectroscopy, and ab initio calculations. Chem. Phys. Lett. 2002, 356, 318. [Google Scholar] [CrossRef]
- Marques, M.P.M.; da Costa, A.M.A.; Ribeiro-Claro, P.J.A. Evidence of C− H⊙⊙⊙ O Hydrogen Bonds in Liquid 4-Ethoxybenzaldehyde by NMR and Vibrational Spectroscopies. J. Phys. Chem. A 2001, 105, 5292. [Google Scholar] [CrossRef]
- Schaeffer, T.; Cox, K.J.; Sebastian, R. Experimental and theoretical conformer distributions of 3-methylbenzaldehyde. Can. J. Chem. 1991, 69, 908. [Google Scholar] [CrossRef]
- Anjaneyulu, A.; Rao, G.R. Vibrational analysis of substituted benzaldehydes: Part I. Vibrational spectra, normal co-ordinate analysis and transferability of force constants of monohalogenated. Spectrochim. Acta A 1999, 55, 749. [Google Scholar]
- Ahmad, S.; Verma, P.K. Laser Raman and infrared and far infrared spectra of 3, 4, 5-trimethoxybenzaldehyde. Ind. J. Phys. 1990, 64B, 50. [Google Scholar]
- Aralakkanavar, M.K.; Katti, N.R.; Jeeragal, P.R.; Kalakoti, G.B.; Rao, R.; Shashidhar, M.A. π*← π systems in the electronic absorption spectra of some trisubstituted benzenes. Spectrochim. Acta 1992, 48A, 983. [Google Scholar]
- Yadav, R.A.; Singh, I.S. Vibrational studies, barrier height and thermodynamic functions for biomolecules: 5-Trifluoromethyl uraci. Ind. J. Phys. 1994, 58B, 556. [Google Scholar]
- Singh, D.N.; Singh, I.D.; Yadav, R.A. Vibrational Spectra and Force Fields for 2, 3-; 2, 4-; 2, 5-and 3, 4-Dihydroxybenzaldehydes. Ind. J. Phys. 2002, 76B, 35. [Google Scholar]
- Hinchliffe, A.; Munn, R.W. Molecular Electromagnetism. In Molecular Electromagnetism; John Wiley and Sons Ltd.: Chichester, UK, 1985. [Google Scholar]
- Lampert, H.; Mikenda, W.; Karpfen, A. Molecular geometries and vibrational spectra of phenol, benzaldehyde, and salicylaldehyde: Experimental versus quantum chemical data. J. Phys. Chem. A 1997, 101, 2254. [Google Scholar]
- Mollendal, H.; Gundersen, S.; Tafipolsky, M.A.; Volden, H.V. The molecular structure of benzene derivatives, part 2: 4-chloro-benzaldehyde by joint analysis of gas electron diffraction, microwave spectroscopy and ab initio molecular orbital calculations. J. Mol. Struct. 1998, 444, 47. [Google Scholar] [CrossRef]
- Bhattacharjee, D.; Ghosh, A.; Mishra, T.N. Solvent-Induced Vibrational Relaxation in Benzaldehyde. Bull. Chem. Soc. Jpn. 1995, 68, 1269. [Google Scholar]
- Das, K.; Kumar, J. Solvent-dependent study of anisotropy shift in the C=O stretching mode of benzaldehyde. Raman Spectrosc. 1999, 30, 563. [Google Scholar]
- Speakman, L.D.; Papas, B.N.; Woodcook, H.L.; Schaefer, H.F. The microwave and infrared spectroscopy of benzaldehyde: Conflict between theory and experimental deductions. J. Chem. Phys. 2004, 120, 4247. [Google Scholar] [CrossRef] [PubMed]
- Kushto, G.P.; Jagodzinski, P.W. Vibrational spectra and normal coordinate analysis of 4-(dimethylamino) benzaldehyde and selected isotopic derivatives. Spectrochim. Acta 1998, 54A, 799. [Google Scholar] [CrossRef]
- Kushto, G.P.; Jagodzinski, P.W. Formation of a ground state twisted-internal-charge-transfer conformer of 4-(dimethylamino) benzaldehyde. J. Mol. Struct. 2000, 516, 215. [Google Scholar]
- Ribeiro-Claro, P.J.A.; Marques, M.P.M.; Amado, A.M. Experimental and Theoretical Evidence of C—H⋅⋅⋅O Hydrogen Bonding in Liquid 4-Fluorobenzaldehyde. ChemPhysChem 2002, 3, 599. [Google Scholar]
- Qayyum, M.; Reddy, B.V.; Rao, G.R. Vibrational analysis of mononitro substituted benzamides, benzaldehydes and toluenes: Part I. Vibrational spectra, normal coordinate analysis and transferability of force constants of nitrobenzamides, nitrobenzaldehydes and nitrotoluenes. Spectrochim. Acta A 2004, 60, 279. [Google Scholar] [CrossRef] [PubMed]
- Akai, N.; Kudoh, S.; Takayanagi, M.; Nakata, M. Photoinduced rotational isomerization mechanism of 2-chlorobenzaldehyde in low-temperature rare-gas matrices by vibrational and electronic spectroscopies. J. Photochem. Photobiol. A 2002, 150, 93. [Google Scholar]
- Jeeragal, P.R.; Kalakoti, G.B.; Navati, M.S.; Aralakkanavar, M.K.; Shashidhar, M.A. FT-Raman and infrared spectra and vibrational assignments for 3-chloro-4-methoxybenzaldehyde, as supported by ab initio, hybrid density functional theory and normal coordinate calculations. Ind. J. Pure Appl. Phys. 1994, 32, 521. [Google Scholar]
- Shashidhar, M.A.; Shanabhag, P.V.; Ayachit, N.H.; Rao, K.S. infrared and electronic absorption-spectra of 3-cyanobenzaldehydes and 4-cyanobenzaldehydes. Ind. J. Pure Appl. Phys. 1984, 22, 433. [Google Scholar]
- Abdulridha, A.A.; Allah, M.A.A.H.; Makki, S.Q.; Sert, Y.; Salman, H.E.; Balakit, A.A. Corrosion Inhibition of Carbon Steel in 1 M H2SO4 Using New Azo Schiff Compound: Electrochemical, Gravimetric, Adsorption, Surface and DFT Studies. J. Mol. Liq. 2020, 315, 113690. [Google Scholar] [CrossRef]
- Green, J.H.S.; Harrison, D.J. Vibrational spectra of benzene derivatives—XVI. Benzaldehyde and mono-substituted benzaldehydes. Spectrochim. Acta A 1976, 32, 1265. [Google Scholar]
- Pinchas, S. Infrared absorption of aldehydic CH group. Anal. Chem. 1957, 29, 334. [Google Scholar]
- Abraham, R.J.; Mobli, M. Prediction of 1H NMR Coupling Constants with Associative Neural Networks Trained for Chemical Shifts 2004. Available online: http://www.spectroscopyeurope.com (accessed on 1 November 2023).
- Alkorta, I.; Perez, J.J. Molecular polarization potential maps of the nucleic acid bases Int. J. Quantum Chem. 1996, 57, 123–135. [Google Scholar] [CrossRef]
- Scrocco, E.; Tomasi, J. Advances in quantum chemistry. In Advances in Quantum Chemistry; Lowdin, P., Ed.; Academic Press: New York, NY, USA, 1978; Volume 2. [Google Scholar]
- Luque, F.J.; Orozco, M.; Bhadane, P.K.; Gadre, S.R. SCRF calculation of the effect of water on the topology of the molecular electrostatic potential. J. Phys. Chem. 1993, 97, 9380–9384. [Google Scholar] [CrossRef]
- Sponer, J.; Hobza, P. DNA base amino groups and their role in molecular interactions: Ab initio and preliminary density functional theory calculations. Int. J. Quantum Chem. 1996, 57, 959–970. [Google Scholar] [CrossRef]
- Murray, J.S.; Sen, K. Molecular electrostatic potentials: Concepts and applications. In Molecular Electrostatic Potentials, Concepts and Applications; Elsevier: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Politzer, P.; Truhlar, D.G. (Eds.) Chemical applications of atomic and molecular electrostatic potentials: Reactivity, structure, scattering, and energetics of organic, inorganic, and biological. In Chemical Application of Atomic and Molecular Electrostatic Potentials; Plenum: New York, NY, USA, 1981. [Google Scholar]
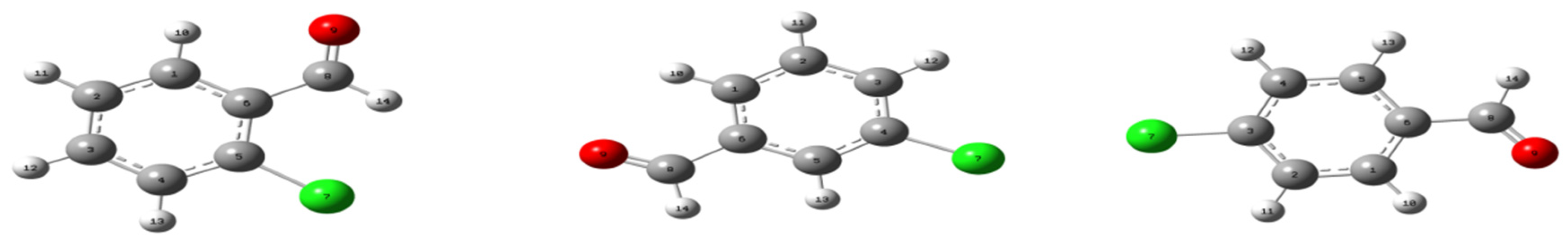

| (a) | |||||
| Bond | Bond Length (Å) | Bond Angle | Value (in 0) | Torsional Angle | Value (in 0) |
| R(1,2) | 1.3892 | A(2,1,6) | 121.242 | D(6,1,2,3) | 0.0 |
| R(1,6) | 1.4055 | A(2,1,10) | 121.7052 | D(6,1,2,11) | 180.0001 |
| R(1,10) | 1.0868 | A(6,1,10) | 117.0528 | D(10,1,2,3) | −180.0 |
| R(2,3) | 1.3987 | A(1,2,3) | 119.5208 | D(10,1,2,11) | 0.0 |
| R(2,11) | 1.0867 | A(1,2,11) | 120.2455 | D(2,1,6,5) | −0.0001 |
| R(3,4) | 1.3949 | A(3,2,11) | 120.2337 | D(2,1,6,8) | −180.0001 |
| R(3,12) | 1.0873 | A(2,3,4) | 120.4875 | D(10,1,6,5) | 180.0 |
| R(4,5) | 1.3948 | A(2,3,12) | 120.2509 | D(10,1,6,8) | 0.0 |
| R(4,13) | 1.0856 | A(4,3,12) | 119.2615 | D(1,2,3,4) | 0.0 |
| R(5,6) | 1.4043 | A(3,4,5) | 119.3706 | D(1,2,3,12) | 180.0 |
| R(5,7) | 1.7638 | A(3,4,13) | 120.9615 | D(11,2,3,4) | 179.9999 |
| R(6,8) | 1.489 | A(5,4,13) | 119.668 | D(11,2,3,12) | −0.0001 |
| R(8,9) | 1.2133 | A(4,5,6) | 121.2421 | D(2,3,4,5) | 0.0 |
| R(8,14) | 1.1071 | A(4,5,7) | 117.5979 | D(2,3,4,13) | −180.0 |
| A(6,5,7) | 121.16 | D(12,3,4,5) | 180.0 | ||
| A(1,6,5) | 118.137 | D(12,3,4,13) | 0.0 | ||
| A(1,6,8) | 118.1085 | D(3,4,5,6) | 0.0 | ||
| A(5,6,8) | 123.7545 | D(3,4,5,7) | −180.0 | ||
| A(6,8,9) | 123.0545 | D(13,4,5,6) | −180.0 | ||
| A(6,8,14) | 115.7743 | D(13,4,5,7) | 0.0 | ||
| A(9,8,14) | 121.1712 | D(4,5,6,1) | 0.0001 | ||
| D(4,5,6,8) | 180.0001 | ||||
| D(7,5,6,1) | 180.0 | ||||
| D(7,5,6,8) | 0.0 | ||||
| D(1,6,8,9) | 0.0021 | ||||
| D(1,6,8,14) | −180.0019 | ||||
| D(5,6,8,9) | 180.0021 | ||||
| D(5,6,8,14) | −0.0019 | ||||
| (b) | |||||
| Bond | Bond Length (Å) | Bond Angle | Value (in 0) | Torsional Angle | Value (in 0) |
| R(1,2) | 1.3911 | A(2,1,6) | 119.6855 | D(6,1,2,3) | −0.0001 |
| R(1,6) | 1.4024 | A(2,1,10) | 121.7567 | D(6,1,2,11) | −180.0001 |
| R(1,10) | 1.0862 | A(6,1,10) | 118.5579 | D(10,1,2,3) | 179.9999 |
| R(2,3) | 1.3993 | A(1,2,3) | 120.4224 | D(10,1,2,11) | −0.0001 |
| R(2,11) | 1.0871 | A(1,2,11) | 120.2862 | D(2,1,6,5) | 0.0001 |
| R(3,4) | 1.3957 | A(3,2,11) | 119.2915 | D(2,1,6,8) | 180.0001 |
| R(3,12) | 1.0857 | A(2,3,4) | 119.3431 | D(10,1,6,5) | −180.0 |
| R(4,5) | 1.3927 | A(2,3,12) | 120.8467 | D(10,1,6,8) | 0.0001 |
| R(4,7) | 1.7585 | A(4,3,12) | 119.8101 | D(1,2,3,4) | 0.0001 |
| R(5,6) | 1.4003 | A(3,4,5) | 121.0311 | D(1,2,3,12) | 180.0001 |
| R(5,13) | 1.0872 | A(3,4,7) | 119.4578 | D(11,2,3,4) | 180.0001 |
| R(6,8) | 1.4847 | A(5,4,7) | 119.5111 | D(11,2,3,12) | 0.0001 |
| R(8,9) | 1.2113 | A(4,5,6) | 119.1364 | D(2,3,4,5) | 0.0 |
| R(8,14) | 1.1145 | A(4,5,13) | 120.3919 | D(2,3,4,7) | −180.0 |
| A(6,5,13) | 120.4717 | D(12,3,4,5) | 180.0 | ||
| A(1,6,5) | 120.3815 | D(12,3,4,7) | 0.0 | ||
| A(1,6,8) | 120.2737 | D(3,4,5,6) | 0.0 | ||
| A(5,6,8) | 119.3448 | D(3,4,5,13) | −180.0001 | ||
| A(6,8,9) | 124.351 | D(7,4,5,6) | 180.0 | ||
| A(6,8,14) | 114.4592 | D(7,4,5,13) | 0.0 | ||
| A(9,8,14) | 121.1898 | D(4,5,6,1) | 0.0 | ||
| D(4,5,6,8) | −180.0 | ||||
| D(13,5,6,1) | −180.0 | ||||
| D(13,5,6,8) | 0.0 | ||||
| D(1,6,8,9) | -0.0004 | ||||
| D(1,6,8,14) | 180.0009 | ||||
| D(5,6,8,9) | −180.0004 | ||||
| D(5,6,8,14) | 0.0009 | ||||
| (c) | |||||
| Bond | Bond Length (Å) | Bond Angle | Value (in 0) | Torsional Angle | Value (in 0) |
| R(1,2) | 1.3896 | A(2,1,6) | 120.3956 | D(6,1,2,3) | −0.0001 |
| R(1,6) | 1.4034 | A(2,1,10) | 121.0573 | D(6,1,2,11) | −180.0001 |
| R(1,10) | 1.0867 | A(6,1,10) | 118.5472 | D(10,1,2,3) | 180.0 |
| R(2,3) | 1.3997 | A(1,2,3) | 118.9399 | D(10,1,2,11) | −0.0001 |
| R(2,11) | 1.0856 | A(1,2,11) | 121.1629 | D(2,1,6,5) | 0.0 |
| R(3,4) | 1.3963 | A(3,2,11) | 119.8973 | D(2,1,6,8) | 180.0001 |
| R(3,7) | 1.7547 | A(2,3,4) | 121.6657 | D(10,1,6,5) | −180.0 |
| R(4,5) | 1.3933 | A(2,3,7) | 119.1476 | D(10,1,6,8) | 0.0 |
| R(4,12) | 1.0854 | A(4,3,7) | 119.1867 | D(1,2,3,4) | 0.0001 |
| R(5,6) | 1.4005 | A(3,4,5) | 118.6961 | D(1,2,3,7) | −179.9999 |
| R(5,13) | 1.0887 | A(3,4,12) | 120.0761 | D(11,2,3,4) | 180.0001 |
| R(6,8) | 1.4813 | A(5,4,12) | 121.2278 | D(11,2,3,7) | 0.0001 |
| R(8,9) | 1.2123 | A(4,5,6) | 120.6123 | D(2,3,4,5) | 0.0 |
| R(8,14) | 1.1148 | A(4,5,13) | 119.7285 | D(2,3,4,12) | −180.0 |
| A(6,5,13) | 119.6591 | D(7,3,4,5) | 180.0 | ||
| A(1,6,5) | 119.6904 | D(7,3,4,12) | 0.0 | ||
| A(1,6,8) | 120.1889 | D(3,4,5,6) | 0.0 | ||
| A(5,6,8) | 120.1207 | D(3,4,5,13) | −180.0 | ||
| A(6,8,9) | 124.4964 | D(12,4,5,6) | 180.0 | ||
| A(6,8,14) | 114.3928 | D(12,4,5,13) | 0.0 | ||
| A(9,8,14) | 121.1107 | D(4,5,6,1) | 0.0 | ||
| D(4,5,6,8) | −180.0 | ||||
| D(13,5,6,1) | −180.0 | ||||
| D(13,5,6,8) | 0.0 | ||||
| D(1,6,8,9) | −0.0005 | ||||
| D(1,6,8,14) | 180.0009 | ||||
| D(5,6,8,9) | −180.0004 | ||||
| D(5,6,8,14) | 0.0009 | ||||
| (a) | |||||
| Dipole Moment | Polarizability | Hyperpolarizability | |||
| µx | −2.7695 | αxx | 111.140 | βxxx | 40.2453 |
| µy | −1.4438 | αyy | −0.255 | βyyy | 133.6728 |
| µz | 0.0824 | αzz | 103.61 | βzzz | −11.011 |
| µ | 3.1243 | αxy | −0.00066 | βxyy | 43.186 |
| αxz | −0.0010 | βxxy | 0.789 | ||
| αyz | 32.396 | βxxz | −0.558 | ||
| α0 | 71.49 | βxzz | −2.142 | ||
| βyzz | −2.189 | ||||
| βyyz | −2.265 | ||||
| βxyz | −0.0012 | ||||
| β0 | 155.86 | ||||
| (b) | |||||
| Dipole Moment | Polarizability | Hyperpolarizability | |||
| µx | 1.4781 | αxx | 124.426 | βxxx | 239.0 |
| µy | 1.1778 | αyy | −0.6923 | βyyy | −47.06 |
| µz | 0.0825 | αzz | 93.120 | βzzz | −95.58 |
| µ | 1.8918 | αxy | −0.0022 | βxyy | −22.57 |
| αxz | 0.0005 | βxxy | −1.57 | ||
| αyz | 32.4588 | βxxz | −0.927 | ||
| α0 | 72.28 | βxzz | −0.31 | ||
| βyzz | 6.567 | ||||
| βyyz | 0.346 | ||||
| βxyz | −0.0004 | ||||
| β0 | 240.81 | ||||
| (c) | |||||
| Dipole Moment | Polarizability | Hyperpolarizability | |||
| µx | −1.1977 | αxx | 136.97 | βxxx | 769.984 |
| µy | 1.7566 | αyy | 0.4221216 | βyyy | 74.092 |
| µz | 0.0824 | αzz | 85.6294302 | βzzz | −9.208 |
| µ | 2.1276 | αxy | 0.0019948 | βxyy | 47.957 |
| αxz | −0.0008 | βxxy | −1.515 | ||
| αyz | 32.4628691 | βxxz | −1.127 | ||
| α0 | 74.34 | βxzz | −0.737 | ||
| βyzz | −2.664 | ||||
| βyyz | 2.678 | ||||
| βxyz | −0.0004 | ||||
| β0 | 820.22 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, R.; Khanam, H.; Pandey, J. Optimization, First-Order Hyperpolarizability Studies of o, m, and p-Cl Benzaldehydes Using DFT Studies. Chem. Proc. 2023, 14, 92. https://doi.org/10.3390/ecsoc-27-16072
Singh R, Khanam H, Pandey J. Optimization, First-Order Hyperpolarizability Studies of o, m, and p-Cl Benzaldehydes Using DFT Studies. Chemistry Proceedings. 2023; 14(1):92. https://doi.org/10.3390/ecsoc-27-16072
Chicago/Turabian StyleSingh, Ruchi, Huda Khanam, and Jyoti Pandey. 2023. "Optimization, First-Order Hyperpolarizability Studies of o, m, and p-Cl Benzaldehydes Using DFT Studies" Chemistry Proceedings 14, no. 1: 92. https://doi.org/10.3390/ecsoc-27-16072
APA StyleSingh, R., Khanam, H., & Pandey, J. (2023). Optimization, First-Order Hyperpolarizability Studies of o, m, and p-Cl Benzaldehydes Using DFT Studies. Chemistry Proceedings, 14(1), 92. https://doi.org/10.3390/ecsoc-27-16072





