The High Performance of Multi-Metal Layered Double Hydroxides (LDHs) in the Removal of Organic Dyes †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of MgNiAl and MgNiFe-LDH
2.2. Characterizations
2.3. Kinetic Studies
2.4. Adsorption Isotherms
3. Results and Discussion
3.1. Characterization of Prepared Samples
3.1.1. XRD Analysis
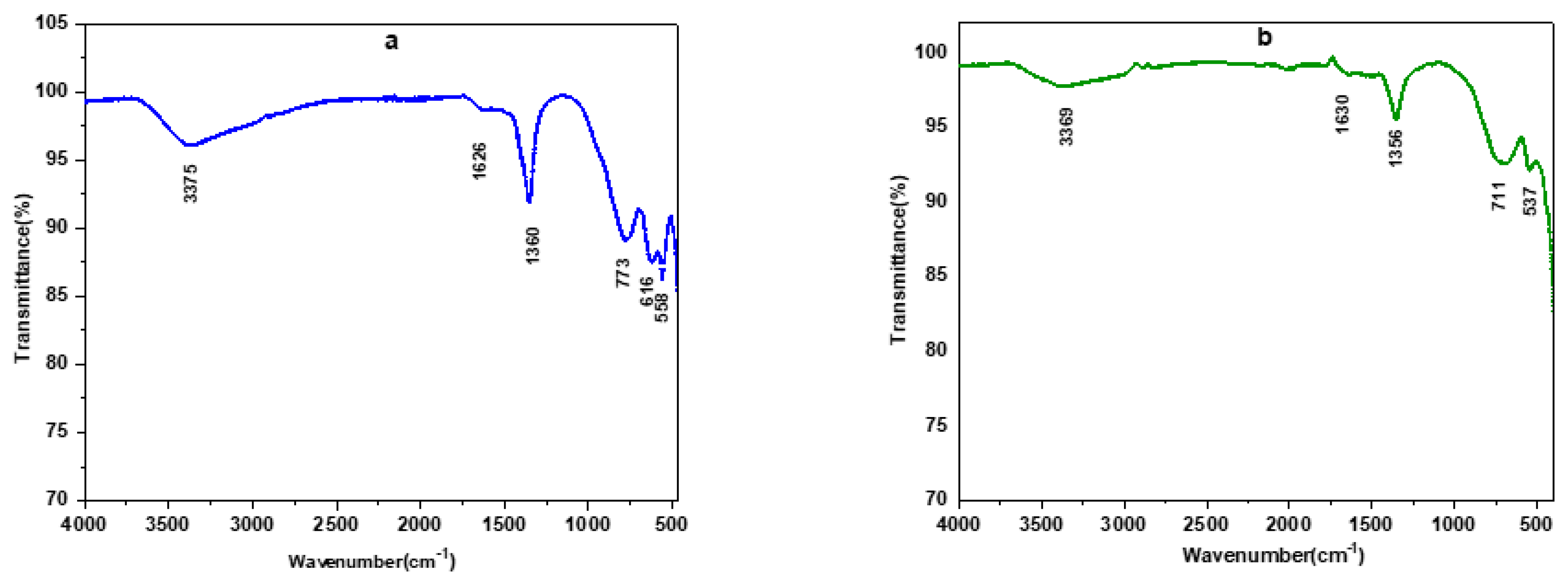
3.1.2. IR Spectroscopy Analysis
3.1.3. TG-DSC
3.2. Kinetic Studies
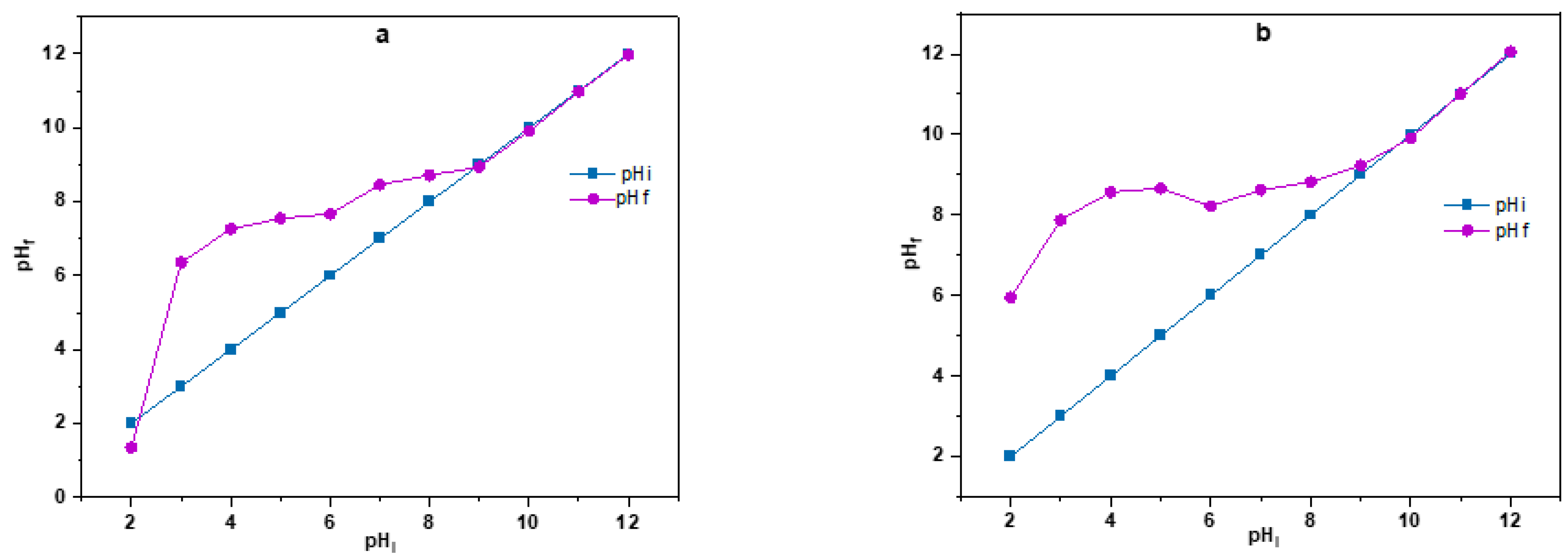
3.2.1. pHpzc
3.2.2. The Effect of Adsorbent Mass
3.2.3. Effect of Contact Time on CR Adsorption
3.2.4. Kinetic Models Applied to the Adsorption of CR onto LDHs
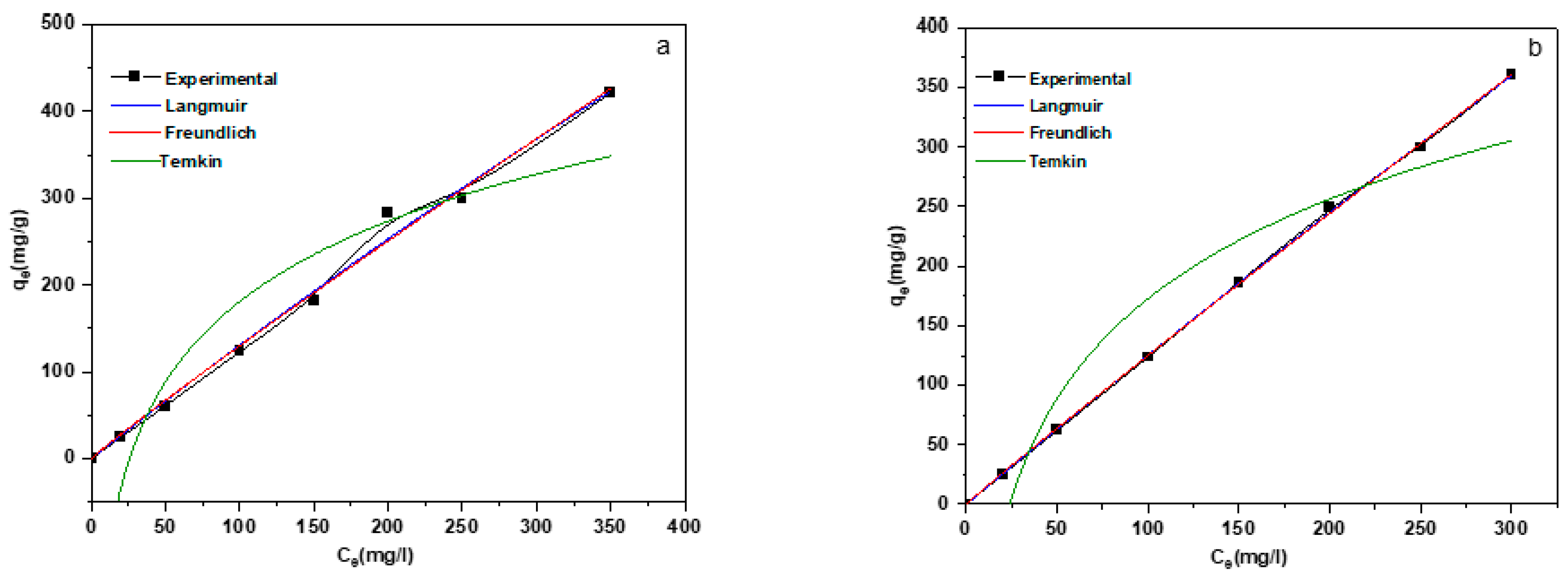
3.3. Adsorption Isotherms
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benkhaya, S.; M’rabet, S.; El Harfi, A. A review on classifications, recent synthesis and applications of textile dyes. Inorg. Chem. Commun. 2020, 115, 107891. [Google Scholar] [CrossRef]
- Krishnamoorthy, R.; Choudhury, A.R.; Jose, P.A.; Suganya, K.; Senthilkumar, M.; Prabhakaran, J.; Gopal, N.O.; Choi, J.; Kim, K.; Anandham, R.; et al. Long-term exposure to azo dyes from textile wastewater causes the abundance of saccharibacteria population. Appl. Sci. 2021, 11, 379. [Google Scholar] [CrossRef]
- Kalwar, N.H.; Sirajuddin Sherazi, S.T.H.; Khaskheli, A.R.; Hallam, K.R.; Scott, T.B.; Tagar, Z.A.; Hassan, S.S.; Soomro, R.A. Fabrication of small l-threonine capped nickel nanoparticles and their catalytic application. Appl. Catal. 2013, 453, 54–55. [Google Scholar] [CrossRef]
- Carneiro, P.A.; Umbuzeiro, G.A.; Oliveira, D.P.; Zanoni, M.V.B. Assessment of water contamination caused by a mutagenic textile effluent/dyehouse effluent bearing disperse dyes. J. Hazard. Mater. 2010, 174, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Holkar, C.R.; Jadhav, A.J.; Pinjari, D.V.; Mahamuni, N.M.; Pandit, A.B. A critical review on textile wastewater treatments: Possible approaches. J. Environ. Manag. 2016, 182, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Ghattas, A.K.; Fischer, F.; Wick, A.; Ternes, T.A. Anaerobic biodegradation of (emerging) organic contaminants in the aquatic environment. Water Res. 2017, 116, 268–295. [Google Scholar] [CrossRef] [PubMed]
- Aboutaleb, W.A.; El-Salamony, R.A. Effect of Fe2O3-CeO2 nanocomposite synthesis method on the Congo red dye photodegradation under visible light irradiation. Mater. Chem. Phys. 2019, 236, 121724. [Google Scholar] [CrossRef]
- Huang, X.; Bo, X.; Zhao, Y.; Gao, B.; Wang, Y.; Sun, S.; Yue, Q.; Li, Q. Effects of compound bioflocculant on coagulation performance and floc properties for dye removal. Bioresour. Technol. 2014, 165, 116–121. [Google Scholar] [CrossRef]
- Dotto, J.; Fagundes-Klen, M.R.; Veit, M.T.; Palácio, S.M.; Bergamasco, R. Performance of different coagulants in the coagulation/flocculation process of textile wastewater. J. Clean. Prod. 2019, 208, 656–665. [Google Scholar] [CrossRef]
- Wang, Y.; Dai, X.; Zhou, Q. Insights into the role of metal cation substitution on the anionic dye removal performance of CoAl-LDH. Colloids Surf A Physicochem. 2022, 636, 128139. [Google Scholar] [CrossRef]
- Sriram, G.; Uthappa, U.T.; Losic, D.; Kigga, M.; Jung, H.Y.; Kurkuri, M.D. MgAl-layered double hydroxide (LDH) modified diatoms for highly efficient removal of Congo red from aqueous solution. Appl. Sci. 2020, 10, 2285. [Google Scholar] [CrossRef]
- Salem, M.A.S.; Khan, A.M.; Manea, Y.K. Nano chromium embedded in f-CNT supported CoBi-LDH nanocomposites for selective adsorption of Pb2+and hazardous organic dyes. Chemosphere 2022, 289, 133073. [Google Scholar] [CrossRef]
- Taher, T.; Rohendi, D.; Mohadi, R.; Lesbani, A. Congo red dye removal from aqueous solution by acid-activated bentonite from sarolangun: Kinetic, equilibrium, and thermodynamic studies. Arab. J. Basic. Appl. Sci. 2019, 26, 125–136. [Google Scholar] [CrossRef]
- El Khanchaoui, A.; Sajieddine, M.; Mansori, M.; Moubarik, A.; Essoumhi, A. Removal of single dye and dye mixture from aqueous solution with alginate-coated calcined layered double hydroxide and illite clay composite beads. Mater. Res. Innov. 2023, 27, 355–370. [Google Scholar] [CrossRef]
- Brahma, D.; Saikia, H. Synthesis of ZrO2/MgAl-LDH composites and evaluation of its isotherm, kinetics and thermodynamic properties in the adsorption of congo red dye. J. Chem. Thermodyn. 2022, 7, 100067. [Google Scholar] [CrossRef]
- Dib, M.; Ouchetto, H.; Akhramez, S.; Fadili, H.; Essoumhi, A.; Ouchetto, K.; Hafid, A.; Sajieddine, M.; Khouili, M. Preparation of Mg/Al-LDH nanomaterials and its application in the condensation of 3-Amino-1-phenyl-2-pyrazolin-5-one with aromatic aldehyde. Mater. Today Proc. 2020, 22, 104–107. [Google Scholar] [CrossRef]
- Ke, G.; Tan, H.; Yang, P.; Pinyu, Z. Preparation and adsorption properties of rod mgnial-ldh. Mater. Sci. Forum. 2019, 956, 294–304. [Google Scholar] [CrossRef]
- Ai, L.; Zhang, C.; Meng, L. Adsorption of methyl orange from aqueous solution on hydrothermal synthesized Mg-Al layered double hydroxide. J. Chem. Eng. Data 2011, 56, 4217–4225. [Google Scholar] [CrossRef]
- Zhong, C.; Su, S.; Xu, L.; Liu, Q.; Zhang, H.; Yang, P.; Zhang, M.; Bai, X.; Wang, J. Preparation of NiAl-LDH/Polypyrrole composites for uranium (VI) extraction from simulated seawater. Colloids Surf. A Physicochem. 2019, 562, 329–335. [Google Scholar] [CrossRef]
- Hu, H.; Wageh, S.; Al-Ghamdi, A.A. NiFeLDH nanosheet/carbon fiber nanocomposite with enhanced anionic dye adsorption performance. Appl. Surf. Sci. 2020, 511, 145570. [Google Scholar] [CrossRef]
- El Khanchaoui, A.; Sajieddine, M.; Mansori, M.; Essoumhi, A. Anionic dye adsorption on ZnAl hydrotalcite-type and regeneration studies based on “memory effect”. J. Environ. Anal. Chem. 2020, 102, 3542–3560. [Google Scholar] [CrossRef]
- Zaghouane-Boudiaf, H.; Boutahala, M.; Arab, L. Removal of methyl orange from aqueous solution by uncalcined and calcined MgNiAl layered double hydroxides (LDHs). J. Chem. Eng. 2012, 187, 142–149. [Google Scholar] [CrossRef]
- Li, S.S.; Jiang, M.; Jiang, T.J.; Liu, J.H.; Guo, Z.; Huang, X.J. Competitive adsorption behavior toward metal ions on nano-Fe/Mg/Ni ternary layered double hydroxide proved by XPS: Evidence of selective and sensitive detection of Pb(II). J. Hazard. Mater. 2017, 338, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ul Hasnain, M.A.; Khoja, A.H.; Butt, F.B.; Ayesha, M.; Saleem, F.; Mehran, M.T.; Liaquat, R.; Ali, M.; Zaki, Z.I. Partial oxidation of methane over CeO2 loaded hydrotalcite (MgNiAl) catalyst for the production of hydrogen rich syngas (H2, CO). Int. J. Hydrog. Energy 2021, 46, 36663–36667. [Google Scholar] [CrossRef]
- Lafi, R.; Charradi, K.; Djebbi, M.A.; Amara, A.B.H.; Hafiane, A. Adsorption study of Congo red dye from aqueous solution to Mg-Al-layered double hydroxide. Adv. Powder Technol. 2016, 27, 232–237. [Google Scholar] [CrossRef]
- Garrison, S. On Points of Zero Charge. Environ. Sci. Technol. 1998, 32, 2815–2819. [Google Scholar] [CrossRef]
- Palapa, N.R.; Taher, T.; Rahayu, B.R.; Mohadi, R.; Rachmat, A.; Lesbani, A. CuAl LDH/Rice husk biochar composite for enhanced adsorptive removal of cationic dye from aqueous solution. Chem. React. Eng. 2020, 15, 527–537. [Google Scholar] [CrossRef]
- Farghali, M.A.; Selim, A.M.; Khater, H.F.; Bagato, M.; Alharbi, W.; Alharbi, K.H.; Radwan, I.T. Optimized adsorption and effective disposal of Congo red dye from wastewater: Hydrothermal fabrication of MgAl-LDH nanohydrotalcite-like materials. Arab. J. Chem. 2022, 15, 104–171. [Google Scholar] [CrossRef]
- Sonal, S.; Prakash, P.; Mishra, B.K.; Nayak, G.C. Synthesis, characterization and sorption studies of a zirconium (IV) impregnated highly functionalized mesoporous actIVated carbonsb. RSC Adv. 2020, 10, 13783–13798. [Google Scholar] [CrossRef]
- Shabbir, R.; Gu, A.; Chen, J.; Khan, M.M.; Wang, P.; Zhang, Z.; Liu, Y.; Yang, Y. Highly efficient removal of congo red and methyl orange by using petal-like Fe-Mg layered double hydroxide. J. Environ. Anal. Chem. 2020, 102, 1060–1077. [Google Scholar] [CrossRef]
- Munir, M.; Nazar, M.F.; Zafar, M.N.; Zubair, M.; Ashfaq, M.; Bandegharaei, A.H.; Khan, S.U.D.; Ahmad, A. Effective Adsorptive Removal of Methylene Blue from Water by Didodecyldimethylammonium Bromide-Modified Brown Clay. ACS Omega 2020, 5, 16711–16721. [Google Scholar] [CrossRef]
- Feng, Z.; Shao, Z.; Yao, J.; Huang, Y.; Chen, Y. Protein adsorption and separation with chitosan-based amphoteric membranes. Polym. J. 2009, 50, 1257–1263. [Google Scholar] [CrossRef]
- Ho, Y.S.; Ofomaja, A.E. Pseudo-second-order model for lead ion sorption from aqueous solutions onto palm kernel fiber. J. Hazard. Mater. 2006, 129, 137–142. [Google Scholar] [CrossRef]
- Wu, F.C.; Tseng, R.L.; Juang, R.S. Initial behavior of intraparticle diffusion model used in the description of adsorption kinetics. J. Chem. Eng. 2009, 153, 1–8. [Google Scholar] [CrossRef]
- Doǧan, M.; Abak, H.; Alkan, M. Adsorption of methylene blue onto hazelnut shell: Kinetics, mechanism and activation parameters. J. Hazard. Mater. 2009, 164, 172–181. [Google Scholar] [CrossRef]
- Kostić, M.; Najdanović, S.; Velinov, N.; Vučić, M.R.; Petrović, M.; Mitrović, J.; Bojić, A. Ultrasound-assisted synthesis of a new material based on MgCoAl-LDH: Characterization and optimization of sorption for progressive treatment of water Environ. Technol. Innov. 2022, 26, 102358. [Google Scholar] [CrossRef]
- Senthil Kumar, P.; Ramalingam, S.; Senthamarai, C. Adsorption of dye from aqueous solution by cashew nut shell: Studies on equilibrium isotherm, kinetics and thermodynamics of interactions. Desalination 2010, 261, 52–60. [Google Scholar] [CrossRef]
- Almoisheer, N.; Alseroury, F.A.; Kumar, R. Adsorption and anion exchange insight of indigo carmine onto CuAl-LDH/SWCNTs nanocomposite: Kinetic, thermodynamic and isotherm analysis. RSC Adv. 2019, 9, 560–568. [Google Scholar] [CrossRef]
- Najafi, M.; Bastami, T.R.; Binesh, N.; Ayati, A.; Emamverdi, S. Sono-sorption versus adsorption for the removal of congo red from aqueous solution using NiFeLDH/Au nanocomposite: Kinetics, thermodynamics, isotherm studies, and optimization of process parameters. J. Ind. Eng. Chem. 2022, 116, 489–503. [Google Scholar] [CrossRef]
- Nyankson, E.; Adjasoo, J.; Efavi, J.K.; Amedalor, R.; Yaya, A.; Manu, G.P.; Asare, K.; Amartey, N.A. Characterization and evaluation of zeolite A/Fe3O4 nanocomposite as a potential adsorbent for removal of organic molecules from wastewater. J. Chem. 2019, 2019, 8090756. [Google Scholar] [CrossRef]
- Crini, G.; Peindy, H.N.; Gimbert, F.; Robert, C. Removal of, C.I. Basic Green 4 (Malachite Green) from aqueous solutions by adsorption using cyclodextrin-based adsorbent: Kinetic and equilibrium studies. Sep. Purif. Technol. 2007, 53, 97–110. [Google Scholar] [CrossRef]
- Khodam, F.; Rezvani, Z.; Amani-Ghadim, A.R. Enhanced adsorption of Acid Red 14 by co-assembled LDH/MWCNTs nanohybrid: Optimization, kinetic and isotherm. J. Ind. Eng. Chem. 2015, 21, 1286–1294. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, B.; Zhang, L.; Yu, J.; Li, Y. Synthesis of MgNiCo LDH hollow structure derived from ZIF-67 as superb adsorbent for Congo red. J. Colloid. Interface Sci. 2022, 612, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Yin, P.; Yang, H. Superb adsorption of organic dyes from aqueous solution on hierarchically porous composites constructed by ZnAl-LDH/Al(OH)3 nanosheets. Microporous Mesoporous Mat. 2018, 259, 123–133. [Google Scholar] [CrossRef]
- Shamsayei, M.; Yamini, Y.; Asiabi, H. Fabrication of zwitterionic histidine/layered double hydroxide hybrid nanosheets for highly efficient and fast removal of anionic dyes. J. Colloid. Interface Sci. 2018, 529, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Tang, M.; Hendriksen, P.V.; Chen, B. Biotemplated fabrication of a 3D hierarchical structure of magnetic ZnFe2O4/MgAl-LDH for efficient elimination of dye from water. J. Alloys Compd. 2020, 829, 154552. [Google Scholar] [CrossRef]
- El Khanchaoui, A.; Sajieddine, M.; Mansori, M.; Essoumhi, A. Calcined ZnAl-LDH trapping performance in alginate beads for adsorption of Congo Red dye. J. Environ. Anal. Chem. 2021. [Google Scholar] [CrossRef]
- Hu, H.; Liu, J.; Xu, Z. Hierarchical porous Ni/Co-LDH hollow dodecahedron with excellent adsorption property for Congo red and Cr(VI) ions. Appl. Surf. Sci. 2019, 478, 981–990. [Google Scholar] [CrossRef]
- El Khanchaoui, A.; Boukontar, N.; Sajieddine, M.; Hnini, K.; Essoumhi, A. Noticeable improvement in adsorption capacity of glycine-modified MgAl-LDH in the removal of Methyl Orange dye compared to urea standard method. Mater. Res. Innov. 2022, 27, 152–162. [Google Scholar] [CrossRef]





| Sample | a (Å) | c (Å) | basal Spacing d003 (Å) | Interlayer Distance d (Å) |
|---|---|---|---|---|
| MgNiAl-LDH | 3.031 | 22.438 | 7.479 | 2.679 |
| MgNiFe-LDH | 3.093 | 23.225 | 7.741 | 2.941 |
| C0 | qe,exp | Pseudo-First Order | Pseudo-Second Order | Intra-particle Diffusion | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| qe,cal | k1 | R2 | qe,cal | k2 | R2 | Kip | C | R2 | ||
| MgNiAl-LDH | ||||||||||
| 50 | 62.287 | 60.890 | 1.158 | 0.993 | 61.791 | 0.059 | 0.997 | 3.179 | 39.069 | 0.340 |
| 100 | 123.650 | 113.477 | 1.508 | 0.998 | 115.477 | 0.035 | 0.978 | 6.522 | 70.254 | 0.400 |
| MgNiFe-LDH | ||||||||||
| 50 | 62.220 | 60.168 | 1.139 | 0.990 | 61.129 | 0.056 | 0.995 | 3.225 | 38.159 | 0.357 |
| 100 | 121.330 | 111.526 | 1.225 | 0.967 | 111.798 | 0.027 | 0.978 | 6.342 | 69.029 | 0.393 |
| Temperature (K) | Langmuir | Freundlich | Temkin | ||||||
|---|---|---|---|---|---|---|---|---|---|
| qm | kL | R2 | KF | 1/n | R2 | KT | B | R2 | |
| MgNiAl-LDH | |||||||||
| 298 | 4043.90 | 3.34035 × 10−4 | 0.991 | 1.638 | 1.053 | 0.988 | 0.038 | 133.436 | 0.899 |
| MgNiFe-LDH | |||||||||
| 298 | 5584.740 | 2.29685 × 10−4 | 0.969 | 1.471 | 1.036 | 0.999 | 0.041 | 120.778 | 0.919 |
| ADSORBENT | ADSORPTION CAPACITY (MG/G) | REFERENCE |
|---|---|---|
| MgNiFe-LDH | 5584 | This work |
| MgNiAl-LDH | 4043 | This work |
| MgNiCo-LDH | 1194 | [43] |
| ZnAl-LDH/Al(OH)3 | 2348 | [44] |
| Histidine/MgAl-LDH | 1112 | [45] |
| ZnFe2O4/MgAl-LDH | 294 | [46] |
| ZnAl-550 | 166 | [47] |
| NiCo-LDH | 909 | [48] |
| ZrO2/MgAl-LDH | 169 | [49] |
| NIFE-LDH | 484 | [20] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talbi, S.; Khanchaoui, A.E.; Bouissane, L.; Hafid, A.; Khouili, M.; Rabi, S.; Essoumhi, A. The High Performance of Multi-Metal Layered Double Hydroxides (LDHs) in the Removal of Organic Dyes. Chem. Proc. 2023, 14, 99. https://doi.org/10.3390/ecsoc-27-16042
Talbi S, Khanchaoui AE, Bouissane L, Hafid A, Khouili M, Rabi S, Essoumhi A. The High Performance of Multi-Metal Layered Double Hydroxides (LDHs) in the Removal of Organic Dyes. Chemistry Proceedings. 2023; 14(1):99. https://doi.org/10.3390/ecsoc-27-16042
Chicago/Turabian StyleTalbi, Soumaya, Amal El Khanchaoui, Latifa Bouissane, Abderrafia Hafid, Mostafa Khouili, Souad Rabi, and Abdellatif Essoumhi. 2023. "The High Performance of Multi-Metal Layered Double Hydroxides (LDHs) in the Removal of Organic Dyes" Chemistry Proceedings 14, no. 1: 99. https://doi.org/10.3390/ecsoc-27-16042
APA StyleTalbi, S., Khanchaoui, A. E., Bouissane, L., Hafid, A., Khouili, M., Rabi, S., & Essoumhi, A. (2023). The High Performance of Multi-Metal Layered Double Hydroxides (LDHs) in the Removal of Organic Dyes. Chemistry Proceedings, 14(1), 99. https://doi.org/10.3390/ecsoc-27-16042






