Markers for Immunological Resilience: Effects of Moderate- and High-Intensity Endurance Exercise on the Kinetic Response of Leukocyte Subsets
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Blood Analysis
2.3. Statistical Analysis and Data
3. Results
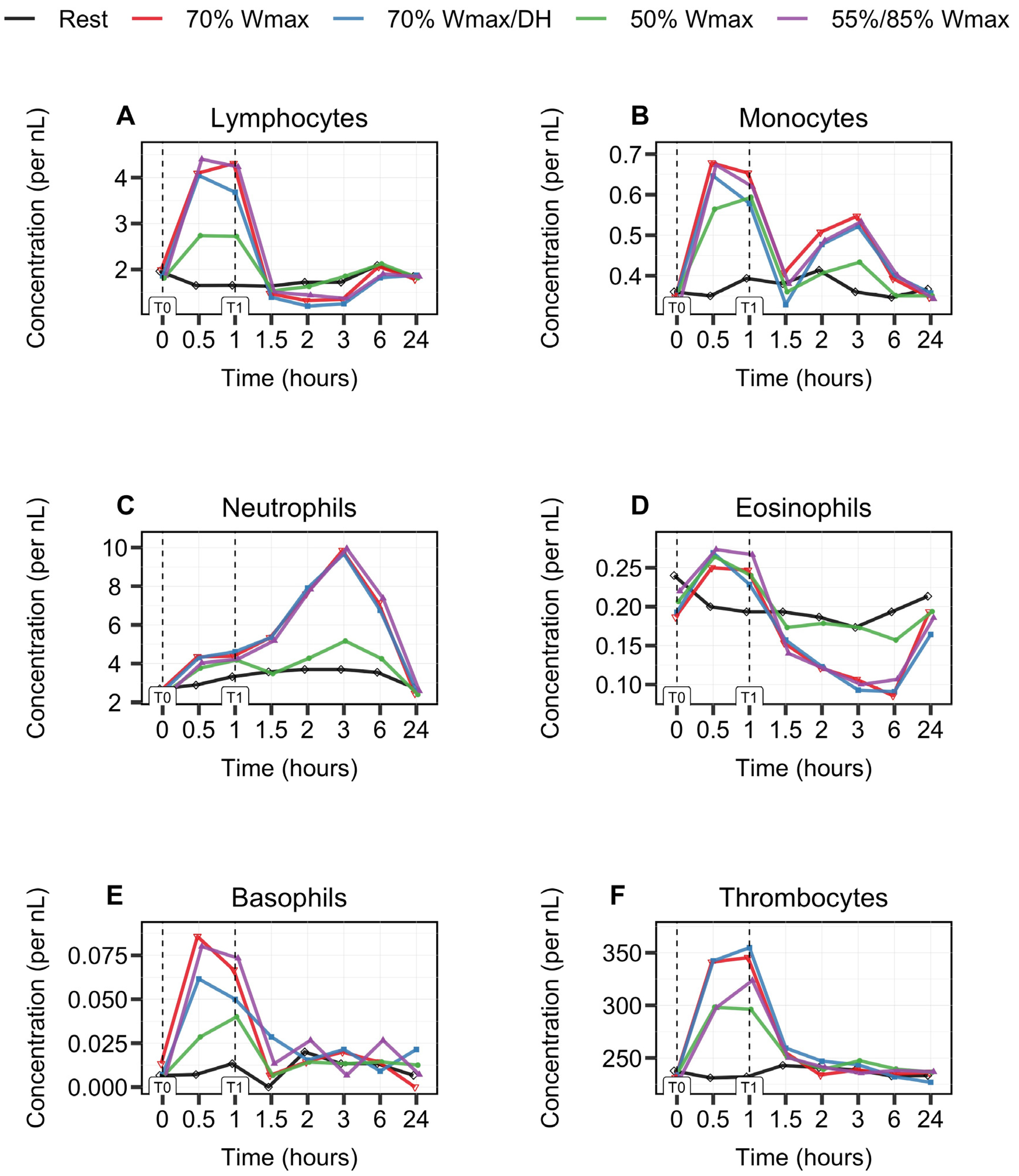
3.1. Biphasic Kinetic Response of Leukocytes
3.2. Leukocyte Subsets Behave Differently during and after 1 h Cycle Exercise
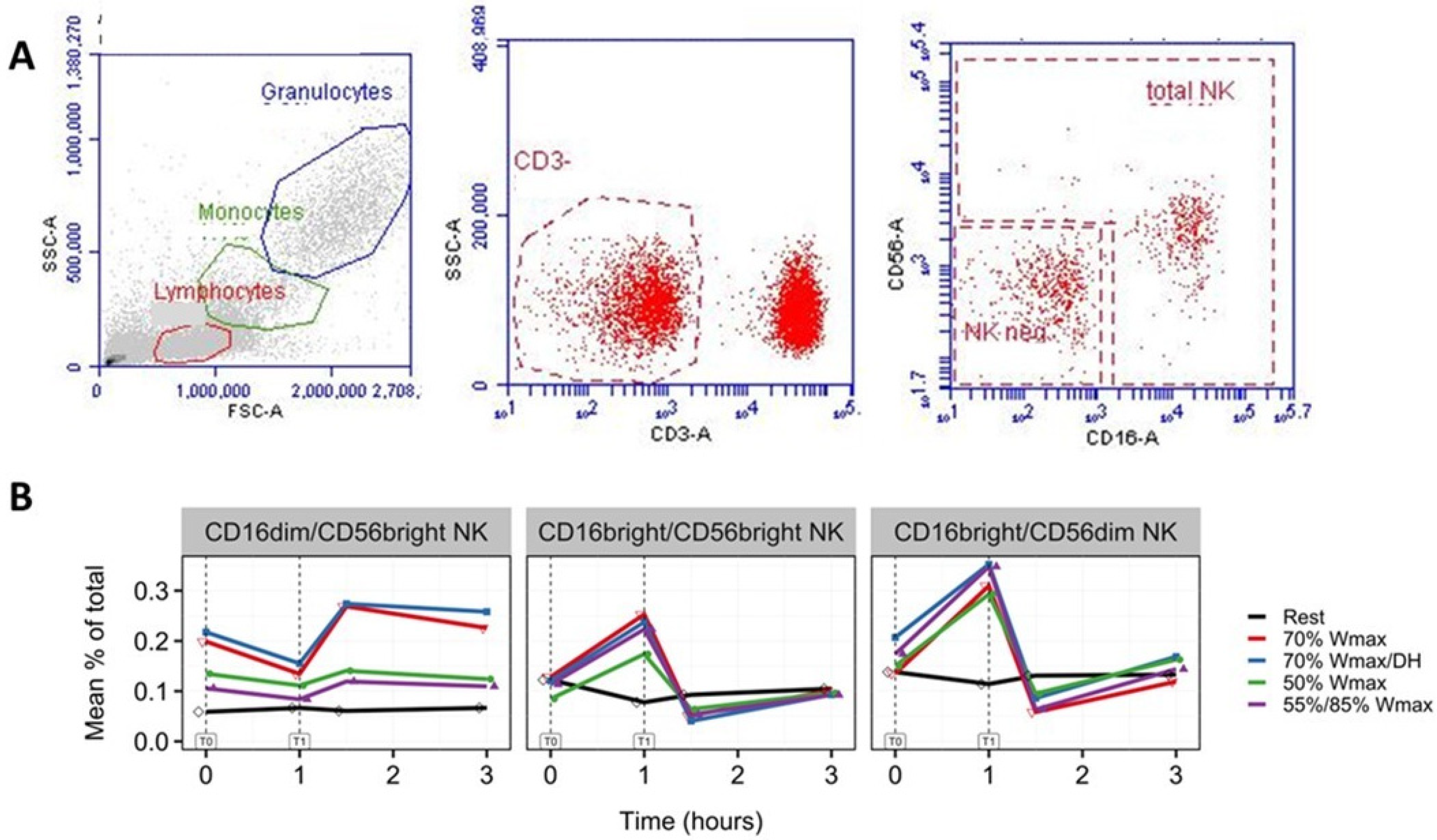
3.3. Increase in NK Cells with Phenotypes CD16brightCD56dim and CD16brightCD56bright
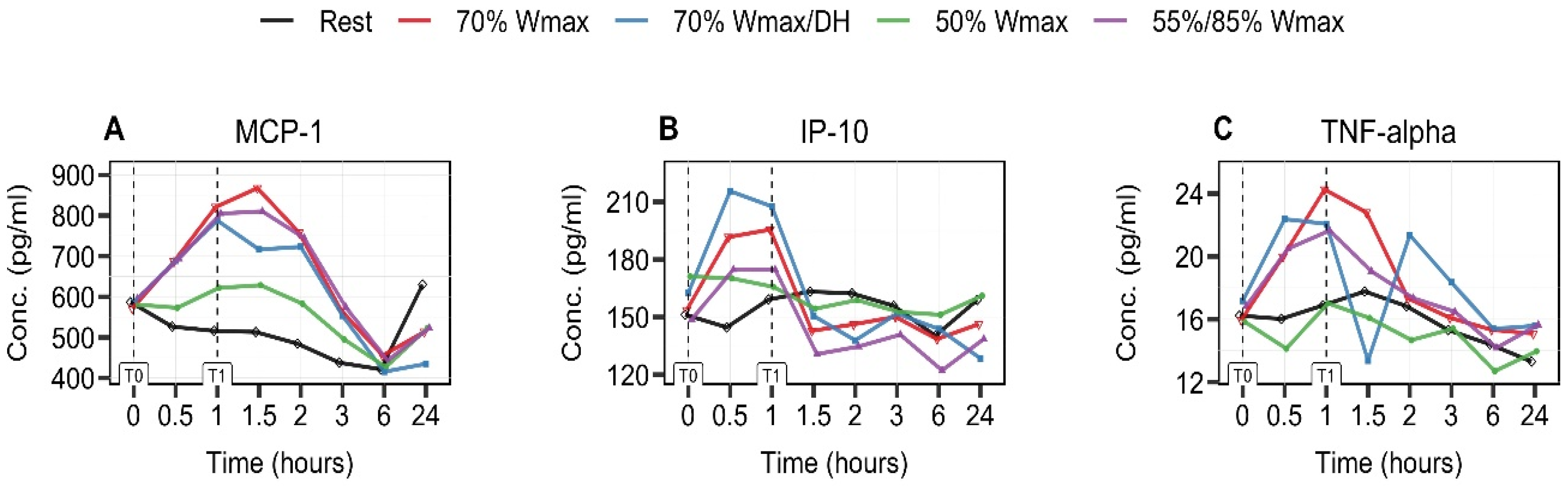
3.4. Exercise-Intensity- and Hydration-Status-Dependent Increase in MCP-1 and IP-10
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walsh, N.P.; Gleeson, M.; Pyne, D.B.; Nieman, D.C.; Dhabhar, F.S.; Shephard, R.J.; Oliver, S.J.; Bermon, S.; Kajeniene, A. Position statement. Part two: Maintaining immune health. Exerc. Immunol. Rev. 2011, 17, 64–103. [Google Scholar]
- de Toda, I.M.; Ceprián, N.; Cerro, E.D.-D.; De la Fuente, M. The Role of Immune Cells in Oxi-Inflamm-Aging. Cells 2021, 10, 2974. [Google Scholar] [CrossRef] [PubMed]
- Petersen, A.M.W.; Pedersen, B.K. The anti-inflammatory effect of exercise. J. Appl. Physiol. 2005, 98, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol 2011, 11, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Pyne, D.B.; Gleeson, M. Effects of Intensive Exercise Training on Immunity in Athletes. Int. J. Sports Med. 1998, 19 (Suppl. S3), S183–S194. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Nehlsen-Cannarella, S.L.; Fagoaga, O.R.; A Henson, D.; Shannon, M.; E Hjertman, J.M.; Schmitt, R.L.; Bolton, M.R.; Austin, M.D.; Schilling, B.K.; et al. Immune function in female elite rowers and non-athletes. Br. J. Sports Med. 2000, 34, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.J.; Katsanis, E. The immunological case for staying active during the COVID-19 pandemic. Brain Behav. Immun. 2020, 87, 6–7. [Google Scholar] [CrossRef]
- Pawelec, G. Age and immunity: What is “immunosenescence”? Exp. Gerontol. 2018, 105, 4–9. [Google Scholar] [CrossRef]
- Nieman, D.C.; Henson, D.A.; Gusewitch, G.; Warren, B.J.; Dotson, R.C.; Butterworth, D.E.; Nehlsen-Cannarella, S.L. Physical activity and immune function in elderly women. Med. Sci. Sports Exerc. 1993, 25, 823–831. [Google Scholar] [CrossRef]
- Suzuki, K.; Totsuka, M.; Nakaji, S.; Yamada, M.; Kudoh, S.; Liu, Q.; Sugawara, K.; Yamaya, K.; Sato, K. Endurance exercise causes interaction among stress hormones, cytokines, neutrophil dynamics, and muscle damage. J. Appl. Physiol. 1999, 87, 1360–1367. [Google Scholar] [CrossRef]
- Carol, A.; Witkamp, R.F.; Wichers, H.J.; Mensink, M. Bovine colostrum supplementation’s lack of effect on immune variables during short-term intense exercise in well-trained athletes. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Hackney, A.C. Exercise as a stressor to the human neuroendocrine system. Medicina 2006, 42, 788–797. [Google Scholar] [PubMed]
- Lee, E.C.; Fragala, M.S.; Kavouras, S.A.; Queen, R.M.; Pryor, J.L.; Casa, D.J. Biomarkers in Sports and Exercise: Tracking Health, Performance, and Recovery in Athletes. J. Strength Cond. Res. 2017, 31, 2920–2937. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K. Influence of Physical Activity on the Cellular Immune System: Mechanisms of Action. Int. J. Sports Med. 1991, 12 (Suppl. S1), S23–S29. [Google Scholar] [CrossRef]
- A McCarthy, D.; Grant, M.; Marbut, M.; Watling, M.; Wade, A.J.; Macdonald, I.; Nicholson, S.; Melsom, R.D.; Perry, J.D. Brief exercise induces an immediate and a delayed leucocytosis. Br. J. Sports Med. 1991, 25, 191–195. [Google Scholar] [CrossRef]
- Ronsen, O.; Pedersen, B.K.; Øritsland, T.R.; Bahr, R.; Kjeldsen-Kragh, J. Leukocyte counts and lymphocyte responsiveness associated with repeated bouts of strenuous endurance exercise. J. Appl. Physiol. 2001, 91, 425–434. [Google Scholar] [CrossRef]
- Saito, Y.; Kusaka, Y.; Shimada, M. Effects of exercise intensity on circulating leukocyte subpopulations. Environ. Health Prev. Med. 2003, 8, 18–22. [Google Scholar] [CrossRef]
- Nieman, D.C. Clinical implications of exercise immunology. J. Sport Health Sci. 2012, 1, 12–17. [Google Scholar] [CrossRef]
- Nigro, E.; Polito, R.; Alfieri, A.; Mancini, A.; Imperlini, E.; Elce, A.; Krustrup, P.; Orrù, S.; Buono, P.; Daniele, A. Molecular mechanisms involved in the positive effects of physical activity on coping with COVID-19. Eur. J. Appl. Physiol. 2020, 120, 2569–2582. [Google Scholar] [CrossRef]
- Scurati, R.; Papini, N.; Giussani, P.; Alberti, G.; Tringali, C. The Challenge of Long COVID-19 Management: From Disease Molecular Hallmarks to the Proposal of Exercise as Therapy. Int. J. Mol. Sci. 2022, 23, 12311. [Google Scholar] [CrossRef] [PubMed]
- Duggal, N.A.; Niemiro, G.; Harridge, S.D.R.; Simpson, R.J.; Lord, J.M. Can physical activity ameliorate immunosenescence and thereby reduce age-related multi-morbidity? Nat. Rev. Immunol. 2019, 19, 563–572. [Google Scholar] [CrossRef]
- Sellami, M.; Gasmi, M.; Denham, J.; Hayes, L.D.; Stratton, D.; Padulo, J.; Bragazzi, N. Effects of acute and chronic exercise on immunological parameters in the elderly aged: Can physical activity counteract the effects of aging? Front. Immunol. 2018, 9, 2187. [Google Scholar] [CrossRef]
- JanssenDuijghuijsen, L.M.; Keijer, J.; Mensink, M.; Lenaerts, K.; Ridder, L.; Nierkens, S.; Kartaram, S.W.; Verschuren, M.C.M.; Pieters, R.H.H.; Bas, R.; et al. Adaptation of exercise-induced stress in well-trained healthy young men. Exp. Physiol. 2017, 102, 86–99. [Google Scholar] [CrossRef]
- JanssenDuijghuijsen, L.M.; Van Norren, K.; Grefte, S.; Koppelman, S.J.; Lenaerts, K.; Keijer, J.; Witkamp, R.F.; Wichers, H.J. Endurance Exercise Increases Intestinal Uptake of the Peanut Allergen Ara h 6 after Peanut Consumption in Humans. Nutrients 2017, 9, 84. [Google Scholar] [CrossRef]
- Kartaram, S.; Mensink, M.; Teunis, M.; Schoen, E.; Witte, G.; Duijghuijsen, L.J.; Verschuren, M.; Mohrmann, K.; M’Rabet, L.; Knipping, K.; et al. Plasma citrulline concentration, a marker for intestinal functionality, reflects exercise intensity in healthy young men. Clin. Nutr. 2019, 38, 2251–2258. [Google Scholar] [CrossRef] [PubMed]
- Kartaram, S.W.; van Norren, K.; Schoen, E.; Teunis, M.; Mensink, M.; Verschuren, M.; M’rabet, L.; der Vaart, I.B.-V.; Mohrmann, K.; Wittink, H.; et al. Kinetics of Physiological Responses as a Measure of Intensity and Hydration Status During Experimental Physical Stress in Human Volunteers. Front. Physiol. 2020, 11, 1006. [Google Scholar] [CrossRef]
- WMA Declaration Of Helsinki – Ethical Principles For Medical Research Involving Human Subjects. Available online: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ (accessed on 17 January 2024).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear Mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- nlme: Linear and Nonlinear Mixed Effects Models. Available online: https://cran.r-project.org/web/packages/nlme/nlme.pdf (accessed on 17 January 2024).
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Gustafson, M.P.; DiCostanzo, A.C.; Wheatley, C.M.; Kim, C.-H.; Bornschlegl, S.; Gastineau, D.A.; Johnson, B.D.; Dietz, A.B. A systems biology approach to investigating the influence of exercise and fitness on the composition of leukocytes in peripheral blood. J. Immunother. Cancer 2017, 5, 30. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Hoffman-Goetz, L. Exercise and the Immune System: Regulation, Integration, and Adaptation. Physiol. Rev. 2000, 80, 1055–1081. [Google Scholar] [CrossRef] [PubMed]
- Sand, K.L.; Flatebo, T.; Andersen, M.B.; A Maghazachi, A. Effects of exercise on leukocytosis and blood hemostasis in 800 healthy young females and males. World J. Exp. Med. 2013, 3, 11–20. [Google Scholar] [CrossRef]
- Neves, P.R.D.S.; Tenório, T.R.D.S.; Lins, T.A.; Muniz, M.T.C.; Pithon-Curi, T.C.; Botero, J.P.; Prado, W.L.D. Acute effects of high- and low-intensity exercise bouts on leukocyte counts. J. Exerc. Sci. Fit. 2015, 13, 24–28. [Google Scholar] [CrossRef]
- Bessa, A.L.; Oliveira, V.N.; Agostini, G.G.; Oliveira, R.J.; Oliveira, A.C.; White, G.E.; Wells, G.D.; Teixeira, D.N.; Espindola, F.S. Exercise Intensity and Recovery. J. Strength Cond. Res. 2016, 30, 311–319. [Google Scholar] [CrossRef]
- Luk, H.-Y.; McKenzie, A.L.; Duplanty, A.A.; Budnar, R.G.; Levitt, D.; Fernandez, A.; Lee, E.C.; Armstrong, L.E.; Vingren, J.L. Leukocyte Subset Changes in Response to a 164-km Road Cycle Ride in a Hot Environment. Int. J. Exerc. Sci. 2016, 9, 34–46. [Google Scholar]
- Suzuki, K.; Nakaji, S.; Yamada, M.; Liu, Q.; Kurakake, S.; Okamura, N.; Kumae, T.; Umeda, T.; Sugawara, K. Impact of a Competitive Marathon Race on Systemic Cytokine and Neutrophil Responses. Med. Sci. Sports Exerc. 2003, 35, 348–355. [Google Scholar] [CrossRef] [PubMed]
- A Cooper, M.; A Fehniger, T.; A Caligiuri, M. The biology of human natural killer-cell subsets. Trends Immunol. 2001, 22, 633–640. [Google Scholar] [CrossRef]
- Timmons, B.W.; Tarnopolsky, M.A.; Bar-Or, O.; Radom-Aizik, S.; Zaldivar, F.; Haddad, F.; Cooper, D.M.; Riddell, M.C.; Devries, M.C.; Hamadeh, M.J.; et al. Sex-based effects on the distribution of NK cell subsets in response to exercise and carbohydrate intake in adolescents. J. Appl. Physiol. 2006, 100, 1513–1519. [Google Scholar] [CrossRef] [PubMed]
- Timmons, B.W.; Bar-Or, O. Evidence of sex-based differences in natural killer cell responses to exercise and carbohydrate intake in children. Eur. J. Appl. Physiol. 2007, 101, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Kakanis, M.W.; Peake, J.; Brenu, E.W.; Simmonds, M.; Gray, B.; Hooper, S.L.; Marshall-Gradisnik, S.M. The open window of susceptibility to infection after acute exercise in healthy young male elite athletes. Exerc. Immunol. Rev. 2010, 16, 119–137. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Febbraio, M.A. Muscle as an Endocrine Organ: Focus on Muscle-Derived Interleukin-6. Physiol. Rev. 2008, 88, 1379–1406. [Google Scholar] [CrossRef]
- Suzuki, K.; Peake, J.; Nosaka, K.; Okutsu, M.; Abbiss, C.R.; Surriano, R.; Bishop, D.; Quod, M.J.; Lee, H.; Martin, D.T.; et al. Changes in markers of muscle damage, inflammation and HSP70 after an Ironman triathlon race. Eur. J. Appl. Physiol. 2006, 98, 525–534. [Google Scholar] [CrossRef]
- Kim, H.-K.; Konishi, M.; Takahashi, M.; Tabata, H.; Endo, N.; Numao, S.; Lee, S.-K.; Kim, Y.-H.; Suzuki, K.; Sakamoto, S. Effects of Acute Endurance Exercise Performed in the Morning and Evening on Inflammatory Cytokine and Metabolic Hormone Responses. PLoS ONE 2015, 10, e0137567. [Google Scholar] [CrossRef]
- Campbell, J.P.; Turner, J.E. Debunking the Myth of Exercise-Induced Immune Suppression: Redefining the Impact of Exercise on Immunological Health Across the Lifespan. Front. Immunol. 2018, 9, 648. [Google Scholar] [CrossRef]
- Farrell, P.A.; Garthwaite, T.L.; Gustafson, A.B. Plasma adrenocorticotropin and cortisol responses to submaximal and exhaustive exercise. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1983, 55, 1441–1444. [Google Scholar] [CrossRef]
- Dearman, J.; Francis, K.T. Plasma levels of catecholamines, cortisol, and beta-endorphins in male athletes after running 26.2, 6, and 2 miles. J. Sports Med. Phys. Fitness 1983, 23, 30–38. [Google Scholar]
- Steensberg, A. The role of IL-6 in exercise-induced immune changes and metabolism. Exerc. Immunol. Rev. 2003, 9, 40–47. [Google Scholar] [PubMed]
- McCarthy, D.A.; Dale, M.M. The leucocytosis of exercise. A review and model. Sports Med. 1988, 6, 333–363. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.O.; Lee, J.B. Leukocyte chemotactic cytokine and leukocyte subset responses during ultra-marathon running. Cytokine 2013, 61, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Dumke, C.L.; Henson, D.A.; McAnulty, S.R.; Gross, S.J.; Lind, R.H. Muscle damage is linked to cytokine changes following a 160-km race. Brain Behav. Immun. 2005, 19, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Raschke, S.; Eckel, J. Adipo-Myokines: Two Sides of the Same Coin—Mediators of Inflammation and Mediators of Exercise. Mediat. Inflamm. 2013, 2013, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Windsor, M.T.; Bailey, T.G.; Perissiou, M.; Meital, L.; Golledge, J.; Russell, F.D.; Askew, C.D. Cytokine Responses to Acute Exercise in Healthy Older Adults: The Effect of Cardiorespiratory Fitness. Front. Physiol. 2018, 9, 203. [Google Scholar] [CrossRef] [PubMed]
- Van Craenenbroeck, A.H.; Van Ackeren, K.; Hoymans, V.Y.; Roeykens, J.; Verpooten, G.A.; Vrints, C.J.; Couttenye, M.M.; Van Craenenbroeck, E.M. Acute Exercise-Induced Response of Monocyte Subtypes in Chronic Heart and Renal Failure. Mediat. Inflamm. 2014, 2014, 1–11. [Google Scholar] [CrossRef]
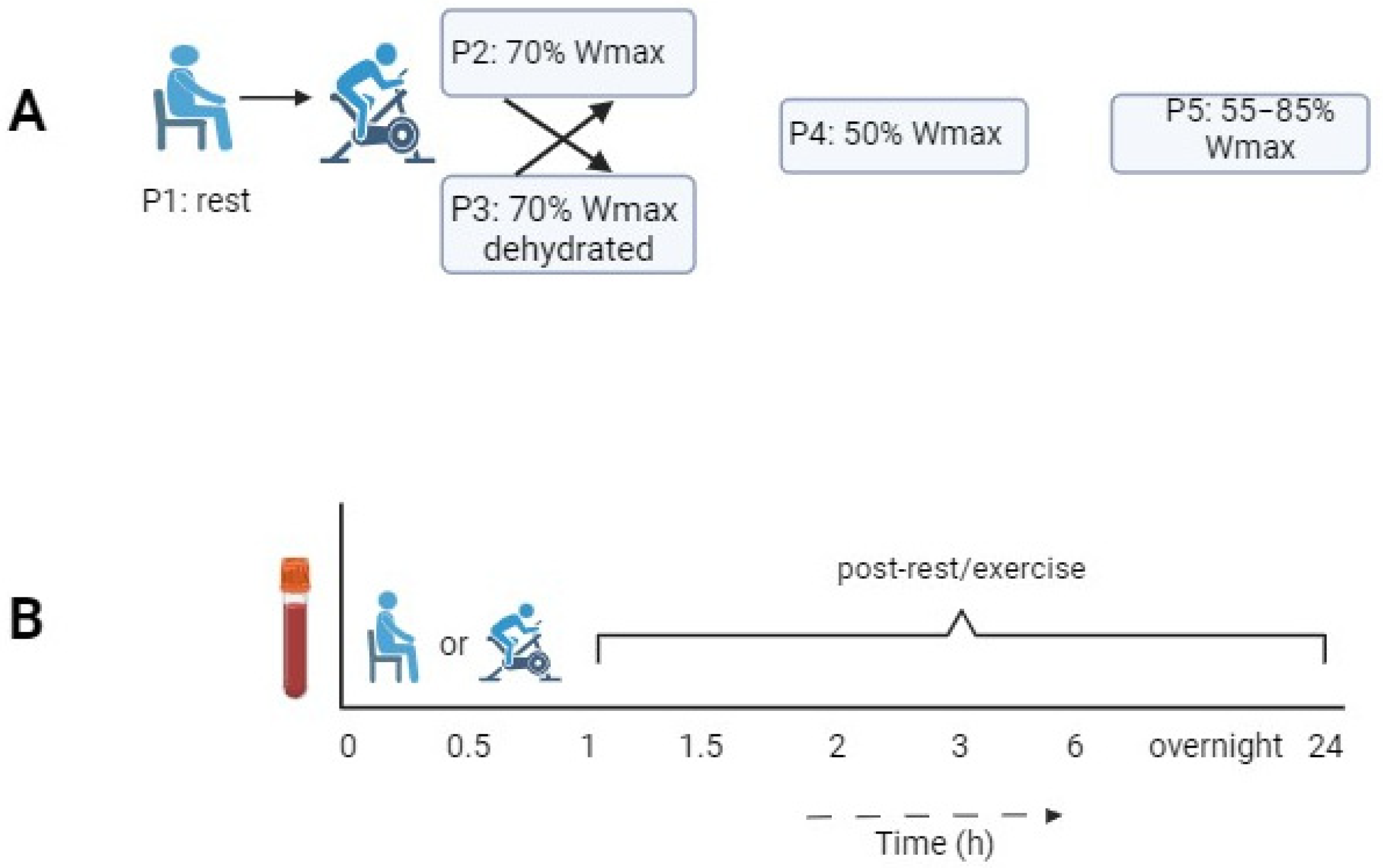
| Protocol | P1 | P2 | P3 | P4 | P5 |
|---|---|---|---|---|---|
| Experimental condition | Rest condition without exercise | 1 h of cycling at 70% of Wmax | 1 h of cycling at 70% of Wmax in dehydrated condition | 1 h of cycling at 50% of Wmax | 1 h of cycling in blocks of 2 min at 55/85% of Wmax |
| Variables | Mean ± SD |
|---|---|
| Age (yrs) | 24.3 ± 2.4 |
| BMI (kg/m2) | 22.5 ± 1.5 |
| Weight (kg) | 75.8 ± 6.7 |
| Length (cm) | 183.4 ± 3.8 |
| VO2max (mL/kg/min) | 56.9 ± 3.9 |
| Wmax (W) | 335.1 ± 39.9 |
| Protocol | T0 | T0.5 | T1 | T1.5 | T2 | T3 | T6 | T24 |
|---|---|---|---|---|---|---|---|---|
| P1 | 5.3 ± 0.84 | 5.1 ± 0.80 | 5.5 ± 1.13 | 5.8 ± 1.07 | 6.0 ± 1.19 | 6.0 ± 1.21 | 6.2 ± 1.14 | 5.2 ± 0.90 |
| P2 | 5.0 ± 1.02 | 9.3 ± 2.0 | 9.6 ± 2.21 | 7.2 ± 2.05 | 9.0 ± 2.27 | 11.4 ± 2.23 | 9.3 ± 1.88 | 4.8 ± 1.14 |
| P3 | 5.0 ± 1.02 | 9.3 ± 1.69 | 9.2 ± 1.99 | 7.4 ± 1.89 | 9.6 ± 2.60 | 11.3 ± 2.44 | 9.0 ± 1.76 | 5.0 ± 0.96 |
| P4 | 4.8 ± 0.76 | 7.4 ± 1.02 | 7.8 ± 1.36 | 5.5 ± 0.87 | 6.4 ± 1.12 | 7.5 ± 1.40 | 6.8 ± 1.35 | 4.8 ± 1.12 |
| P5 | 4.7 ± 0.65 | 9.4 ± 1.40 | 9.4 ± 1.48 | 7.2 ± 3.42 | 9.9 ± 4.3 | 12.0 ± 3.06 | 9.8 ± 2.20 | 4.9 ± 0.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kartaram, S.W.; Teunis, M.; van Norren, K.; Smits, M.; M’Rabet, L.; Verschuren, M.C.M.; Mohrmann, K.; Garssen, J.; Witkamp, R.; Pieters, R. Markers for Immunological Resilience: Effects of Moderate- and High-Intensity Endurance Exercise on the Kinetic Response of Leukocyte Subsets. Immuno 2024, 4, 43-56. https://doi.org/10.3390/immuno4010003
Kartaram SW, Teunis M, van Norren K, Smits M, M’Rabet L, Verschuren MCM, Mohrmann K, Garssen J, Witkamp R, Pieters R. Markers for Immunological Resilience: Effects of Moderate- and High-Intensity Endurance Exercise on the Kinetic Response of Leukocyte Subsets. Immuno. 2024; 4(1):43-56. https://doi.org/10.3390/immuno4010003
Chicago/Turabian StyleKartaram, Shirley W., Marc Teunis, Klaske van Norren, Mieke Smits, Laura M’Rabet, Martie C. M. Verschuren, Karin Mohrmann, Johan Garssen, Renger Witkamp, and Raymond Pieters. 2024. "Markers for Immunological Resilience: Effects of Moderate- and High-Intensity Endurance Exercise on the Kinetic Response of Leukocyte Subsets" Immuno 4, no. 1: 43-56. https://doi.org/10.3390/immuno4010003
APA StyleKartaram, S. W., Teunis, M., van Norren, K., Smits, M., M’Rabet, L., Verschuren, M. C. M., Mohrmann, K., Garssen, J., Witkamp, R., & Pieters, R. (2024). Markers for Immunological Resilience: Effects of Moderate- and High-Intensity Endurance Exercise on the Kinetic Response of Leukocyte Subsets. Immuno, 4(1), 43-56. https://doi.org/10.3390/immuno4010003





