Dilatational and Shear Interfacial Properties of Pea Protein Isolate Systems with Transglutaminase at the Air–Water Interface
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Preparation
2.2. Measurements of ζ- Potential
2.3. Interfacial Measurements
2.4. Bulk Viscosity
2.5. Data Analysis
3. Results and Discussion
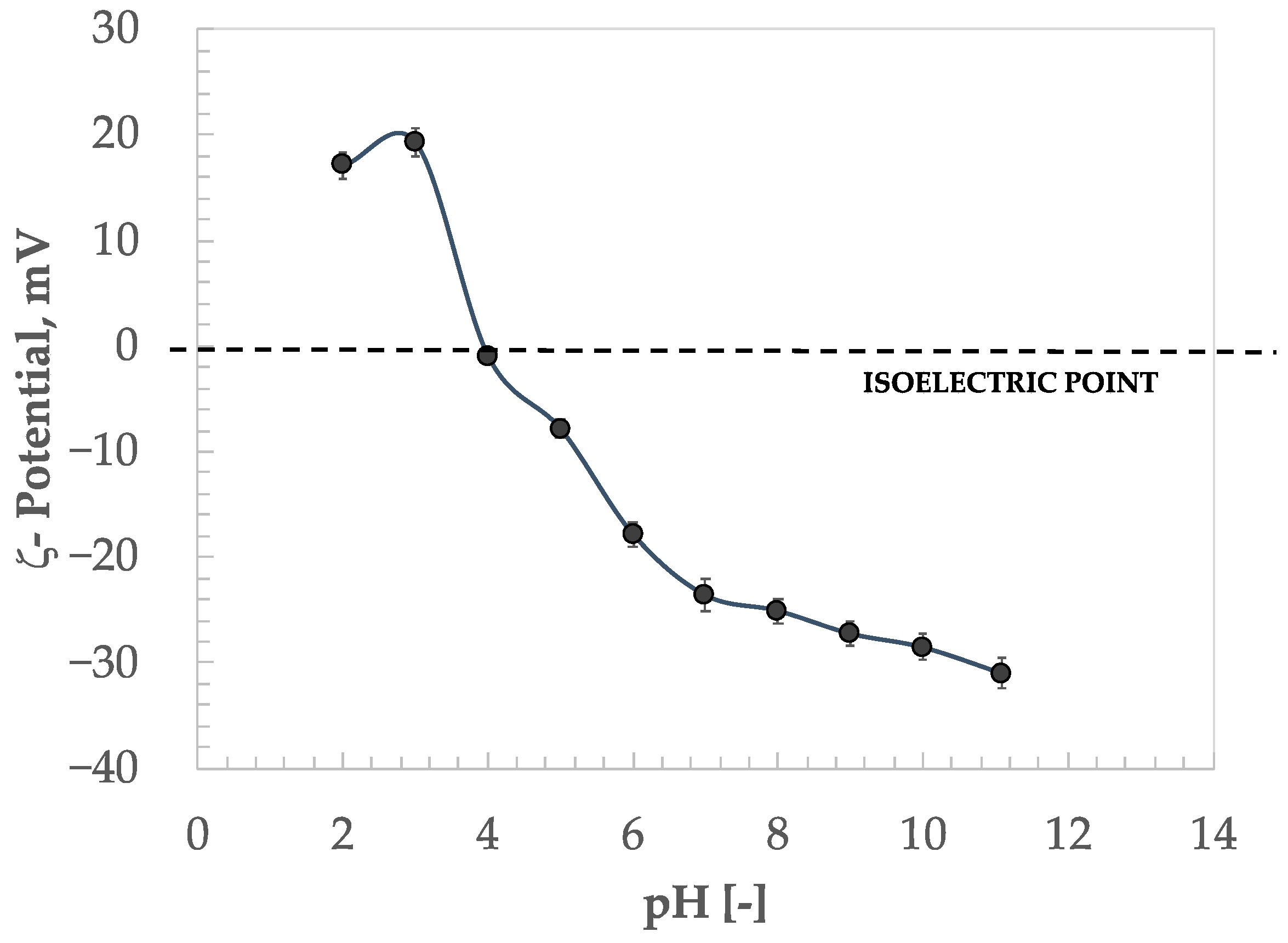
3.1. Results of ζ- Potential Analysis
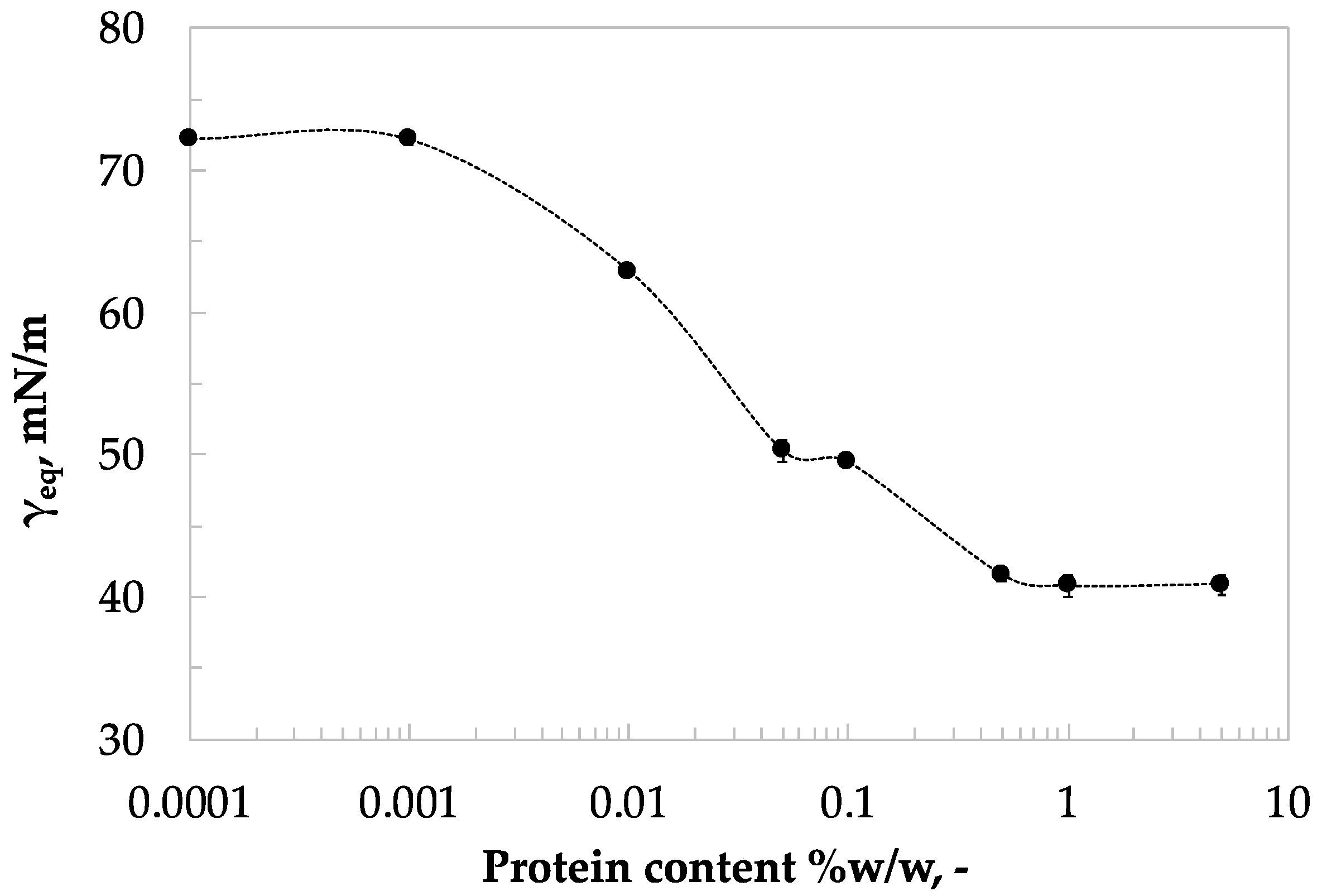
3.2. Static Surface Analysis
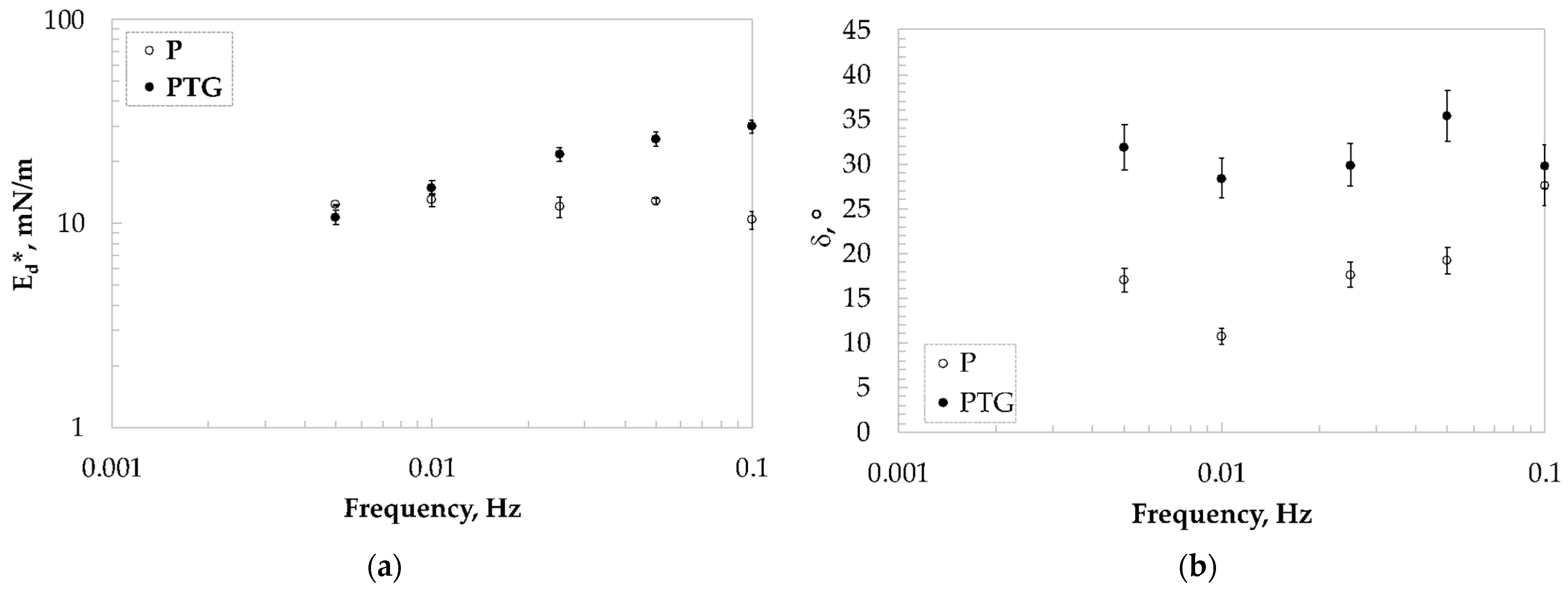
3.3. Interfacial Dilatational and Shear Measurements
3.4. Bulk Shear Viscosity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Golovinskaia, O.; Wang, C.-K. The hypoglycemic potential of phenolics from functional foods and their mechanisms. Food Sci. Hum. Wellness 2023, 12, 986–1007. [Google Scholar] [CrossRef]
- Bauer-Estrada, K.; Sandoval-Cuellar, C.; Rojas-Muñoz, Y.; Quintanilla-Carvajal, M.X. The modulatory effect of encapsulated bioactives and probiotics on gut microbiota: Improving health status through functional food. Food Funct. 2023, 14, 32–55. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Das, M. Functional foods: An overview. Food Sci. Biotechnol. 2011, 20, 861–875. [Google Scholar] [CrossRef]
- Baldino, N.; Carnevale, I.; Laitano, F.; Lupi, F.R.; Curcio, S.; Gabriele, D. Formulation of bread model doughs with resistant starch, vegetable proteins and transglutaminase. Eur. Food Res. Technol. 2020, 246, 397–408. [Google Scholar] [CrossRef]
- Kaczynska, K.; Wouters, A.G.; Delcour, J.A. Microbial transglutaminase induced modification of wheat gliadin based nanoparticles and its impact on their air-water interfacial properties. Food Hydrocoll. 2022, 127, 107471. [Google Scholar] [CrossRef]
- Lu, S.; Xiong, W.; Yao, Y.; Zhang, J.; Wang, L. Investigating the physicochemical properties and air-water interface adsorption behavior of transglutaminase-crosslinking rapeseed protein isolate. Food Res. Int. 2023, 174, 113505. [Google Scholar] [CrossRef] [PubMed]
- Mileti, O.; Baldino, N.; Carmona, J.A.; Lupi, F.R.; Muñoz, J.; Gabriele, D. Shear and dilatational rheological properties of vegetable proteins at the air/water interface. Food Hydrocoll. 2022, 126, 107472. [Google Scholar] [CrossRef]
- Mastromatteo, M.; Danza, A.; Guida, M.; Del Nobile, M.A. Formulation optimisation of vegetable flour-loaded functional bread Part I: Screening of vegetable flours and structuring agents. Int. J. Food Sci. Technol. 2012, 47, 1313–1320. [Google Scholar] [CrossRef]
- Shen, Q.; Zheng, W.; Han, F.; Zuo, J.; Dai, J.; Tang, C.; Song, R.; Li, B.; Chen, Y. Air-water interfacial properties and quantitative description of pea protein isolate-Tween 20. Food Hydrocoll. 2023, 139, 108568. [Google Scholar] [CrossRef]
- Shen, Q.; Li, J.; Shen, X.; Zhu, X.; Dai, J.; Tang, C.; Song, R.; Li, B.; Chen, Y. Linear and nonlinear interface rheological behaviors and structural properties of pea protein (vicilin, legumin, albumin). Food Hydrocoll. 2023, 139, 108500. [Google Scholar] [CrossRef]
- Liang, H.-N.; Tang, C.-H. pH-dependent emulsifying properties of pea [Pisum sativum (L.)] proteins. Food Hydrocoll. 2013, 33, 309–319. [Google Scholar] [CrossRef]
- A Rubio, L.; Pérez, A.; Ruiz, R.; Guzmán, M.A.; Aranda-Olmedo, I.; Clemente, A. Characterization of pea (Pisum sativum) seed protein fractions. J. Sci. Food Agric. 2014, 94, 280–287. [Google Scholar] [CrossRef]
- Shen, Q.; Luo, Y.; Zheng, W.; Xiong, T.; Han, F.; Zuo, J.; Dai, J.; Li, B.; Chen, Y. Nonlinear rheological behavior and quantitative proteomic analysis of pea protein isolates at the air-water interface. Food Hydrocoll. 2023, 135, 108115. [Google Scholar] [CrossRef]
- Sharif, H.R.; Williams, P.A.; Sharif, M.K.; Abbas, S.; Majeed, H.; Masamba, K.G.; Safdar, W.; Zhong, F. Current progress in the utilization of native and modified legume proteins as emulsifiers and encapsulants—A review. Food Hydrocoll. 2018, 76, 2–16. [Google Scholar] [CrossRef]
- Yang, J.; Zamani, S.; Liang, L.; Chen, L. Extraction methods significantly impact pea protein composition, structure and gelling properties. Food Hydrocoll. 2021, 117, 106678. [Google Scholar] [CrossRef]
- Gao, Z.; Shen, P.; Lan, Y.; Cui, L.; Ohm, J.-B.; Chen, B.; Rao, J. Effect of alkaline extraction pH on structure properties, solubility, and beany flavor of yellow pea protein isolate. Food Res. Int. 2020, 131, 109045. [Google Scholar] [CrossRef] [PubMed]
- Stone, A.K.; Karalash, A.; Tyler, R.T.; Warkentin, T.D.; Nickerson, M.T. Functional attributes of pea protein isolates prepared using different extraction methods and cultivars. Food Res. Int. 2015, 76, 31–38. [Google Scholar] [CrossRef]
- Tanger, C.; Engel, J.; Kulozik, U. Influence of extraction conditions on the conformational alteration of pea protein extracted from pea flour. Food Hydrocoll. 2020, 107, 105949. [Google Scholar] [CrossRef]
- Savoca, M.P.; Tonoli, E.; Atobatele, A.G.; Verderio, E.A.M. Biocatalysis by Transglutaminases: A Review of Biotechnological Applications. Micromachines 2018, 9, 562. [Google Scholar] [CrossRef]
- Partanen, R.; Paananen, A.; Forssell, P.; Linder, M.B.; Lille, M.; Buchert, J.; Lantto, R. Effect of transglutaminase-induced cross-linking of sodium caseinate on the properties of equilibrated interfaces and foams. Colloids Surfaces A Physicochem. Eng. Asp. 2009, 344, 79–85. [Google Scholar] [CrossRef]
- Tamm, F.; Drusch, S. Impact of enzymatic hydrolysis on the interfacial rheology of whey protein/pectin interfacial layers at the oil/water-interface. Food Hydrocoll. 2017, 63, 8–18. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, B.-Y.; Sun, X.; Liu, Y.-A.; Yu, Z.; Wang, X.; Xu, N. Freeze-thaw stability of transglutaminase-induced soy protein-maltose emulsion gel: Focusing on morphology, texture properties, and rheological characteristics. Int. J. Biol. Macromol. 2024, 261, 129716. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Zhao, J.; Wang, L.; Jiang, J. Modified pea protein coupled with transglutaminase reduces phosphate usage in low salt myofibrillar gel. Food Hydrocoll. 2023, 139, 108577. [Google Scholar] [CrossRef]
- Moreno, H.M.; Tovar, C.A.; Domínguez-Timón, F.; Cano-Báez, J.; Díaz, M.T.; Pedrosa, M.M.; Borderías, A.J. Gelation of commercial pea protein isolate: Effect of microbial transglutaminase and thermal processing. Food Sci. Technol. 2020, 40, 800–809. [Google Scholar] [CrossRef]
- Shand, P.; Ya, H.; Pietrasik, Z.; Wanasundara, P. Transglutaminase treatment of pea proteins: Effect on physicochemical and rheological properties of heat-induced protein gels. Food Chem. 2008, 107, 692–699. [Google Scholar] [CrossRef]
- Romero, A.; Beaumal, V.; David-Briand, E.; Cordobes, F.; Guerrero, A.; Anton, M. Interfacial and emulsifying behaviour of rice protein concentrate. Food Hydrocoll. 2012, 29, 1–8. [Google Scholar] [CrossRef]
- Seta, L.; Baldino, N.; Gabriele, D.; Lupi, F.R.; de Cindio, B. The effect of surfactant type on the rheology of ovalbumin layers at the air/water and oil/water interfaces. Food Hydrocoll. 2012, 29, 247–257. [Google Scholar] [CrossRef]
- Seta, L.; Baldino, N.; Gabriele, D.; Lupi, F.R.; de Cindio, B. The influence of carrageenan on interfacial properties and short-term stability of milk whey proteins emulsions. Food Hydrocoll. 2013, 32, 373–382. [Google Scholar] [CrossRef]
- Wüstneck, R.; Enders, P.; Ebisch, T.; Miller, R. Axialsymmetric stress relaxation and surface dilation rheology of docosanic acid monolayers spread at the interface of pendant drops in the short time region. Thin Solid Films 1997, 298, 39–46. [Google Scholar] [CrossRef]
- Ward, A.F.H.; Tordai, L. Time-Dependence of Boundary Tensions of Solutions I. The Role of Diffusion in Time-Effects. J. Chem. Phys. 1946, 14, 453–461. [Google Scholar] [CrossRef]
- Mileti, O.; Baldino, N.; Lupi, F.R.; Gabriele, D. Interfacial behavior of vegetable protein isolates at sunflower oil/water interface. Colloids Surf. B Biointerfaces 2023, 221, 113035. [Google Scholar] [CrossRef] [PubMed]
- Saulnier, P.; Boury, F.; Malzert, A.; Heurtault, B.; Ivanova, T.; Cagna, A.; Panaïotov, I.; Proust, J.E. Rheological Model for the Study of Dilational Properties of Monolayers. Comportment of Dipalmitoylphosphatidylcholine (DPPC) at the Dichloromethane (DCM)/Water Interface under Ramp Type or Sinusoidal Perturbations. Langmuir 2001, 17, 8104–8111. [Google Scholar] [CrossRef]
- Ivanov, I.B.; Danov, K.D.; Ananthapadmanabhan, K.P.; Lips, A. Interfacial rheology of adsorbed layers with surface reaction: On the origin of the dilatational surface viscosity. Adv. Colloid Interface Sci. 2005, 114–115, 61–92. [Google Scholar] [CrossRef] [PubMed]
- Doan, C.D.; Ghosh, S. Formation and Stability of Pea Proteins Nanoparticles Using Ethanol-Induced Desolvation. Nanomaterials 2019, 9, 949. [Google Scholar] [CrossRef]
- Barac, M.; Cabrilo, S.; Pesic, M.; Stanojevic, S.; Zilic, S.; Macej, O.; Ristic, N. Profile and Functional Properties of Seed Proteins from Six Pea (Pisum sativum) Genotypes. Int. J. Mol. Sci. 2010, 11, 4973–4990. [Google Scholar] [CrossRef]
- Tang, C.-H.; Ten, Z.; Wang, X.-S.; Yang, X.-Q. Physicochemical and Functional Properties of Hemp (Cannabis sativa L.) Protein Isolate. J. Agric. Food Chem. 2006, 54, 8945–8950. [Google Scholar] [CrossRef] [PubMed]
- XCao, X.; Wen, H.; Li, C.; Gu, Z. Differences in functional properties and biochemical characteristics of congenetic rice proteins. J. Cereal Sci. 2009, 50, 184–189. [Google Scholar] [CrossRef]
- Karaca, A.C.; Low, N.; Nickerson, M. Emulsifying properties of chickpea, faba bean, lentil and pea proteins produced by isoelectric precipitation and salt extraction. Food Res. Int. 2011, 44, 2742–2750. [Google Scholar] [CrossRef]
- Sejersen, M.T.; Salomonsen, T.; Ipsen, R.; Clark, R.; Rolin, C.; Engelsen, S.B. Zeta potential of pectin-stabilised casein aggregates in acidified milk drinks. Int. Dairy J. 2007, 17, 302–307. [Google Scholar] [CrossRef]
- Rodrigueznino, M.; Sánchez, C.C.; Ruizhenestrosa, V.; Patino, J.M.R. Milk and soy protein films at the air–water interface. Food Hydrocoll. 2005, 19, 417–428. [Google Scholar] [CrossRef]
- Martin, A.; Bos, M.; van Vliet, T. Interfacial rheological properties and conformational aspects of soy glycinin at the air/water interface. Food Hydrocoll. 2002, 16, 63–71. [Google Scholar] [CrossRef]
- Mileti, O.; Baldino, N.; Luzzi, S.; Lupi, F.R.; Gabriele, D. Interfacial Rheological Study of β-Casein/Pectin Mixtures at the Air/Water Interface. Gels 2024, 10, 41. [Google Scholar] [CrossRef] [PubMed]
- Patino, J.M.R.; Pilosof, A.M. Protein–polysaccharide interactions at fluid interfaces. Food Hydrocoll. 2011, 25, 1925–1937. [Google Scholar] [CrossRef]
- Camino, N.A.; Pérez, O.E.; Sanchez, C.C.; Patino, J.M.R.; Pilosof, A.M. Hydroxypropylmethylcellulose surface activity at equilibrium and adsorption dynamics at the air–water and oil–water interfaces. Food Hydrocoll. 2009, 23, 2359–2368. [Google Scholar] [CrossRef]
- Pérez, O.E.; Sánchez, C.C.; Pilosof, A.M.; Patino, J.M.R. Kinetics of adsorption of whey proteins and hydroxypropyl-methyl-cellulose mixtures at the air–water interface. J. Colloid Interface Sci. 2009, 336, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Perez, A.A.; Carrara, C.R.; Sánchez, C.C.; Santiago, L.G.; Patino, J.M.R. Interfacial dynamic properties of whey protein concentrate/polysaccharide mixtures at neutral pH. Food Hydrocoll. 2009, 23, 1253–1262. [Google Scholar] [CrossRef]
- Færgemand, M.; Murray, B.S.; Dickinson, E. Cross-Linking of Milk Proteins with Transglutaminase at the Oil−Water Interface. J. Agric. Food Chem. 1997, 45, 2514–2519. [Google Scholar] [CrossRef]
- Bergfreund, J.; Bertsch, P.; Fischer, P. Effect of the hydrophobic phase on interfacial phenomena of surfactants, proteins, and particles at fluid interfaces. Curr. Opin. Colloid Interface Sci. 2021, 56, 101509. [Google Scholar] [CrossRef]
- Sun, X.D.; Arntfield, S.D. Gelation properties of myofibrillar/pea protein mixtures induced by transglutaminase crosslinking. Food Hydrocoll. 2012, 27, 394–400. [Google Scholar] [CrossRef]
- Tanimoto, S.Y.; Kinsella, J.E. Enzymic modification of proteins: Effects of transglutaminase cross-linking on some physical properties of .beta.-lactoglobulin. J. Agric. Food Chem. 1988, 36, 281–285. [Google Scholar] [CrossRef]
- Shen, Y.; Hong, S.; Singh, G.; Koppel, K.; Li, Y. Improving functional properties of pea protein through “green” modifications using enzymes and polysaccharides. Food Chem. 2022, 385, 132687. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, E. Enzymic crosslinking as a tool for food colloid rheology control and interfacial stabilization. Trends Food Sci. Technol. 1997, 8, 334–339. [Google Scholar] [CrossRef]
- Bönisch, M.P.; Huss, M.; Weitl, K.; Kulozik, U. Transglutaminase cross-linking of milk proteins and impact on yoghurt gel properties. Int. Dairy J. 2007, 17, 1360–1371. [Google Scholar] [CrossRef]
- Yaputri, B.P.; Feyzi, S.; Ismail, B.P. Transglutaminase-Induced Polymerization of Pea and Chickpea Protein to Enhance Functionality. Gels 2023, 10, 11. [Google Scholar] [CrossRef]
- Qin, J.; Zhao, Y.; Zhou, J.; Zhang, G.; Li, J.; Liu, X. Rheological properties of transglutaminase-treated concentrated pea protein under conditions relevant to high-moisture extrusion processing. Front. Nutr. 2022, 9, 970010. [Google Scholar] [CrossRef]
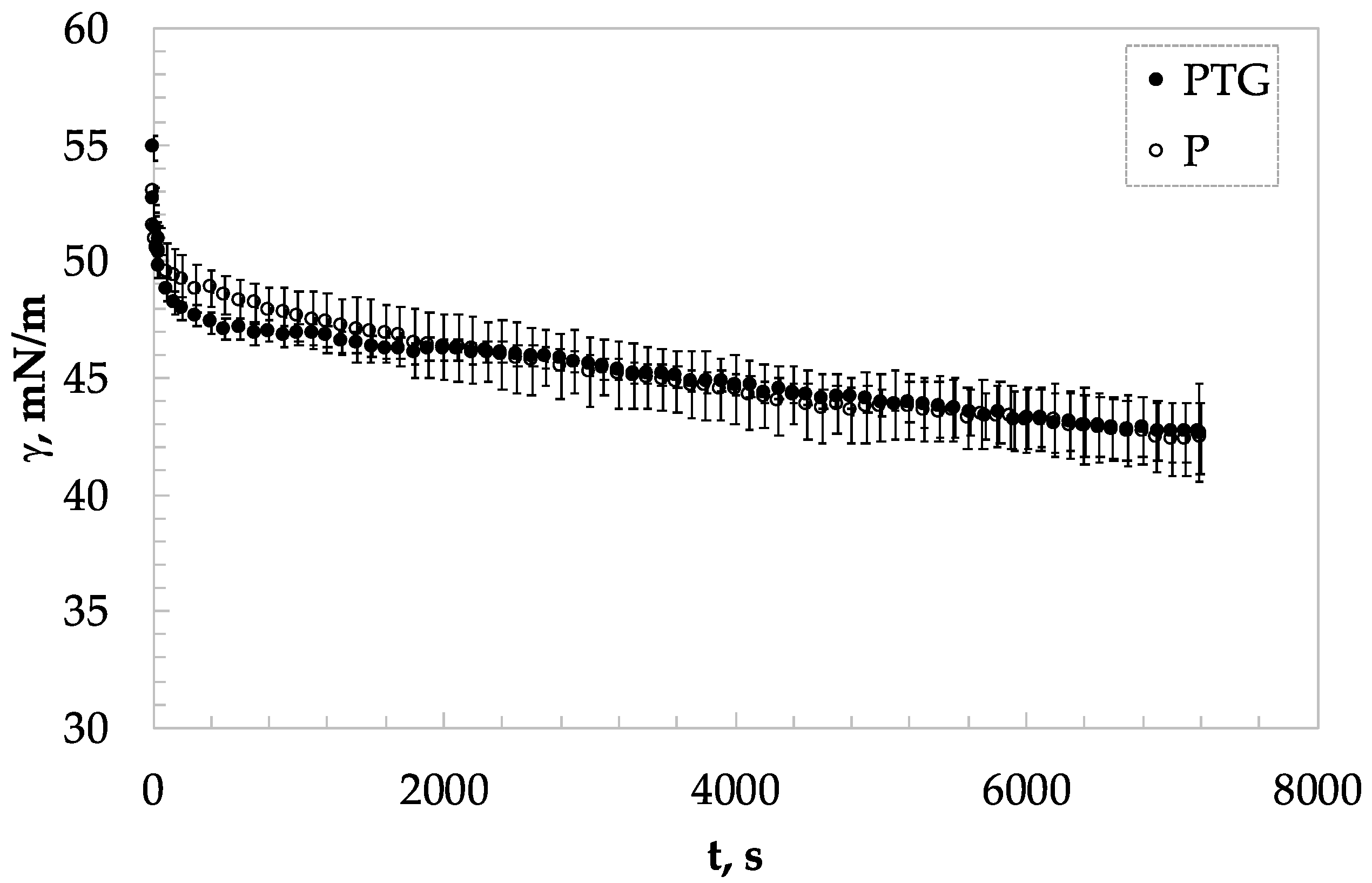
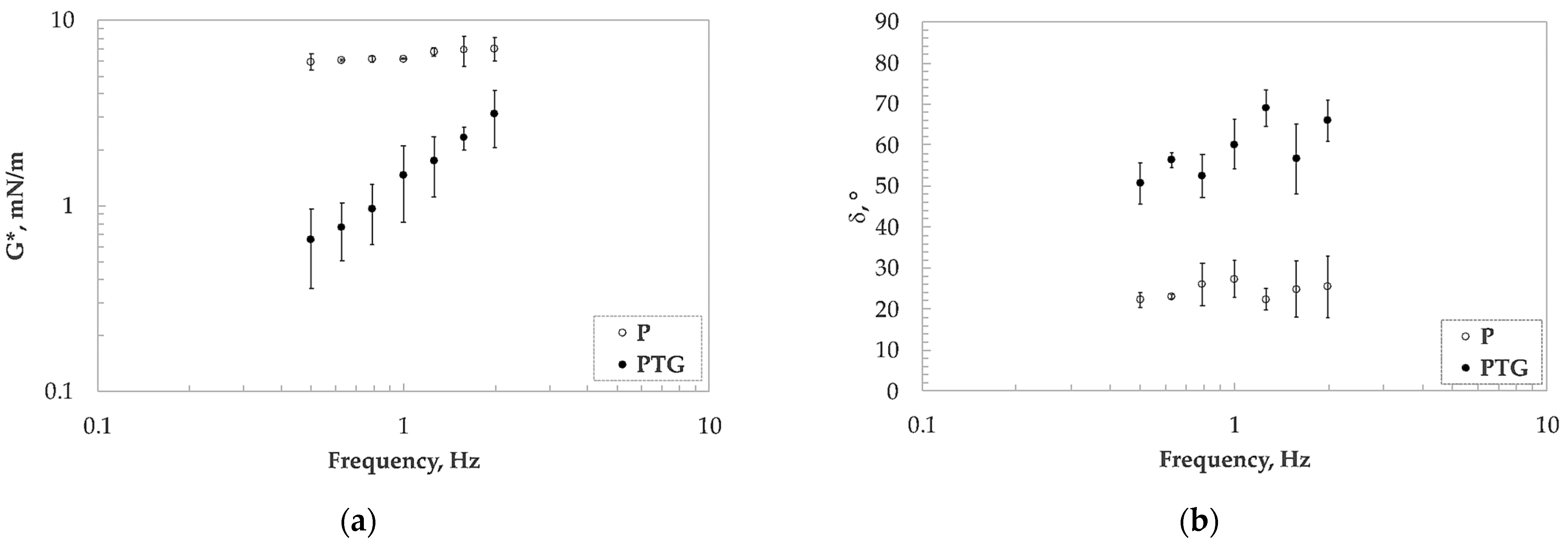
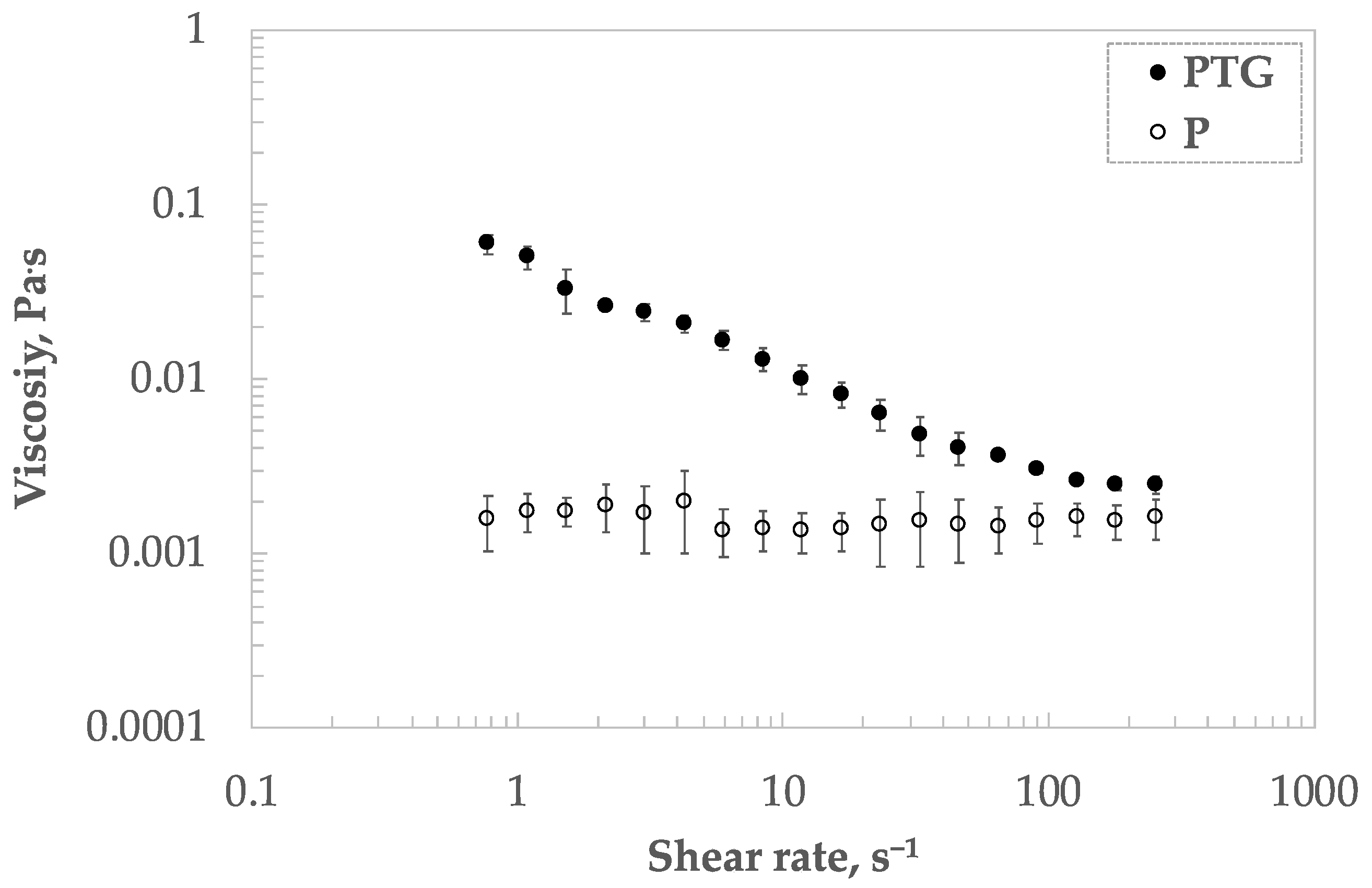
| TG Concentration, % w/w | Protein Concentration, % w/w | kdiff (m·Nm−1·s−0.5) | kads·104 (s−1) | krearr·104 (s−1) |
|---|---|---|---|---|
| 0 | 0.001 | Not detectable | Not detectable | Not detectable |
| 0 | 0.01 | 0.17 ± 0.01 a | Not detectable | Not detectable |
| 0 | 0.05 | 0.94 ± 0.01 b | 3.35 ± 0.02 a | 5.69 ± 0.02 c |
| 0 | 0.1 | 1.11 ± 0.01 c | 2.50 ± 0.70 b | 9.70 ± 0.70 c |
| 0 | 0.5 | too fast | 2.11 ± 0.03 b | 8.63 ± 0.04 c |
| 0 | 1 | too fast | 2.60 ± 0.03 b | 24.8 ± 0.3 a |
| 0.25 | 1 | too fast | 2.59 ± 0.35 b | 16.5 ± 1.0 b |
| Sample | kd, mN/m·sn | nd, - | ks, mN/m·sn | ns, - |
|---|---|---|---|---|
| P | 13.2 ± 0.5 a | 0.10 ± 0.01 b | 0.43 ± 0.02 a | 0.12 ± 0.02 b |
| PTG | 71.0 ± 3.0 b | 0.34 ± 0.04 a | 0.13 ± 0.06 b | 0.66 ± 0.05 a |
| Sample | Ed, mN/m | , mN/(ms) | , s | β, - |
|---|---|---|---|---|
| P | 27.0 ± 2.0 a | 22 ± 1 a | 43.0 ± 2.0 a | 0.38 ± 0.02 a |
| PTG | 21.0 ± 3.0 a | 13 ± 2 b | 34.1 ± 0.2 b | 0.21 ± 0.02 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldino, N.; Mileti, O.; Paleologo, M.F.O.; Lupi, F.R.; Gabriele, D. Dilatational and Shear Interfacial Properties of Pea Protein Isolate Systems with Transglutaminase at the Air–Water Interface. Macromol 2024, 4, 227-239. https://doi.org/10.3390/macromol4020012
Baldino N, Mileti O, Paleologo MFO, Lupi FR, Gabriele D. Dilatational and Shear Interfacial Properties of Pea Protein Isolate Systems with Transglutaminase at the Air–Water Interface. Macromol. 2024; 4(2):227-239. https://doi.org/10.3390/macromol4020012
Chicago/Turabian StyleBaldino, Noemi, Olga Mileti, Mario F. O. Paleologo, Francesca R. Lupi, and Domenico Gabriele. 2024. "Dilatational and Shear Interfacial Properties of Pea Protein Isolate Systems with Transglutaminase at the Air–Water Interface" Macromol 4, no. 2: 227-239. https://doi.org/10.3390/macromol4020012







