Enzymatic Oxidation of Ferulic Acid as a Way of Preparing New Derivatives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Enzyme
2.2. Laccase Characterization
2.3. FA-enzymatic Oxidation
2.4. Oxidation Kinetics by HPLC
2.5. Measurement of Reaction Mixture Color
2.6. Characterization of FA Products
2.6.1. Recovery of FA-Oxidation Products
2.6.2. Purification of FA-Products
2.6.3. Liquid Chromatography-Mass Spectrometry (LC-MS)
2.6.4. Nuclear Magnetic Resonance Spectroscopy (NMR) Analysis
2.7. Physical Properties of Oxidation Products
2.7.1. Lipophilicity (logP)
2.7.2. Measurement of Melting Point
2.8. Antiradical Properties Using ABTS
2.9. Anti-Proliferative Activity
2.9.1. Cells and Cell Culture
2.9.2. Determination of Cell Viability
2.10. Statistical Analysis
3. Results and Discussion
3.1. Characterisation of Laccase
3.2. Oxidation Kinetics
3.3. Color Measurement
3.4. Characterization of Laccase-Catalyzed Oxidation Products
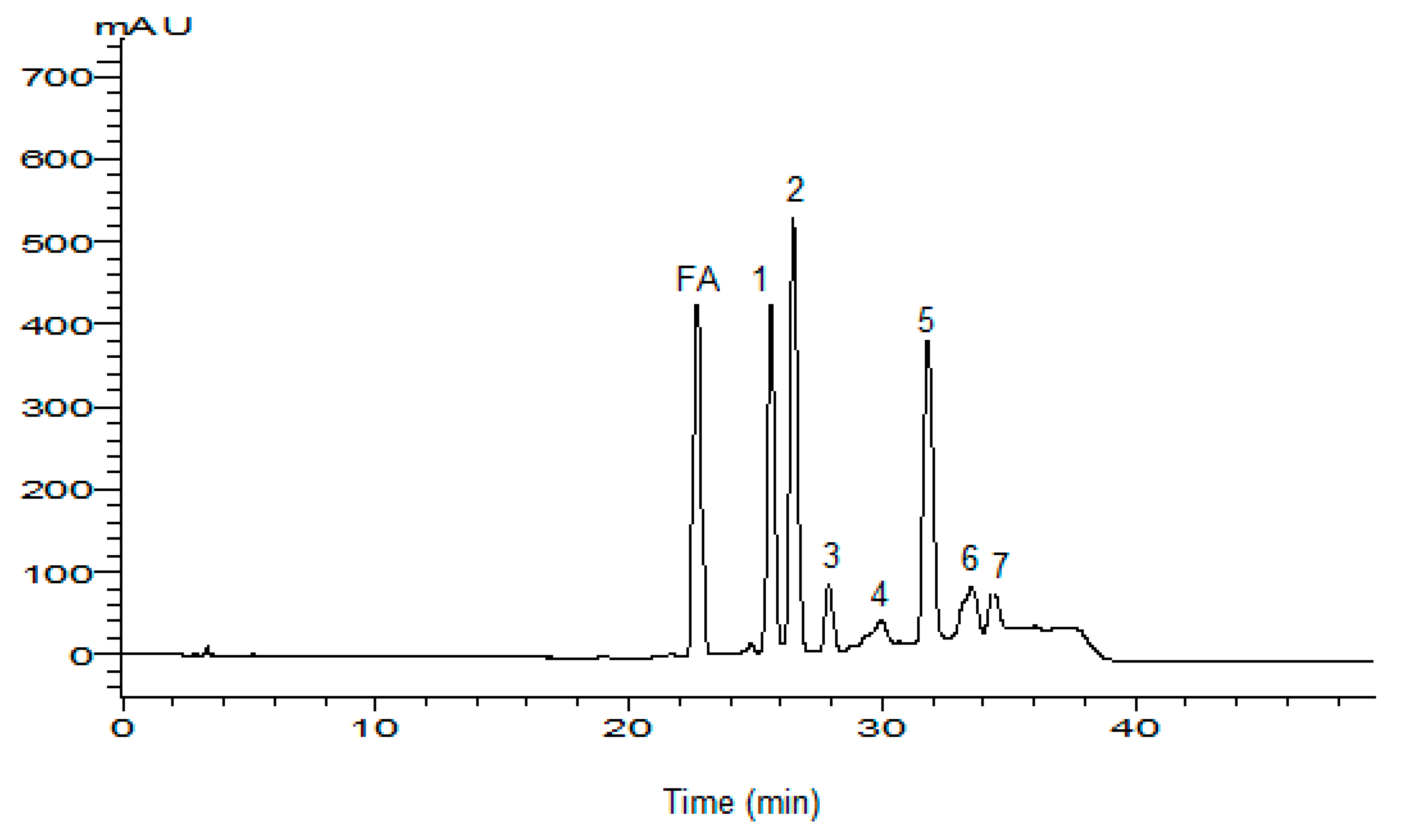
3.4.1. HPLC Analysis
3.4.2. Purification of FA-Products
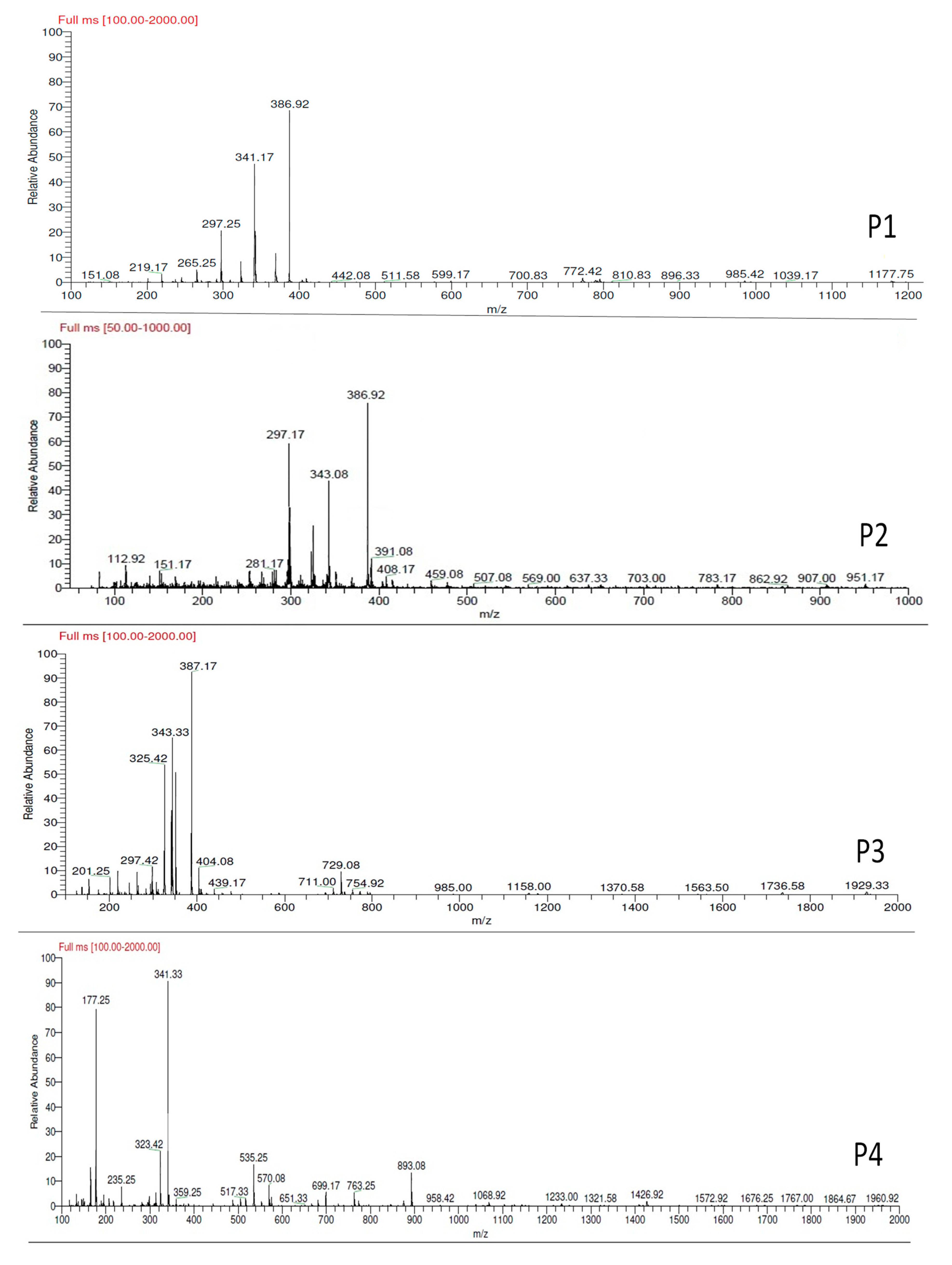
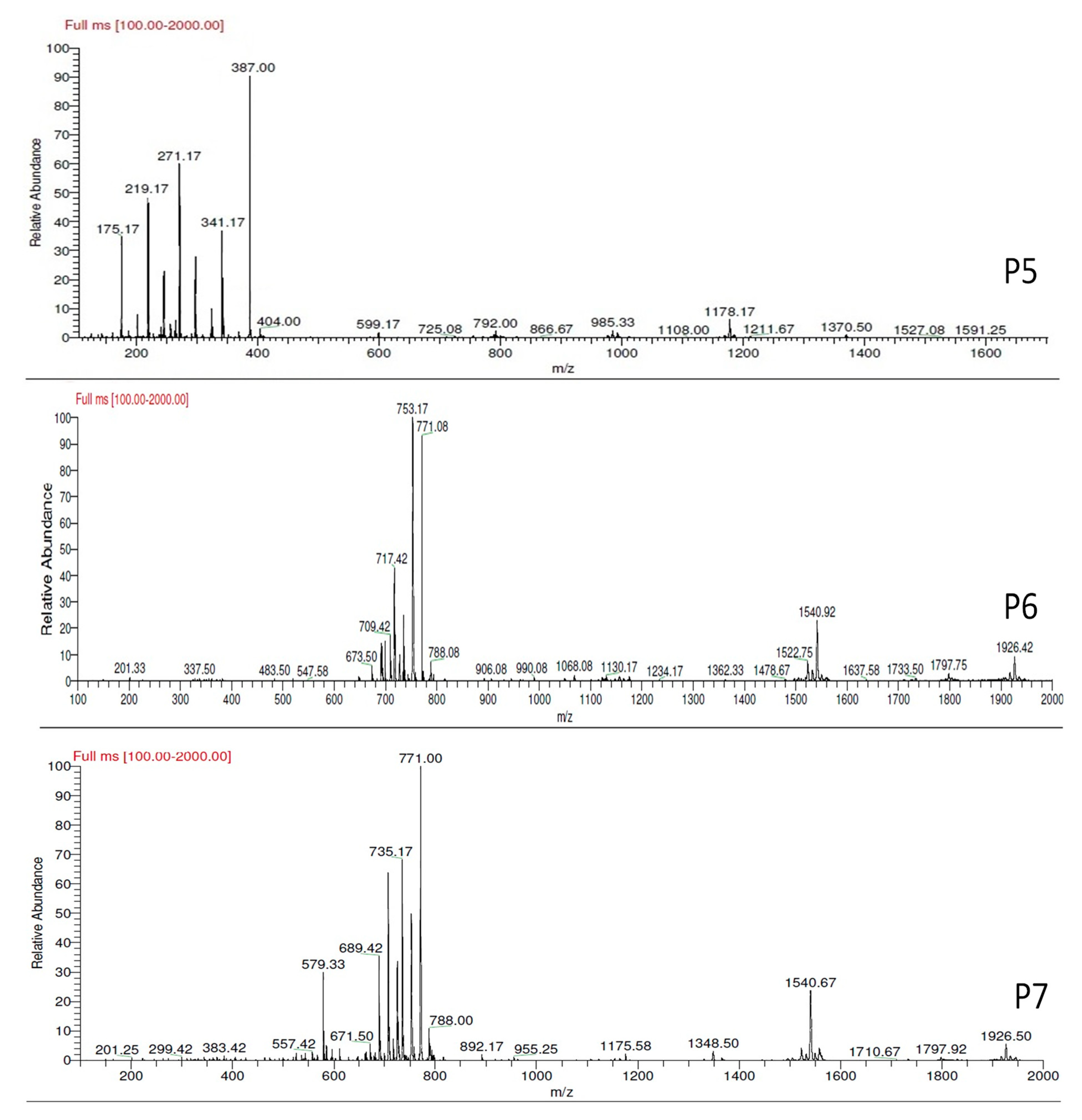
3.4.3. LC-MS Analysis
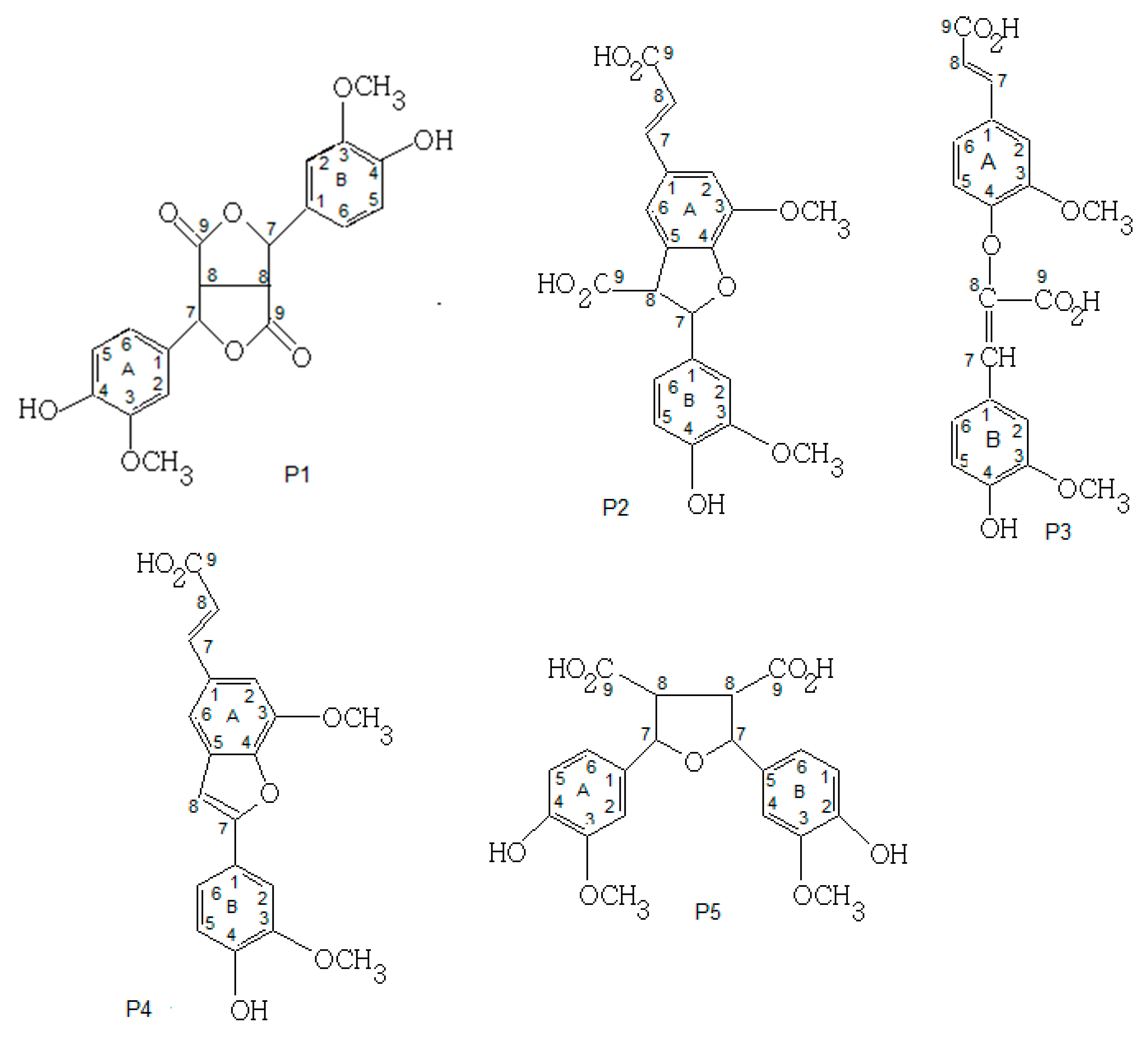
3.4.4. NMR Analysis
3.5. Physic Properties of Oxidation Products
3.5.1. Lipophilicity (LogP)
3.5.2. Melting Point
3.6. Antioxidant Properties of FA Products
3.7. Anti-Proliferative Activity of FA Products
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aljawish, A.; Paris, C.; Chevalot, I.; Muniglia, L. Green synthesis of glyco-phenol by enzymatic coupling between ferulic acid and glucosamine: An ecofriendly procedure. Biotechnol. Appl. Biochem. 2021, 69, 1438–1450. [Google Scholar] [CrossRef]
- Aljawish, A.; Chevalot, I.; Jasniewski, J.; Paris, C.; Scher, J.; Muniglia, L. Laccase-catalysed oxidation of ferulic acid and ethyl ferulate in aqueous medium: A green procedure for the synthesis of new compounds. Food Chem. 2014, 145, 1046–1054. [Google Scholar] [CrossRef]
- Adelakun, O.E.; Kudanga, T.; Parker, A.; Green, I.R.; le Roes-Hill, M.; Burton, S.G. Laccase-catalyzed dimerization of ferulic acid amplifies antioxidant activity. J. Mol. Cat. B-Enzym. 2012, 74, 29–35. [Google Scholar] [CrossRef]
- Li, M.; Karboune, S.; Liu, L.; Light, K.; L’Hocine, L.; Achouri, A.; Pitre, M.; Mateo, C. Combining phenolic grafting and laccase-catalyzed cross-linking: Effects on structures, technofunctional properties and human immunoglobulin E binding capacity of egg white proteins. Food Chem. 2021, 355, 129587. [Google Scholar] [CrossRef]
- Guerberoff, G.K.; Camusso, C.C. Effect of laccase from Trametes versicolor on the oxidative stability of edible vegetable oils. Food Sci. Hum. Wellness 2019, 8, 356–361. [Google Scholar] [CrossRef]
- Mustafa, R.; Muniglia, L.; Rovel, B.; Girardin, M. Phenolic colorants obtained by enzymatic synthesis using a fungal laccase in a hydro-organic biphasic system. Food Res. Inter. 2005, 38, 995–1000. [Google Scholar] [CrossRef]
- Riebel, M.; Sabel, A.; Claus, H.; Fronk, P.; Xia, N.; Li, H.; König, H.; Decker, H. Influence of Laccase and Tyrosinase on the Antioxidant Capacity of Selected Phenolic Compounds on Human Cell Lines. Molecules 2015, 20, 17194–17207. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.S.; Xing, T.L.; Tang, R.C. Enzymatic dyeing and functional finishing of textile fibres with ferulic acid. Indian J. Fibre Text. Res. 2015, 40, 62–69. [Google Scholar]
- Brugnari, T.; Braga, D.M.; dos Santos CS, A.; Torres BH, C.; Modkovski, T.A.; Haminiuk CW, I.; Maciel, G.M. Laccases as green and versatile biocatalysts: From lab to enzyme market—An overview. Bioresour. Bioprocess. 2021, 8, 131. [Google Scholar] [CrossRef]
- Demolliens, A.; Boucher, C.; Durocher, Y.; Jolicoeur, M.; Buschmann, M.D.; De Crescenzo, G. Tyrosinase-catalyzed synthesis of a universal coil-chitosan bioconjugate for protein immobilization. Bioconjug. Chem. 2021, 19, 1849–1854. [Google Scholar] [CrossRef]
- de Aguiar, V.M.; Esquinelato Silva, R.; Leao RA, C.; de Souza RO v, M.A.; Gonçalves RS, B.; de Mariz e Miranda, L.S. Studies on the laccases catalyzed oxidation of norbelladine like acetamides. Mol. Catal. 2020, 485, 110788. [Google Scholar] [CrossRef]
- Aljawish, A.; Chevalot, I.; Piffaut, B.; Rondeau-Mouro, C.; Girardin, M.; Jasniewski, J.; Scher, J.; Muniglia, L. Functionalization of chitosan by laccase-catalyzed oxidation of ferulic acid and ethyl ferulate under heterogeneous reaction conditions. Carbohydr. Polym. 2012, 87, 537–544. [Google Scholar] [CrossRef]
- Huber, D.; Tegl, G.; Baumann, M.; Sommer, E.; Gorji, E.G.; Borth, N.; Schleining, G.; Nyanhongo, G.S.; Guebitz, G.M. Chitosan hydrogel formation using laccase activated phenolics as cross-linkers. Carbohydr. Polym. 2017, 10, 814–822. [Google Scholar] [CrossRef]
- Yu, C.; Liu, X.; Pei, J.; Wang, Y. Grafting of laccase-catalysed oxidation of butyl paraben and p-coumaric acid onto chitosan to improve its antioxidant and antibacterial activities. React. Funct. Polym. 2020, 149, 104511. [Google Scholar] [CrossRef]
- Mathew, S.; Abraham, T.E. Ferulic acid: An antioxidant found naturally in plant cell walls and feruloyl esterases involved in its release and their applications. Crit. Rev. Biotechnol. 2004, 24, 59–83. [Google Scholar] [CrossRef]
- Zhao, Z.H.; Moghadasian, M.H. Chemistry, natural sources, dietary intake and pharmacokinetic properties of ferulic acid: A review. Food Chem. 2008, 109, 691–702. [Google Scholar] [CrossRef]
- Carunchio, F.; Crescenzi, C.; Girelli, A.M.; Messina, A.; Tarola, A.M. Oxidation of ferulic acid by laccase: Identification of the products and inhibitory effects of some dipeptides. Talanta 2001, 55, 189–200. [Google Scholar] [CrossRef]
- Slocum, T.L.; Deupree, J.D. Interference of Biogenic-Amines with the Measurement of Proteins Using Bicinchoninic Acid. Anal. Biochem. 1991, 195, 14–17. [Google Scholar] [CrossRef]
- Lathasree, S.; Rao, A.N.; SivaSankar, B.; Sadasivam, V.; Rengaraj, K. Heterogeneous photocatalytic mineralisation of phenols in aqueous solutions. J. Mol. Catal. A Chem. 2004, 223, 101–105. [Google Scholar] [CrossRef]
- Whitaker, J.R. Polyphenol oxidase. In Food Enzymes; Springer: Boston, MA, USA, 1995; pp. 271–307. [Google Scholar]
- Rojo, M.; Gomez, M.; Isorna, P.; Estrada, P. Micellar catalysis of polyphenol oxidase in AOT/cyclohexane. J. Mol. Catal. B Enzym. 2001, 11, 857–865. [Google Scholar] [CrossRef]
- Ralph, J.; Bunzel, M.; Marita, J.; Hatfield, R.; Lu, F.; Kim, H.; Schatz, P.; Grabber, J.; Steinhart, H. Peroxidase-dependent cross-linking reactions of p-hydroxycinnamates in plant cell walls. Phytochem. Rev. 2004, 3, 79–96. [Google Scholar] [CrossRef]
- Fulcrand, H.; Cheminat, A.; Brouillard, R.; Cheynier, V. Characterization of compounds obtained by chemical oxidation of caffeic acid in acidic conditions. Phytochemistry 1994, 35, 499–505. [Google Scholar] [CrossRef]
- Ward, G.; Hadar, Y.; Bilkis, I.; Konstantinovsky, L.; Dosoretz, C.G. Initial steps of ferulic acid polymerization by lignin peroxidase. J. Bio. Chem. 2001, 276, 18734–18741. [Google Scholar] [CrossRef] [Green Version]
- Micard, V.; Grabber, J.H.; Ralph, J.; Renard, C.; Thibault, J.F. Dehydrodiferulic acids from sugar-beet pulp. Phytochemistry 1997, 44, 1365–1368. [Google Scholar] [CrossRef]
- Nenadis, N.; Zhang, H.Y.; Tsimidou, M.Z. Structure-antioxidant activity relationship of ferulic acid derivatives: Effect of carbon side chain characteristic groups. J. Agric. Food Chem. 2003, 51, 1874–1879. [Google Scholar] [CrossRef]
- Ota, A.; Abramovic, H.; Abram, V.; Ulrih, N.P. Interactions of p-coumaric, caffeic and ferulic acids and their styrenes with model lipid membranes. Food Chem. 2011, 125, 1256–1261. [Google Scholar] [CrossRef]
- Brown RJ, C.; Brown RF, C. Melting point and molecular symmetry. J. Chem. Educ. 2000, 77, 724–731. [Google Scholar] [CrossRef]
- Perez-Jimenez, J.; Saura-Calixto, F. Antioxidant capacity of dietary polyphenols determined by ABTS assay: A kinetic expression of the results. Int. J. Food Sci. Technol. 2008, 43, 185–191. [Google Scholar] [CrossRef]
- Sanchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. A procedure to measure the antiradical efficiency of polyphenols. J. Sci. Food Agric. 1998, 76, 270–276. [Google Scholar] [CrossRef]
- Pekkarinen, S.S.; Stockmann, H.; Schwarz, K.; Heinonen, I.M.; Hopia, A.I. Antioxidant activity and partitioning of phenolic acids in bulk and emulsified methyl linoleate. J. Agric. Food Chem. 1999, 47, 3036–3043. [Google Scholar] [CrossRef]
- Matsuura, T.; Ohkatsu, Y. Phenolic antioxidants: Effect of o-benzyl substituents. Polym. Degrad. Stab. 2000, 70, 59–63. [Google Scholar] [CrossRef]
- Srinivasan, M.; Sudheer, A.R.; Menon, V.P. Ferulic acid: Therapeutic potential through its antioxidant property. J. Clin. Biochem. Nutr. 2007, 40, 92–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.L.; Liu, J.L.; Hu, Y.B.; Song, X.; Zhao, Y.R. An antioxidant exopolysaccharide devoid of pro-oxidant activity produced by the soil bacterium Bordetella sp B. Bioresour. Technol. 2012, 124, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Sheih, I.C.; Wu, T.K.; Fang, T.J. Antioxidant properties of a new antioxidative peptide from algae protein waste hydrolysate in different oxidation systems. Bioresour. Technol. 2009, 100, 3419–3425. [Google Scholar] [CrossRef] [PubMed]
- Kanski, J.; Aksenova, M.; Stoyanova, A.; Butterfield, D.A. Ferulic acid antioxidant protection against hydroxyl and peroxyl radical oxidation in synaptosomal and neuronal cell culture systems in vitro: Structure-activity studies. J. Nutr. Biochem. 2002, 13, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Loo, G. Redox-sensitive mechanisms of phytochemical-mediated inhibition of cancer cell proliferation (Review). J. Nutr. Biochem. 2003, 14, 64–73. [Google Scholar] [CrossRef]
- Janicke, B.; Onning, G.; Oredsson, S.M. Differential effects of ferulic acid and p-coumaric acid on S phase distribution and length of S phase in the human colonic cell line Caco. J. Agric. Food Chem. 2005, 53, 6658–6665. [Google Scholar] [CrossRef]

| A | P1 | P5 | ||
|---|---|---|---|---|
| Position | δ1H (ppm) | δ13C | δ1H (ppm) | δ13C |
| A1, B1 | - | 130.1 | - | 130.6 |
| A2, B2 | 6.79 (2H) | 107.6 | 7.06 (2H) | 109.5 |
| A3, B3 | - | 146.5 | - | 146.8 |
| A4, B4 | - | 147.2 | - | 147.8 |
| A5, B5 | 6.93 (2H) | 115.1 | 6.96 (2H) | 116.3 |
| A6, B6 | 6.84 (2H) | 117.5 | 6.83 (2H) | 118.2 |
| A7, B7 | 5.69 (2H) | 82.5 | 5.78 (2H) | 82.9 |
| A8, B8 | 3.56 (2H) | 48.6 | 4.08 (2H) | 49.1 |
| OMe A3, B3 | 3.91 (3H) | 56.4 | 3.87 (2H) | 56.7 |
| OH A4, B4 | 5.86 (2H) | - | 5.91 (2H) | - |
| A9, B9 | - | 174.5 | 9.04 | 173.1 |
| B | P2 | P3 | P4 | |||
|---|---|---|---|---|---|---|
| Position | δ1H (ppm) | δ13C | δ1H (ppm) | δ13C | δ1H (ppm) | δ13C |
| A1 | - | 129.3 | - | 129.8 | - | 128.5 |
| A2 | 6.99 | 112.42 | 6.85 | 110.8 | 7.39 (1H) | 110.9 |
| A5 | - | 128.07 | 6.9 | 127.1 | - | 125.8 |
| A6 | 7.02 (1H) | 113.17 | 7.15 | 115.2 | 7.32 (1H) | 115.9 |
| A7 | 7.68 | 146.03 | - | 149.4 | 6.88 | 150.0 |
| A8 | 6.3 | 117.2 | 6.5 | 119.1 | 7.33 (1H) | 118.6 |
| B1 | - | 131.67 | - | 129.3 | - | 131.4 |
| B2 | 7.17 (1H) | 108.41 | 7.1 | 106.7 | 7.32 (1H) | 105.6 |
| B5 | 6.99 (1H) | 116.7 | 6.89 | 115.8 | 6.92 (1H) | 116.2 |
| B6 | 7.23 (1H) | 117.7 | 7.3 | 118.1 | 7.44 (1H) | 121.3 |
| B7 | 5.84 (1H) | 86.2 | 5.79 | 86.9 | - | 88.1 |
| B8 | 3.86 (1H) | 53.31 | - | 52.6 | 3.75 | 56.4 |
| A3 | - | 145.3 | - | 147.2 | - | 146.5 |
| B3 | - | 148.8 | - | 147.8 | - | 148.0 |
| A4 | - | 146.2 | - | 147.6 | - | 147.9 |
| B4 | - | 146.6 | - | 149.1 | - | 148.5 |
| OMe A3 | 3.86 (3H) | 55.9 | 3.82 | 54.9 | 3.87 (3H) | 55.84 |
| OMe B3 | 3.86 (3H) | 55.95 | 3.80 | 55.6 | 3.82 (3H) | 55.93 |
| OH A4 | - | - | - | - | 9.86 (1H) | - |
| OH B4 | 9.01 | - | 8.99 | - | 9.69 | - |
| A9 | 9.01 | 168.3 | - | 166.9 | 9.05 | 169.4 |
| B9 | 8.98 | 172.9 | - | 169.3 | - | - |
| LogP | Melting Point (°C) | |
|---|---|---|
| FA | 1.45 ± 0.04 f | 173.4 ± 3.3 c |
| P1 | 1.51± 0.02 e | 183.5 ± 4.1 b |
| P2 | 1.59 ± 0.02 d | 155.8 ± 4.3 d |
| P3 | 1.67 ± 0.05 c | 136.4 ± 3.7 e |
| P4 | 1.74 ± 0.04 b | 149.4 ± 2.6 d |
| P5 | 1.79 ± 0.06 b | 201.2 ± 3.4 a |
| P6 | 1.93 ± 0.06 a | 206.9 ± 2.9 a |
| P7 | 1.96 ± 0.04 a | 203.5 ± 1.6 a |
| Antiradical Activity (ABTS) | Anti-Proliferative Activity (Neutral Red) Assay | ||
|---|---|---|---|
| IC50 (µM) | TEAC | IC50 (mM) | |
| FA | 7.2 ± 0.1 e | 1.24 ± 0.04 b | 3.6 ± 0.3 c |
| P1 | 24.4 ± 0.8 a | 0.36 ± 0.04 d | 7.9 ± 0.4 a |
| P2 | 6.4 ± 0.2 f | 1.39 ± 0.05 a | 1.8 ± 0.2 f |
| P3 | 6.6 ± 0.3 f | 1.35 ± 0.04 a | 2.1 ± 0.2 e |
| P4 | 19.9 ± 1.1 b | 0.45 ± 0.05 d | 3.2 ± 0.3 c |
| P5 | 11.9 ± 0.4 c | 0.75 ± 0.05 c | 4.7 ± 0.3 b |
| P6 | 6.5 ± 0.2 f | 1.37 ± 0.03 a | 2.1 ± 0.1 e |
| P7 | 7.1 ± 0.1 e | 1.26 ± 0.04 b | 2.5 ± 0.2 d |
| Trolox | 8.9 ± 0.2 d | 1 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aljawish, A.; Chevalot, I.; Paris, C.; Muniglia, L. Enzymatic Oxidation of Ferulic Acid as a Way of Preparing New Derivatives. BioTech 2022, 11, 55. https://doi.org/10.3390/biotech11040055
Aljawish A, Chevalot I, Paris C, Muniglia L. Enzymatic Oxidation of Ferulic Acid as a Way of Preparing New Derivatives. BioTech. 2022; 11(4):55. https://doi.org/10.3390/biotech11040055
Chicago/Turabian StyleAljawish, Abdulhadi, Isabelle Chevalot, Cédric Paris, and Lionel Muniglia. 2022. "Enzymatic Oxidation of Ferulic Acid as a Way of Preparing New Derivatives" BioTech 11, no. 4: 55. https://doi.org/10.3390/biotech11040055





