Soil Colour and Plant-Wax Markers: Application in Forensic Investigations under Urban Subtropical Environments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Sites and Sampling
2.2. Colour Spectroscopy
2.3. Plant-Wax Compound Determination by Gas Chromatography (GC/MS)
2.4. Statistical Analysis
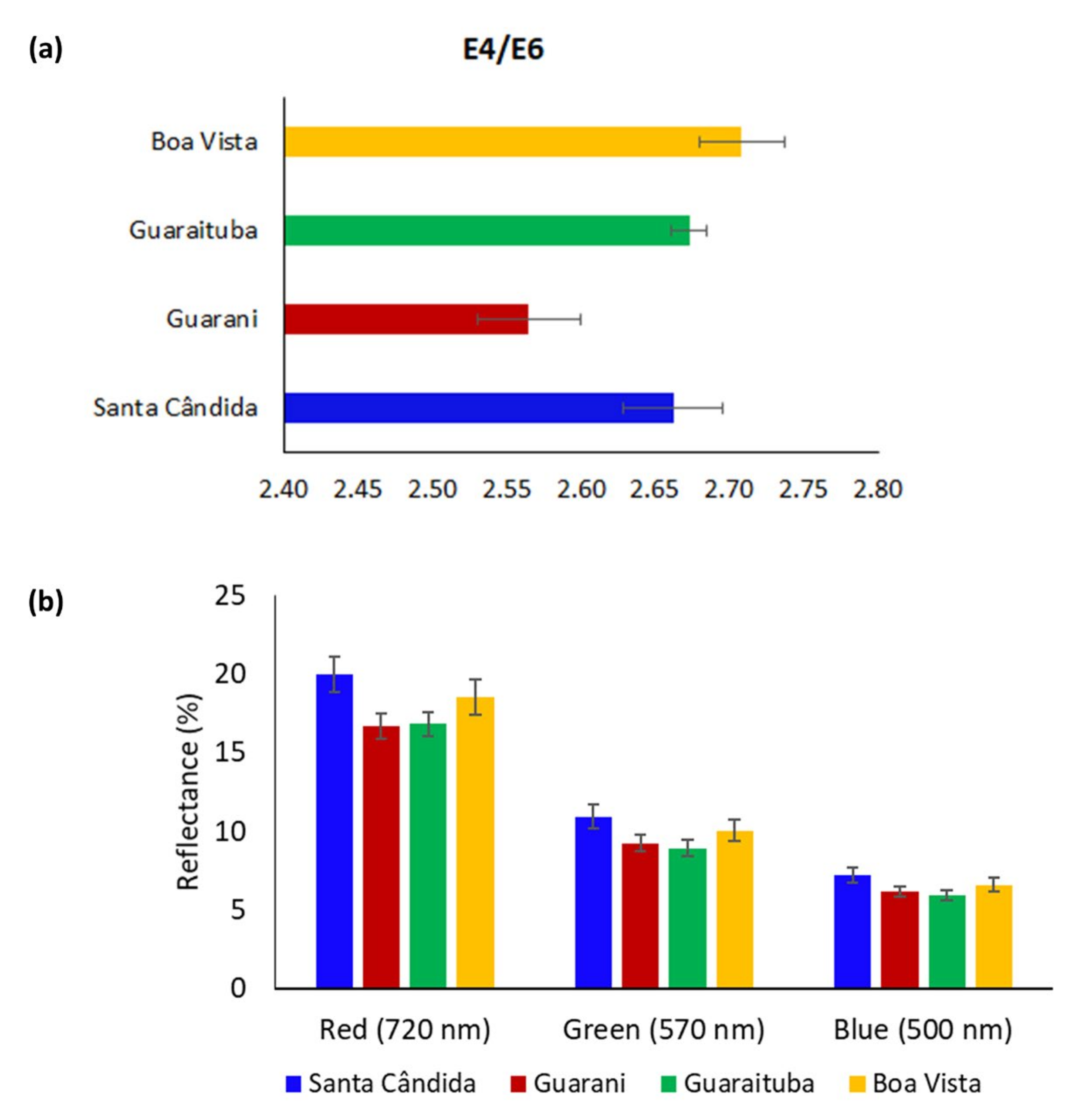
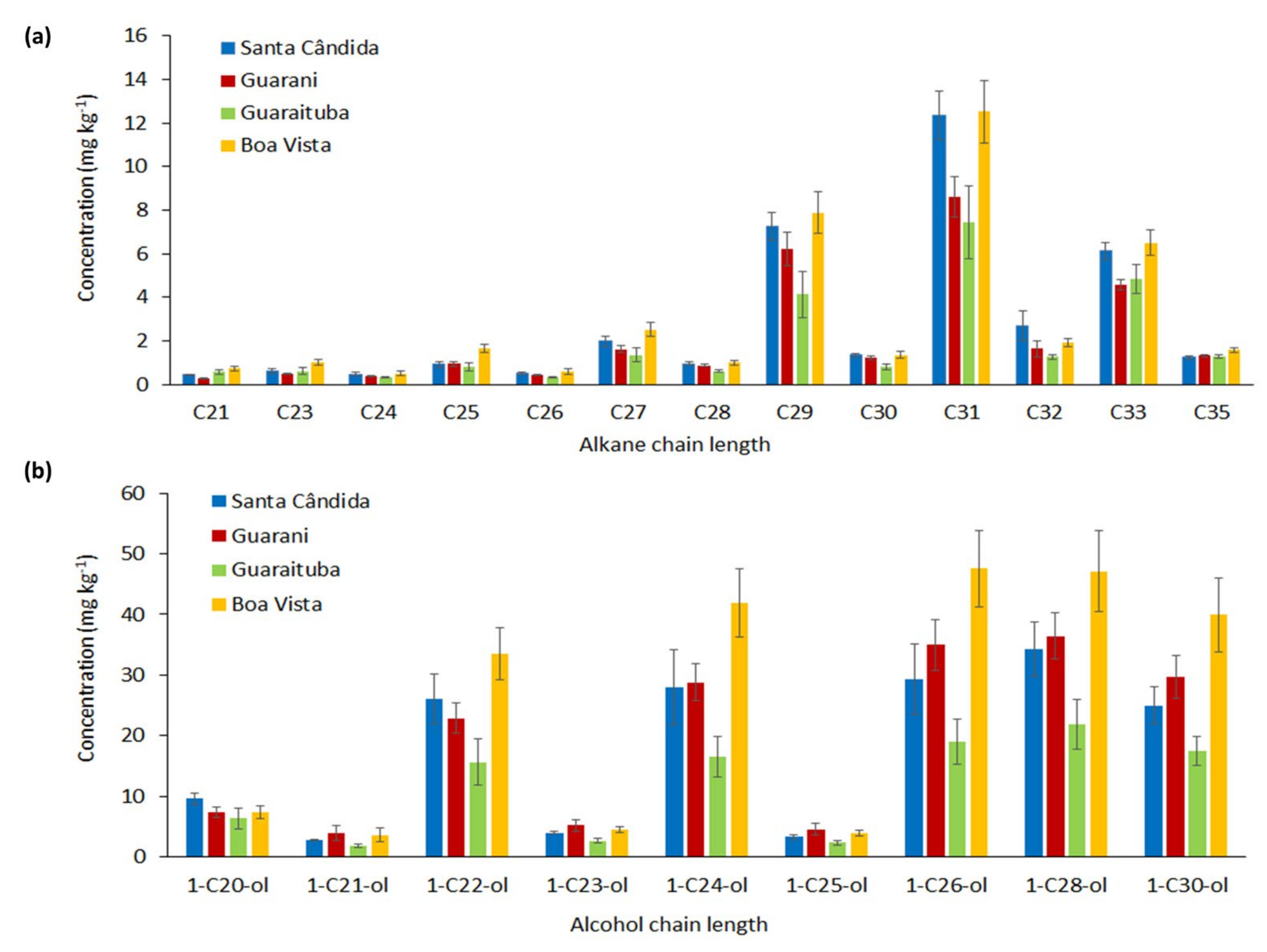
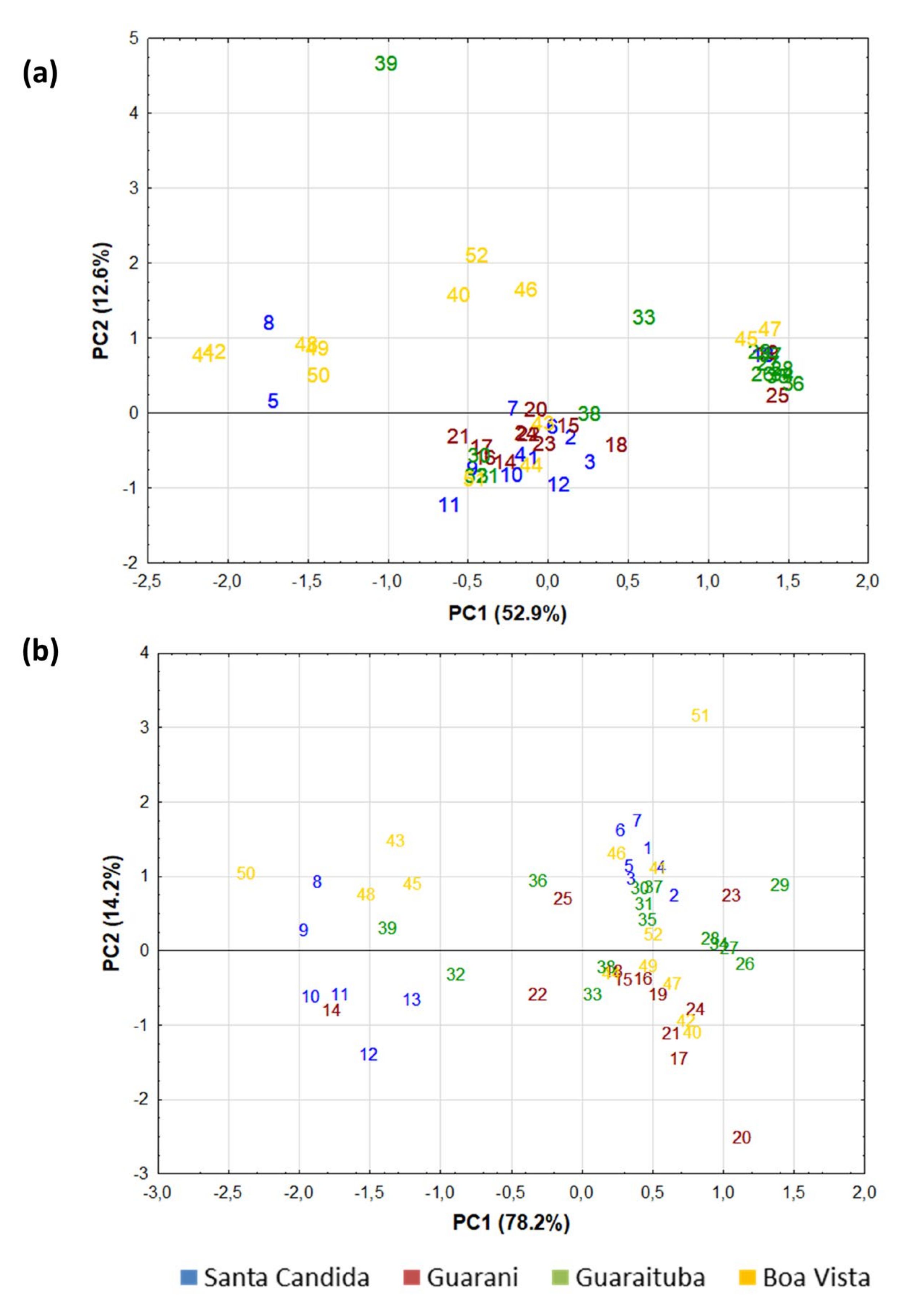
3. Results
- (a)
- Samples collected over long and medium distances (kilometres and metres)—in this comparison, samples from the same neighbourhood (corners and centre of the quadrant) must be placed in the same homogeneous group if the sample comes from the same place on the ground. Santa Cândida (intragroup similarities): [10]—95%; the present study (Figure 6a)—42%. Guarani (intragroup similarities): [10]—97%; present study (Figure 6a)—42%. Guaraituba (intragroup similarities): [10]—97%; present study (Figure 6a)—42%. Boa Vista (intragroup similarities): [10]—93%; present study (Figure 6a)—42%.
- (b)
- Samples collected over a short distance from the sole of a boot (centimetres)—in this comparison, samples collected from the same boot sole should be placed in the same homogeneous group if the sample comes from the same place on the ground. Santa Cândida (intragroup similarities): [10]—97%; present study (Figure 6a)—42%. Guarani (intragroup similarities): [10]—98%; present study (Figure 6a)—42%. Guaraituba (intragroup similarities): [10]—98%; present study (Figure 6a)—42%. Boa Vista (intragroup similarities): [10]—94%; present study (Figure 6a)—73%.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morgan, R.M.; Bull, P.A. Data Interpretation in Forensic Sediment and Soil Geochemistry. Environ. Forensics 2006, 7, 325–334. [Google Scholar] [CrossRef] [Green Version]
- Ritz, K.; Dawson, L.; Miller, D. Criminal and Environmental Soil Forensics; Springer: New York, NY, USA, 2008. [Google Scholar]
- Dawson, L.A.; Hillier, S. Measurement of soil characteristics for forensic applications. Surf. Interface Anal. 2010, 42, 363–377. [Google Scholar] [CrossRef]
- Fitzpatrick, R.W.; Raven, M.D. Guidelines for Conducting Criminal and Environmental Soil Forensic Investigations: Version 7.0.076; Acid Sulfate Soils Centre: Adelaide, Australia, 2012. [Google Scholar]
- Murray, K.R.; Fitzpatrick, R.W.; Bottrill, R.S.; Berry, R.; Kobus, H. Soil transference patterns on bras: Image processing and laboratory dragging experiments. Forensic Sci. Int. 2016, 258, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, R.; Kumar, R.; Diwan, P.; Sharma, V. Thermogravimetric analysis and chemometric based methods for soil examination: Application to soil forensics. Forensic Chem. 2020, 17, 100191. [Google Scholar] [CrossRef]
- Nakai, I.; Furuya, S.; Bong, W.; Abe, Y.; Osaka, K.; Matsumoto, T.; Itou, M.; Ohta, A.; Ninomiya, T. Quantitative analysis of heavy elements and semi-quantitative evaluation of heavy mineral compositions of sediments in Japan for construction of a forensic soil database using synchrotron radiation X-ray analyses. X-ray Spectrom. 2014, 43, 38–48. [Google Scholar] [CrossRef]
- Melo, V.F.; Testoni, S.A.; Dawson, L.; de Lara, A.G.; da Silva Salvador, F.A. Can analysis of a small clod of soil help to solve a murder case? Sci. Justice 2019, 59, 667–677. [Google Scholar] [CrossRef]
- Testoni, S.A.; Melo, V.F.; Dawson, L.A.; Salvador, F.A.D.S.; Kunii, P.A. Validation of a Standard Operating Procedure (SOP) for Forensic Soils Investigation in Brazil. Revista Brasileira de Ciência do Solo 2019, 43, e0190010. [Google Scholar] [CrossRef] [Green Version]
- Prandel, L.; Melo, V.F.; Testoni, S.A.; Brinatti, A.M.; Saab, S.D.C.; Dawson, L.A. Spectroscopic techniques applied to discriminate soils for forensic purposes. Soil Res. 2020, 58, 151. [Google Scholar] [CrossRef]
- Dawson, L.A.; Mayes, R.W. Criminal and Environmental Soil Forensics: Soil as Physical Evidence in Forensic Investigations, Introduction to Environmental Forensics, 3rd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; pp. 457–486. [Google Scholar] [CrossRef]
- Dawson, L. Soil organic characterisation in forensic case work. J. Int. Geosci. 2017, 40, 157–165. [Google Scholar] [CrossRef]
- Melo, V.; Mazzetto, J.M.; Dieckow, J.; Bonfleur, E.J. Factor analysis of organic soils for site discrimination in a forensic setting. Forensic Sci. Int. 2018, 290, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Mazzetto, J.M.; Melo, V.F.; Bonfleur, E.; Vidal-Torrado, P.; Dieckow, J. Potential of soil organic matter molecular chemistry determined by pyrolysis-gas chromatography/mass spectrometry for forensic investigations. Sci. Justice 2019, 59, 635–642. [Google Scholar] [CrossRef]
- Schellekens, J.; Almeida-Santos, T.; Macedo, R.S.; Buurman, P.; Kuyper, T.W.; Vidal-Torrado, P. Molecular composition of several soil organic matter fractions from anthropogenic black soils (Terra Preta de Índio) in Amazonia—A pyrolysis-GC/MS study. Geoderma 2017, 288, 154–165. [Google Scholar] [CrossRef]
- Dawson, L.A.; Towers, W.; Mayes, R.W.; Craig, J.; Väisänen, R.K.; Waterhouse, E.C. The use of plant hydrocarbon signatures in characterizing soil organic matter. Geol. Soc. Spec. Publ. 2004, 232, 269–276. [Google Scholar] [CrossRef]
- Carvalho, Á.; Ribeiro, H.; Mayes, R.; Guedes, A.; Abreu, I.; Noronha, F.; Dawson, L. Organic matter characterization of sediments in two river beaches from northern Portugal for forensic application. Forensic Sci. Int. 2013, 233, 403–415. [Google Scholar] [CrossRef]
- Madureira-Carvalho, Á.; Ribeiro, H.; Newman, G.; Brewer, M.J.; Guedes, A.; Abreu, I.; Noronha, F.; Dawson, L. Geochemical analysis of sediment samples for forensic purposes: Characterisation of two river beaches from the Douro River, Portugal. Aust. J. Forensic Sci. 2018, 52, 222–234. [Google Scholar] [CrossRef]
- Mcculloch, G.; Dawson, L.A.; Ross, J.M.; Morgan, R.M. The discrimination of geoforensic trace material from close proximity locations by organic pro fi ling using HPLC and plant wax marker analysis by GC. Forensic Sci. Int. 2018, 288, 310–326. [Google Scholar] [CrossRef] [PubMed]
- Demanèche, S.; Schauser, L.; Dawson, L.; Franqueville, L.; Simonet, P. Microbial soil community analyses for forensic science: Application to a blind test. Forensic Sci. Int. 2016, 270, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Meyers, P.A.; Ishiwatari, R. Lacustrine organic geochemistry-an overview of indicators of organic matter sources and dia-genesis in lake sediments. Org. Geochem. 1993, 20, 867–900. [Google Scholar] [CrossRef] [Green Version]
- Meyers, P.A. Application of organic geochemistry to paleolimnological reconstruction: A summary of examples from the Laurention Great Lakes. Org. Geochem. 2003, 34, 261–289. [Google Scholar] [CrossRef]
- Schellekens, J.; Buurman, P.; Pontevedra-Pombal, X. Selecting parameters for the environmental interpretation of peat mo-lecular chemistry–A pyrolysis-GC/MS study. Org. Geochem. 2009, 40, 678–691. [Google Scholar] [CrossRef]
- Dove, H.; Mayes, R.; Freer, M. Effects of species, plant part, and plant age on the n-alkane concentrations in the cuticular wax of pasture plants. Aust. J. Agric. Res. 1996, 47, 1333–1347. [Google Scholar] [CrossRef]
- Dove, H.; Mayes, R.W. Protocol for the analysis of n-alkanes and other plant-wax compounds and for their use as markers for quantifying the nutrient supply of large mammalian herbivores. Nat. Protoc. 2006, 1, 1680–1697. [Google Scholar] [CrossRef] [PubMed]
- Mayes, R.W.; Macdonald, L.M.; Ross, J.M.; Dawson, L.A. Discrimination of Domestic Garden Soils Using Plant Wax Compounds as Markers. In Criminal and Environmental Soil Forensics; Ritz, K., Dawson, L.A., Miller, D., Eds.; Springer: Cham, Switzerland, 2008; pp. 463–476. [Google Scholar] [CrossRef]
- McCulloch, G.; Dawson, L.; Brewer, M.; Morgan, R. The identification of markers for Geoforensic HPLC profiling at close proximity sites. Forensic Sci. Int. 2017, 272, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.G.; Mayes, R.W.; Raats, J.G. Effect of species, plant part, and season of harvest on n-alkane concentrations in the cuticular wax of common rangeland grasses from southern Africa. Aust. J. Agric. Res. 2001, 52, 875–882. [Google Scholar] [CrossRef]
- Thanasoulias, N.C.; Piliouris, E.T.; Kotti, E.M.-S.; Evmiridis, N.P. Application of multivariate chemometrics in forensic soil discrimination based on the UV-Vis spectrum of the acid fraction of humus. Forensic Sci. Int. 2002, 130, 73–82. [Google Scholar] [CrossRef]
- Fitzpatrick, R.; Raven, M. A Criminal Case Study Involving Transference of Acid Sulfate Soil Material from a Crime Scene to Forensic Evidence. In Inland Acid Sulfate Soil Systems Across Australia; CRC LEMEL: Perth, Australia, 2009; Chapter 8; pp. 151–161. [Google Scholar]
- Cheshire, K.; Morgan, R.; Holmes, J. The potential for geochemical discrimination of single- and mixed-source soil samples from close proximity urban parkland locations. Aust. J. Forensic Sci. 2017, 49, 161–174. [Google Scholar] [CrossRef] [Green Version]
- Bourguignon, L.; Dawson, L.A.; Hellmann, A.; Marlof, A.; Fernadez Rodriquez, A.; Repele, M.; Utehaag, S. Best Practice Manual for the Forensic Comparison of Soil Traces; European Network of Forensic Science Institutes: Wiesbaden, Germany, 2019. [Google Scholar]
- Cheng, L.; Shi, S.; Li, Q.; Chen, J.; Zhang, H.; Lu, Y. Progressive Degradation of Crude Oil n-Alkanes Coupled to Methane Production under Mesophilic and Thermophilic Conditions. PLoS ONE 2014, 9, e113253. [Google Scholar] [CrossRef]
- Soil Survey Staff. Keys to Soil Taxonomy; Natural Resources Consersation Services: Washington, DC, USA, 2014. [Google Scholar]
- Dos Santos, H.G.; Jacomine, P.K.T.; dos Anjos, L.H.C.; de Oliveira, V.A.; Lumbreras, J.F.; Coelho, M.R.; de Almeida, J.A.; de Araujo Filho, J.C.; de Oliveira, J.B.; Cunha, T.J.F. Sistema Brasileiro de Classificação de Solos; Empresa Brasileira de Pesquisa Agropecuária: Brasilia, Brasil, 2018. [Google Scholar]
- Kumar, R.; Sharma, V. Chemometrics in forensic science. TrAC Trends Anal. Chem. 2018, 105, 191–201. [Google Scholar] [CrossRef]
- Morgan, R.M.; Scott, K.R.; Ainley, J.; Bull, P.A. Journey history reconstruction from the soils and sediments on footwear: An empirical approach. Sci. Justice 2019, 59, 306–316. [Google Scholar] [CrossRef]
- Chen, Y.; Senesi, N.; Schnitzer, M. Information provided on humic substances by E4/E6 ratio. Soil Sci. Soc. Am. J. 1977, 41, 352–358. [Google Scholar] [CrossRef]
- Chauhan, R.; Kumar, R.; Sharma, V. Soil forensics: A spectroscopic examination of trace evidence. Microchem. J. 2020, 139, 74–84. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontologia Electronica 2001, 4, 9. [Google Scholar]
- StatSoft. Statistica (Data Analysis Software System); StatSoft: Tulsa, OK, USA, 2011. [Google Scholar]
- Zalba, P.; Amiotti, N.M.; Galantini, J.A.; Pistola, S. Soil Humic and Fulvic Acids from Different Land-Use Systems Evaluated by E4/E6 Ratios. Commun. Soil Sci. Plant Anal. 2016, 47, 1675–1679. [Google Scholar] [CrossRef]
- Oktaba, L.; Odrobińska, D.; Uzarowicz, Ł. The impact of different land uses in urban area on humus quality. J. Soils Sediments 2018, 18, 2823–2832. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, R.; Kumar, R.; Kumar, V.; Sharma, K.; Sharma, V. On the discrimination of soil samples by derivative diffuse reflectance UV–vis-NIR spectroscopy and chemometric methods. Forensic Sci. Int. 2020, 319, 110655. [Google Scholar] [CrossRef] [PubMed]
- Senesi, N.; Sipos, S. Molecular-weight distribution, analytical and spectroscopic characterization of humic fractions sequentialy isolated by organic-solvents from a brown coal acid. Org. Geochem. 1985, 8, 157–162. [Google Scholar] [CrossRef]
- Mbarek, B.H.; Gargouri, K.; Mbadra, C.; Chaker, R.; Souidi, Y.; Abbas, O.; Baeten, V.; Rigane, H. Change and spatial variability of soil organic matter humification after long-term tillage and olive mill wastewater application in arid regions. Soil Res. 2020, 58, 388. [Google Scholar] [CrossRef]
- Sharma, V.; Chauhan, R.; Kumar, R. Spectral characteristics of organic soil matter: A comprehensive review. Microchem. J. 2021, 171, 106836. [Google Scholar] [CrossRef]
- Sachse, D.; Billault, I.; Bowen, G.J.; Chikaraishi, Y.; Dawson, T.E.; Feakins, S.J.; Freeman, K.H.; Magill, C.R.; McInerney, F.A.; van der Meer, M.T.; et al. Molecular Paleohydrology: Interpreting the Hydrogen-Isotopic Composition of Lipid Biomarkers from Photosynthesizing Organisms. Annu. Rev. Earth Planet. Sci. 2012, 40, 221–249. [Google Scholar] [CrossRef] [Green Version]
- Vogts, A.; Moossen, H.; Rommerskirchen, F.; Rullkötter, J. Distribution patterns and stable carbon isotopic composition of alkanes and alkan-1-ols from plant waxes of African rain forest and savanna C3 species. Org. Geochem. 2009, 40, 1037–1054. [Google Scholar] [CrossRef]
- Holtvoeth, J.; Rushworth, D.; Copsey, H.; Imeri, A.; Cara, M.; Vogel, H.; Wagner, T.; Wolff, G.A. Improved end-member characterisation of modern organic matter pools in the Ohrid Basin (Albania, Macedonia) and evaluation of new palaeoenvironmental proxies. Biogeosciences 2016, 13, 795–816. [Google Scholar] [CrossRef] [Green Version]




| UTM (22J) | |||||
|---|---|---|---|---|---|
| Sample | Position 1 | Place | E-W | N-S | Parent Material |
| Site 1 | |||||
| 1 | Bottom right | Santa Cândida | 678,203 | 7,192,226 | Claystone |
| 2 | Bottom left | Santa Cândida | 678,203 | 7,192,226 | Claystone |
| 3 | Top right | Santa Cândida | 678,203 | 7,192,226 | Claystone |
| 4 | Top left | Santa Cândida | 678,203 | 7,192,226 | Claystone |
| 5 | Bottom right | Santa Cândida | 678,203 | 7,192,226 | Claystone |
| 6 | Bottom left | Santa Cândida | 678,203 | 7,192,226 | Claystone |
| 7 | Top right | Santa Cândida | 678,203 | 7,192,226 | Claystone |
| 8 | Top left | Santa Cândida | 678,203 | 7,192,226 | Claystone |
| 9 | Footprint/toes | Santa Cândida | 678,203 | 7,192,226 | Claystone |
| 10 | Footprint/heel | Santa Cândida | 678,203 | 7,192,226 | Claystone |
| 11 | Footprint/heel | Santa Cândida | 678,203 | 7,192,226 | Claystone |
| 12 | Footprint/toes | Santa Cândida | 678,203 | 7,192,226 | Claystone |
| 13 | Footprint/heel | Santa Cândida | 678,203 | 7,192,226 | Claystone |
| Site 2 | |||||
| 14 | Bottom right | Guarani | 682,747 | 7,192,633 | Limestone |
| 15 | Bottom left | Guarani | 682,747 | 7,192,633 | Limestone |
| 16 | Top right | Guarani | 682,747 | 7,192,633 | Limestone |
| 17 | Top left | Guarani | 682,747 | 7,192,633 | Limestone |
| 18 | Bottom right | Guarani | 682,747 | 7,192,633 | Limestone |
| 19 | Bottom left | Guarani | 682,747 | 7,192,633 | Limestone |
| 20 | Top right | Guarani | 682,747 | 7,192,633 | Limestone |
| 21 | Top left | Guarani | 682,747 | 7,192,633 | Limestone |
| 22 | Footprint/toes | Guarani | 682,747 | 7,192,633 | Limestone |
| 23 | Footprint/heel | Guarani | 682,747 | 7,192,633 | Limestone |
| 24 | Footprint/heel | Guarani | 682,747 | 7,192,633 | Limestone |
| 25 | Footprint/toes | Guarani | 682,747 | 7,192,633 | Limestone |
| Site 3 | |||||
| 26 | Bottom right | Guaraituba | 683,189 | 7,195,492 | Limestone |
| 27 | Bottom left | Guaraituba | 683,189 | 7,195,492 | Limestone |
| 28 | Top right | Guaraituba | 683,189 | 7,195,492 | Limestone |
| 29 | Top left | Guaraituba | 683,189 | 7,195,492 | Limestone |
| 30 | Bottom right | Guaraituba | 683,189 | 7,195,492 | Limestone |
| 31 | Bottom left | Guaraituba | 683,189 | 7,195,492 | Limestone |
| 32 | Top right | Guaraituba | 683,189 | 7,195,492 | Limestone |
| 33 | Top left | Guaraituba | 683,189 | 7,195,492 | Limestone |
| 34 | Footprint/toes | Guaraituba | 683,189 | 7,195,492 | Limestone |
| 35 | Footprint/heel | Guaraituba | 683,189 | 7,195,492 | Limestone |
| 36 | Footprint/heel | Guaraituba | 683,189 | 7,195,492 | Limestone |
| 37 | Footprint/toes | Guaraituba | 683,189 | 7,195,492 | Limestone |
| 38 | Footprint/heel | Guaraituba | 683,189 | 7,195,492 | Limestone |
| 39 | Footprint/heel | Guaraituba | 683,189 | 7,195,492 | Limestone |
| Site 4 | |||||
| 40 | Bottom right | Boa Vista | 676,186 | 7,190,193 | Claystone |
| 41 | Bottom left | Boa Vista | 676,186 | 7,190,193 | Claystone |
| 42 | Top right | Boa Vista | 676,186 | 7,190,193 | Claystone |
| 43 | Top left | Boa Vista | 676,186 | 7,190,193 | Claystone |
| 44 | Bottom right | Boa Vista | 676,186 | 7,190,193 | Claystone |
| 45 | Bottom left | Boa Vista | 676,186 | 7,190,193 | Claystone |
| 46 | Top right | Boa Vista | 676,186 | 7,190,193 | Claystone |
| 47 | Top left | Boa Vista | 676,186 | 7,190,193 | Claystone |
| 48 | Footprint/toes | Boa Vista | 676,186 | 7,190,193 | Claystone |
| 49 | Footprint/heel | Boa Vista | 676,186 | 7,190,193 | Claystone |
| 50 | Footprint/heel | Boa Vista | 676,186 | 7,190,193 | Claystone |
| 51 | Footprint/toes | Boa Vista | 676,186 | 7,190,193 | Claystone |
| 52 | Footprint/heel | Boa Vista | 676,186 | 7,190,193 | Claystone |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Testoni, S.; Dawson, L.; Melo, V.; Lopes-Mazzetto, J.; Ramalho, B.; Salvador, F. Soil Colour and Plant-Wax Markers: Application in Forensic Investigations under Urban Subtropical Environments. Forensic Sci. 2022, 2, 57-71. https://doi.org/10.3390/forensicsci2010005
Testoni S, Dawson L, Melo V, Lopes-Mazzetto J, Ramalho B, Salvador F. Soil Colour and Plant-Wax Markers: Application in Forensic Investigations under Urban Subtropical Environments. Forensic Sciences. 2022; 2(1):57-71. https://doi.org/10.3390/forensicsci2010005
Chicago/Turabian StyleTestoni, Samara, Lorna Dawson, Vander Melo, Josiane Lopes-Mazzetto, Bruna Ramalho, and Fábio Salvador. 2022. "Soil Colour and Plant-Wax Markers: Application in Forensic Investigations under Urban Subtropical Environments" Forensic Sciences 2, no. 1: 57-71. https://doi.org/10.3390/forensicsci2010005







