Kinetic Interjoint Coordination in Lower Limbs during Gait in Patients with Hemiparesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Gait Analysis
2.3. PCA Using Singular Value Decomposition (SVD)
2.4. Statistical Analysis
3. Results
3.1. Gait Speed and Spatiotemporal Parameters
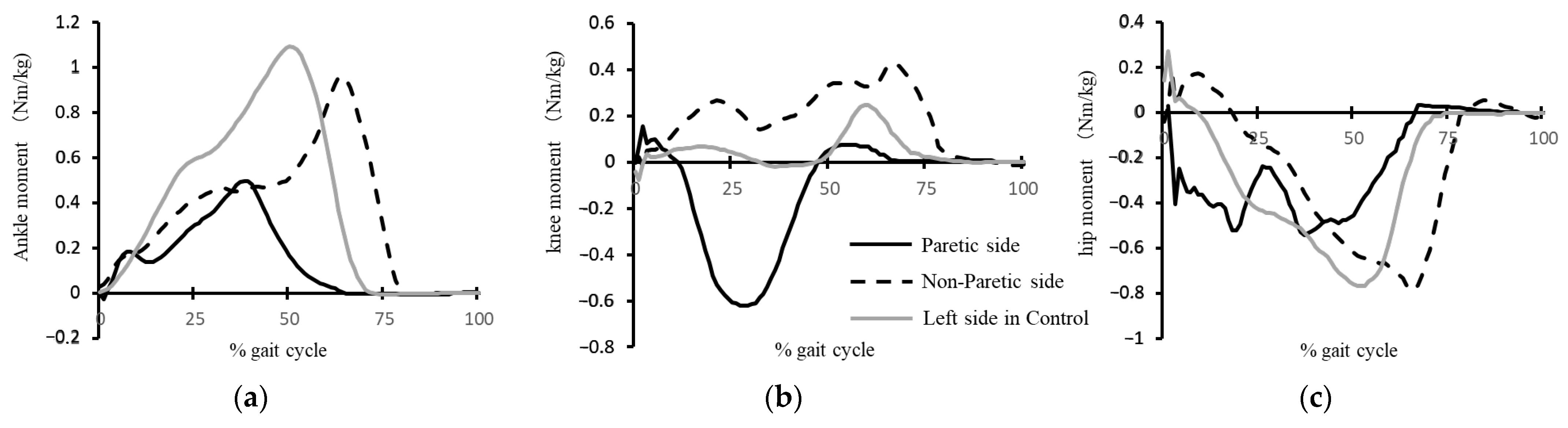
3.2. Kinetic Parameters
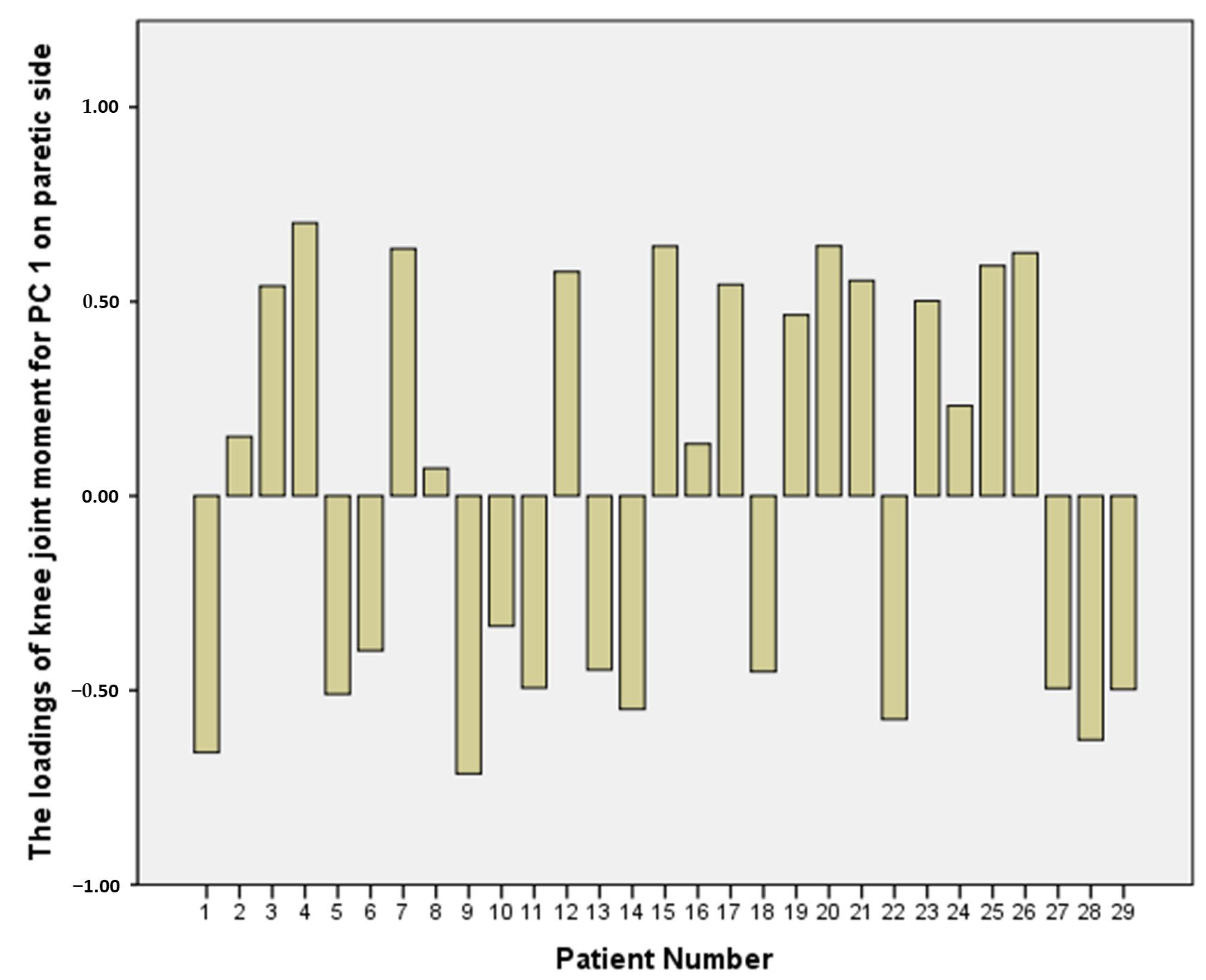
3.3. PCA-Related Parameters
4. Discussion
4.1. Percent Variance Explained by the PCs
4.2. Impaired Temporal Interjoint Coordination on the PS
4.3. Various Knee Moment Pattern Types on the PS
4.4. Kinetic Interjoint Coordination on the Non-PS
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lord, S.; McPherson, K.; McNaughton, H.; Rochester, L.; Weatherall, M. Community ambulation after stroke: How important and obtainable is it and what measures appear predictive? Arch. Phys. Med. Rehabil. 2004, 85, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Thilarajah, S.; Mentiplay, B.F.; Bower, K.J.; Tan, D.; Pua, Y.H.; Williams, G.; Koh, G.; Clark, R.A. Factors Associated With Post-Stroke Physical Activity: A Systematic Review and Meta-Analysis. Arch. Phys. Med. Rehabil. 2018, 99, 1876–1889. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.; Garrett, M.; Gronley, J.; Mulroy, S. Classification of walking handicap in the stroke population. Stroke 1995, 26, 982–989. [Google Scholar] [CrossRef]
- Franceschini, M.; Rampello, A.; Agosti, M.; Massucci, M.; Bovolenta, F.; Sale, P. Walking performance: Correlation between energy cost of walking and walking participation. new statistical approach concerning outcome measurement. PLoS ONE 2013, 8, e56669. [Google Scholar] [CrossRef]
- Fulk, G.D.; He, Y.; Boyne, P.; Dunning, K. Predicting Home and Community Walking Activity Poststroke. Stroke 2017, 48, 406–411. [Google Scholar] [CrossRef]
- Fulk, G.D.; Reynolds, C.; Mondal, S.; Deutsch, J.E. Predicting home and community walking activity in people with stroke. Arch. Phys. Med. Rehabil. 2010, 91, 1582–1586. [Google Scholar] [CrossRef] [PubMed]
- Awad, L.N.; Binder-Macleod, S.A.; Pohlig, R.T.; Reisman, D.S. Paretic Propulsion and Trailing Limb Angle Are Key Determinants of Long-Distance Walking Function After Stroke. Neurorehabil. Neural Repair 2015, 29, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Sibley, K.M.; Tang, A.; Patterson, K.K.; Brooks, D.; McIlroy, W.E. Changes in spatiotemporal gait variables over time during a test of functional capacity after stroke. J. Neuroeng. Rehabil. 2009, 6, 27. [Google Scholar] [CrossRef]
- Turns, L.J.; Neptune, R.R.; Kautz, S.A. Relationships between muscle activity and anteroposterior ground reaction forces in hemiparetic walking. Arch. Phys. Med. Rehabil. 2007, 88, 1127–1135. [Google Scholar] [CrossRef]
- Ivanenko, Y.P.; Poppele, R.E.; Lacquaniti, F. Five basic muscle activation patterns account for muscle activity during human locomotion. J. Physiol. 2004, 556, 267–282. [Google Scholar] [CrossRef]
- Clark, D.J.; Ting, L.H.; Zajac, F.E.; Neptune, R.R.; Kautz, S.A. Merging of healthy motor modules predicts reduced locomotor performance and muscle coordination complexity post-stroke. J. Neurophysiol. 2010, 103, 844–857. [Google Scholar] [CrossRef] [PubMed]
- Neptune, R.R.; Clark, D.J.; Kautz, S.A. Modular control of human walking: A simulation study. J. Biomech. 2009, 42, 1282–1287. [Google Scholar] [CrossRef] [PubMed]
- Brough, L.G.; Kautz, S.A.; Bowden, M.G.; Gregory, C.M.; Neptune, R.R. Merged plantarflexor muscle activity is predictive of poor walking performance in post-stroke hemiparetic subjects. J. Biomech. 2018, 82, 361–367. [Google Scholar] [CrossRef]
- Routson, R.L.; Clark, D.J.; Bowden, M.G.; Kautz, S.A.; Neptune, R.R. The influence of locomotor rehabilitation on module quality and post-stroke hemiparetic walking performance. Gait Posture 2013, 38, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Peterson, C.L.; Cheng, J.; Kautz, S.A.; Neptune, R.R. Leg extension is an important predictor of paretic leg propulsion in hemiparetic walking. Gait Posture 2010, 32, 451–456. [Google Scholar] [CrossRef]
- Sadeghi, H.; Sadeghi, S.; Prince, F.; Allard, P.; Labelle, H.; Vaughan, C.L. Functional roles of ankle and hip sagittal muscle moments in able-bodied gait. Clin. Biomech. 2001, 16, 688–695. [Google Scholar] [CrossRef]
- Shemmell, J.; Johansson, J.; Portra, V.; Gottlieb, G.L.; Thomas, J.S.; Corcos, D.M. Control of interjoint coordination during the swing phase of normal gait at different speeds. J. Neuroeng. Rehabil. 2007, 4, 10. [Google Scholar] [CrossRef]
- Olney, S.; Griffin, M.; Monga, T.; McBride, I. Work and power in gait of stroke patients. Arch. Phys. Med. Rehabil. 1991, 72, 309–314. [Google Scholar]
- Olney, S.J.; Griffin, M.P.; McBride, I.D. Temporal, kinematic, and kinetic variables related to gait speed in subjects with hemiplegia: A regression approach. Phys. Ther. 1994, 74, 872–885. [Google Scholar] [CrossRef]
- Kim, C.M.; Eng, J.J. Magnitude and pattern of 3D kinematic and kinetic gait profiles in persons with stroke: Relationship to walking speed. Gait Posture 2004, 20, 140–146. [Google Scholar] [CrossRef]
- Farris, D.J.; Hampton, A.; Lewek, M.D.; Sawicki, G.S. Revisiting the mechanics and energetics of walking in individuals with chronic hemiparesis following stroke: From individual limbs to lower limb joints. J. Neuroeng. Rehabil. 2015, 12, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mentiplay, B.F.; Williams, G.; Tan, D.; Adair, B.; Pua, Y.H.; Bok, C.W.; Bower, K.J.; Cole, M.H.; Ng, Y.S.; Lim, L.S.; et al. Gait Velocity and Joint Power Generation After Stroke: Contribution of Strength and Balance. Am. J. Phys. Med. Rehabil. 2019, 98, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, Y.; Honda, K.; Owaki, D.; Izumi, S.I. Classification of Ankle Joint Stiffness during Walking to Determine the Use of Ankle Foot Orthosis after Stroke. Brain Sci. 2021, 11, 1512. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, Y.; Muraki, T.; Kuramatsu, Y.; Furusawa, Y.; Izumi, S. The contribution of quasi-joint stiffness of the ankle joint to gait in patients with hemiparesis. Clin. Biomech. 2012, 27, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, Y.; Muraki, T.; Tanaka, N.; Izumi, S. Relationship between activation of ankle muscles and quasi-joint stiffness in early and middle stances during gait in patients with hemiparesis. Gait Posture 2015, 42, 348–353. [Google Scholar] [CrossRef]
- Fujita, K.; Miaki, H.; Fujimoto, A.; Hori, H.; Fujimoto, H.; Kobayashi, Y. Factors affecting premature plantarflexor muscle activity during hemiparetic gait. J. Electromyogr. Kinesiol. 2018, 39, 99–103. [Google Scholar] [CrossRef]
- Brunnstrom, S. Recovery stages and evaluation procedures. In Movement Therapy in Hemiplegia: A Neurophysiological Approach; Harper & Row: New York, NY, USA, 1970; pp. 34–55. [Google Scholar]
- Tsuji, T.; Liu, M.; Sonoda, S.; Domen, K.; Chino, N. The stroke impairment assessment set: Its internal consistency and predictive validity. Arch. Phys. Med. Rehabil. 2000, 81, 863–868. [Google Scholar] [CrossRef]
- Linacre, J.M.; Heinemann, A.W.; Wright, B.D.; Granger, C.V.; Hamilton, B.B. The structure and stability of the Functional Independence Measure. Arch. Phys. Med. Rehabil. 1994, 75, 127–132. [Google Scholar] [CrossRef]
- Dumas, R.; Cheze, L.; Verriest, J.P. Adjustments to McConville et al. and Young et al. body segment inertial parameters. J. Biomech. 2007, 40, 543–553. [Google Scholar] [CrossRef]
- Winter, D.A. (Ed.) Biomechanics and Motor Control of Human Movement, 4th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009. [Google Scholar]
- Selbie, W.S.; Hamill, J.; Kepple, T.M. Three-Dimensional Kinetics; Human Kinetics: Champaign, IL, USA, 2013. [Google Scholar]
- Kinsella, S.; Moran, K. Gait pattern categorization of stroke participants with equinus deformity of the foot. Gait Posture 2008, 27, 144–151. [Google Scholar] [CrossRef]
- Funato, T.; Aoi, S.; Oshima, H.; Tsuchiya, K. Variant and invariant patterns embedded in human locomotion through whole body kinematic coordination. Exp. Brain Res. 2010, 205, 497–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Troje, N.F. Decomposing biological motion: A framework for analysis and synthesis of human gait patterns. J. Vis. 2002, 2, 371–387. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.W.; Stokic, D.S. Intersegmental coordination of gait after hemorrhagic stroke. Exp. Brain Res. 2015, 233, 125–135. [Google Scholar] [CrossRef]
- Cheron, G.; Bouillot, E.; Dan, B.; Bengoetxea, A.; Draye, J.P.; Lacquaniti, F. Development of a kinematic coordination pattern in toddler locomotion: Planar covariation. Exp. Brain Res. 2001, 137, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Ivanenko, Y.P.; d’Avella, A.; Poppele, R.E.; Lacquaniti, F. On the origin of planar covariation of elevation angles during human locomotion. J. Neurophysiol. 2008, 99, 1890–1898. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, H.; Prince, F.; Sadeghi, S.; Labelle, H. Principal component analysis of the power developed in the flexion/extension muscles of the hip in able-bodied gait. Med. Eng. Phys. 2000, 22, 703–710. [Google Scholar] [CrossRef]
- Adams, R.W.; Gandevia, S.C.; Skuse, N.F. The distribution of muscle weakness in upper motoneuron lesions affecting the lower limb. Brain 1990, 113 Pt 5, 1459–1476. [Google Scholar] [CrossRef]
- Sekiguchi, Y.; Owaki, D.; Honda, K.; Izumi, S. The contribution of intralimb kinetic coordination in lower limb to control of propulsion and weight support at a wide range of gait speed in young and elderly people. In Proceedings of the ISPGR World Congress, Edinburgh, UK, 30 June–4 July 2019; pp. 305–306. [Google Scholar]
- Den Otter, A.R.; Geurts, A.C.; Mulder, T.; Duysens, J. Abnormalities in the temporal patterning of lower extremity muscle activity in hemiparetic gait. Gait Posture 2007, 25, 342–352. [Google Scholar] [CrossRef]
- Dyer, J.O.; Maupas, E.; de Andrade Melo, S.; Bourbonnais, D.; Nadeau, S.; Forget, R. Changes in activation timing of knee and ankle extensors during gait are related to changes in heteronymous spinal pathways after stroke. J. Neuroeng. Rehabil. 2014, 11, 148. [Google Scholar] [CrossRef]
- Raja, B.; Neptune, R.R.; Kautz, S.A. Coordination of the non-paretic leg during hemiparetic gait: Expected and novel compensatory patterns. Clin. Biomech. 2012, 27, 1023–1030. [Google Scholar] [CrossRef]
- Milovanovic, I.; Popovic, D.B. Principal component analysis of gait kinematics data in acute and chronic stroke patients. Comput. Math. Methods Med. 2012, 2012, 649743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldberg, S.R.; Stanhope, S.J. Sensitivity of joint moments to changes in walking speed and body-weight-support are interdependent and vary across joints. J. Biomech. 2013, 46, 1176–1183. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Hemiparesis | Control | |
|---|---|---|
| N | 29 | 12 |
| Gender | 25 M/4 F | 5 M/7 F |
| Age (years) | 57.9 (SD 11.1) | 52.8 (SD 10.1) |
| Height (cm) | 166.4 (SD 8.2) | 166.3 (SD 8.7) |
| Weight (kg) | 65.9 (SD 8.5) | 61.4 (SD 12.5) |
| Diagnosis | Cerebral hemorrhage 19 Cerebral infarction 10 | |
| Paretic side | 19 R/10 L | |
| Time Since neurologic event (Month) | 43 (SD 49) | |
| Brunnstrom stage in the lower limb (3/4/5/6) a | 14/4/10/1 | |
| SIAS b | ||
| Muscle tone in lower limb (0/1/2/3) | 6/15/8 | |
| Total score | 51.2 (SD 9.6) | |
| Walking item of FIM c (0/1/2/3/4/5/6/7) | 0/0/0/0/0/4/21/4 |
| Segment | Placement of Markers |
|---|---|
| Trunk | Spinous process of the 7th cervical vertebrae, spinous process of the 10th thoracic vertebrae, jugular notch where the clavicles meet the sternum, xiphoid process of the sternum, and the position in the middle of the right scapula. |
| Upper arm | Both acromions and both elbow lateral epicondyles |
| Forearm | Both elbow lateral epicondyles and ulnar styloid processes and radius |
| Pelvis | Both anterior superior iliac spines and both posterior superior iliac spines |
| Thigh | Both greater trochanters and both knee lateral and medial epicondyles |
| Shank | Both knee lateral epicondyles and both lateral and medial malleoli |
| Foot | Both the 1st and 5th metatarsal heads, both lateral and medial malleoli, and both calcanei |
| Control | Paretic Side | Non-Paretic Side | |
|---|---|---|---|
| Gait speed (cm/s) | 53.4 (SD 17.4) | 42.2 (SD 20.9) | |
| Stride time (s) | 1.67 (SD 0.37) | 1.67 (SD 0.51) | |
| Stride length (cm) | 90.9 (SD 16.9) a | 67.8 (SD 24.3) a | |
| Step length (cm) | 44.5 (SD 8.6) b | 35.1 (SD 10.4) | 30.1 (SD 13.8) b |
| Stance time (s) | 1.09 (SD 0.27) | 1.09 (SD 0.49) | 1.25 (SD 0.48) |
| Swing time (s) | 0.57 (SD 0.10) b | 0.58 (SD 0.11) c | 0.40 (SD 0.15) bc |
| Single support time (s) | 0.57 (SD 0.09) ab | 0.43 (SD 0.14) a | 0.40 (SD 0.15) b |
| Swing time asymmetry | 1.02 (SD 0.06) a | 1.51 (SD 0.61) a | |
| Step length asymmetry | 1.03(SD0.08) | 1.37 (SD 0.53) a | |
| Peak propulsion (N/kg) | 1.00(SD0.44) | 0.61 (SD 0.37) ac | 0.99 (SD0.42) c |
| Maximum hip extension moment in the early stance (Nm/kg) | 0.38 (SD 0.23) | 0.28 (SD 0.24) c | 0.47 (SD 0.22) c |
| Maximum hip flexion moment in the stance phase (Nm/kg) | 0.66 (SD 0.19) | 0.87 (SD 0.33) | 0.77 (SD 0.41) |
| Maximum knee extension moment in the early stance (Nm/kg) | 0.19 (SD 0.18) | 0.27 (SD 0.18) | 0.33 (SD 0.23) |
| Maximum knee flexion moment in the stance phase (Nm/kg) | 0.17 (SD 0.12) | 0.27 (SD 0.22) c | 0.14 (SD 0.14) c |
| Maximum knee extension moment in the late stance (Nm/kg) | 0.27 (SD 0.09) b | 0.30 (SD 0.20) c | 0.45 (SD 0.25) bc |
| Maximum ankle dorsiflexion moment in the early stance (Nm/kg) | 0.05 (SD 0.04) | 0.02 (SD 0.06) | 0.04 (SD 0.04) |
| Maximum ankle plantarflexion moment in the stance phase (Nm/kg) | 1.05 (SD 0.16) a | 0.71 (SD 0.28) ac | 0.90 (SD 0.24) c |
| Control | Paretic Side | Non-Paretic Side | |
|---|---|---|---|
| Variance explained by PC1 (%) | 81 (SD 6) | 81 (SD 12) | 75 (SD 14) |
| Variance explained by PC2 (%) | 17 (SD 6) | 18 (SD 12) | 20 (SD 11) |
| Variance explained by PC1 + PC2 (%) | 98 (SD 1) b | 99 (SD 1) c | 95 (SD 5) bc |
| Timing of peak PC1 (% gait cycle) | 52 (SD 3) a | 43 (SD 9) ac | 55 (SD11) c |
| Loadings of hip joint moment in PC1 | −0.63 (SD 0.07) | −0.47 (SD 0.23) | −0.42 (SD 0.35) |
| Loadings of knee joint moment in PC1 | 0.26 (SD 0.37) | 0.03 (SD 0.53) c | 0.35 (SD 0.45) c |
| Loadings of ankle joint moment in PC1 | 0.63 (SD 0.09) | 0.67 (SD 0.10) | 0.61 (SD 0.15) |
| Loadings of hip joint moment in PC2 | 0.06 (SD 0.36) | −0.31 (SD 0.52) c | 0.07 (SD 0.67) c |
| Loadings of knee joint moment in PC2 | 0.86 (SD 0.10) ab | 0.67 (SD 0.22) ac | 0.51 (SD 0.28) bc |
| Loadings of ankle joint moment in PC2 | −0.28 (SD 0.23) | −0.13 (SD 0.37) | −0.11 (SD 0.48) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sekiguchi, Y.; Owaki, D.; Honda, K.; Izumi, S.-I. Kinetic Interjoint Coordination in Lower Limbs during Gait in Patients with Hemiparesis. Biomechanics 2022, 2, 466-477. https://doi.org/10.3390/biomechanics2030036
Sekiguchi Y, Owaki D, Honda K, Izumi S-I. Kinetic Interjoint Coordination in Lower Limbs during Gait in Patients with Hemiparesis. Biomechanics. 2022; 2(3):466-477. https://doi.org/10.3390/biomechanics2030036
Chicago/Turabian StyleSekiguchi, Yusuke, Dai Owaki, Keita Honda, and Shin-Ichi Izumi. 2022. "Kinetic Interjoint Coordination in Lower Limbs during Gait in Patients with Hemiparesis" Biomechanics 2, no. 3: 466-477. https://doi.org/10.3390/biomechanics2030036







