Capture and Reaction of CO2 and H2 Catalyzed by a Complex of Coronene: A Computational Study
Abstract
:1. Introduction
2. Methods
3. Results and Discussion
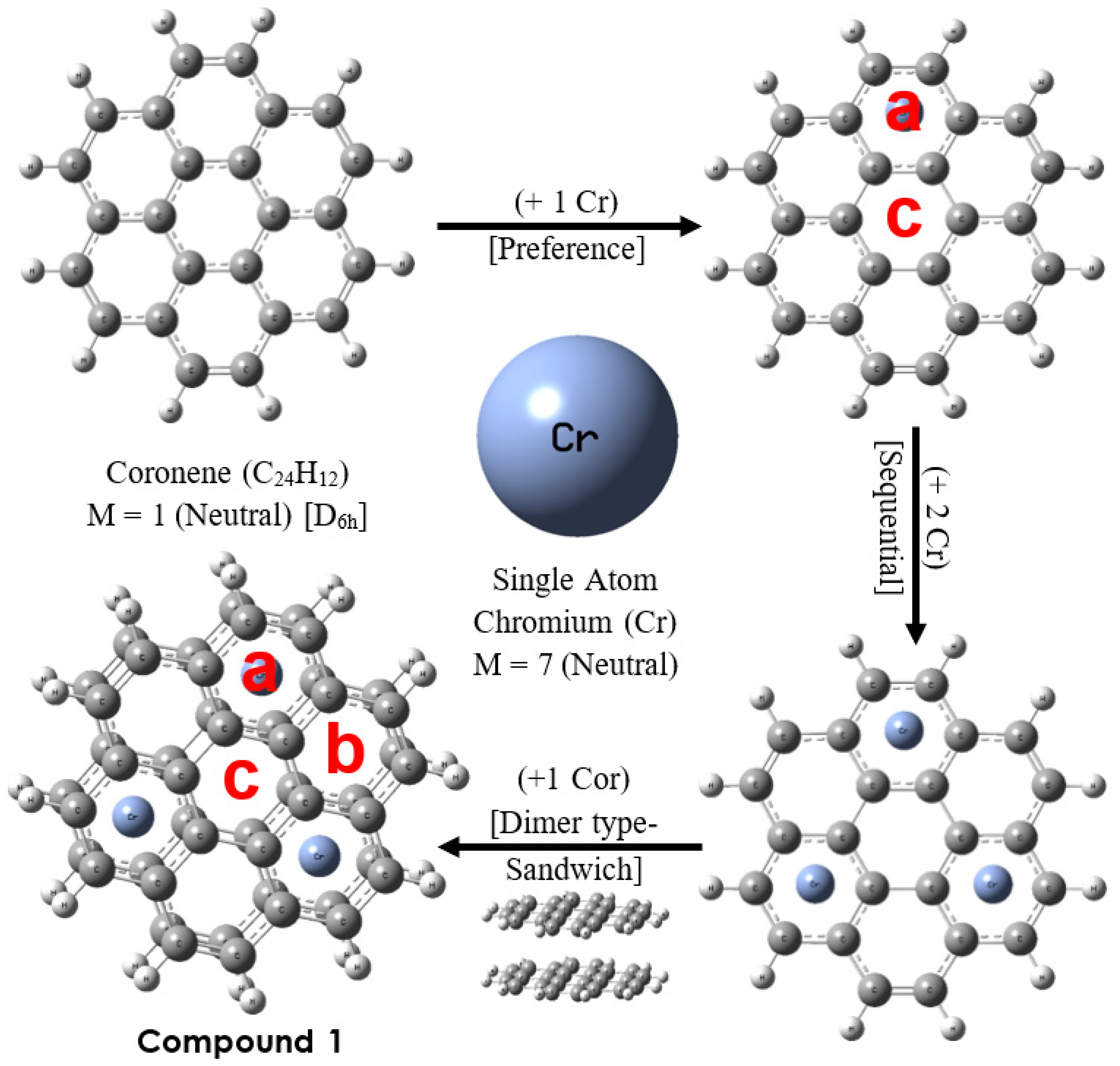
3.1. Structure and Aromaticity
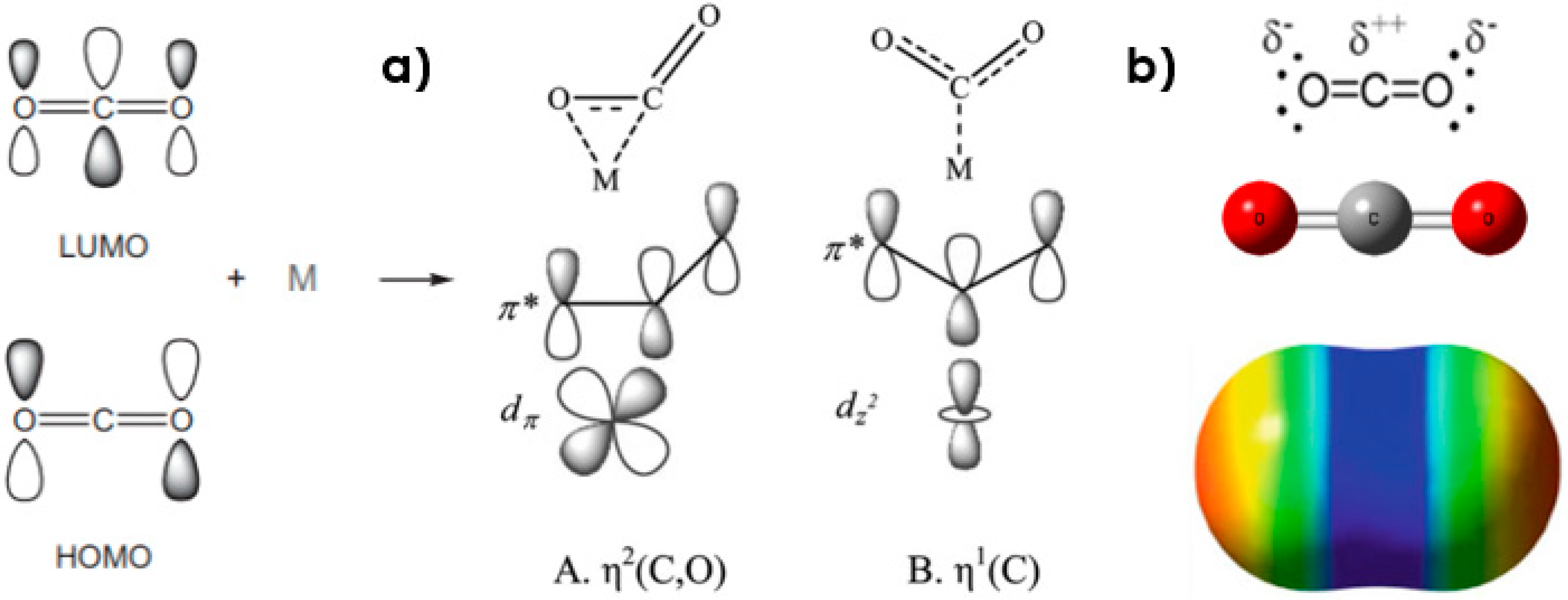
3.2. Electronic and Bonding Properties
3.3. Energies and Interactions in the Cr3-Cor2 Complex
3.4. Hydrogenation of CO2 to Formic Acid
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Falkowski, P.; Scholes, R.J.; Boyle, E.; Canadell, J.; Canfield, D.; Elser, J.; Gruber, N.; Hibbard, K.; Högberg, P.; Linder, S.; et al. The Global Carbon Cycle: A Taste of Our Knowledge of Earth as A System. Science 2000, 290, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Aresta, M.; Dibenedetto, A. The Carbon Dioxide Revolution: Challenges and Perspectives for a Global Society; Springer International Publishing: Berlin, Germany, 2021. [Google Scholar]
- Cuéllar-Franca, R.M.; Azapagic, A. Carbon Capture, Storage and Utilisation Technologies: A Critical Analysis and Comparison of Their Life Cycle Environmental Impacts. J. CO2 Util. 2015, 9, 82–102. [Google Scholar] [CrossRef]
- Goldblatt, C.; Watson, A.J. The Runaway Greenhouse: Implications for Future Climate Change, Geoengineering and Planetary Atmospheres. Philos. Trans. R. Soc. A 2012, 370, 4197–4216. [Google Scholar] [CrossRef]
- Otto, A.; Grube, T.; Schiebahn, S.; Stolten, D. Closing the Loop: Captured CO2 as a Feedstock in the Chemical Industry. Energy Environ. Sci. 2015, 8, 3283–3297. [Google Scholar] [CrossRef]
- NASA. Global Climate Change: Vital Signs of the Planet. 2014. Available online: https://climate.nasa.gov/400ppmquotes/ (accessed on 4 December 2018).
- Aresta, M. Carbon Dioxide as Chemical Feedstock; Wiley-VCH: Weinheim, Germany, 2010. [Google Scholar]
- Artz, J.; Müller, T.E.; Thenert, K.; Kleinekorte, J.; Meys, R.; Sternberg, A.; Bardow, A.; Leitner, W. Sustainable Conversion of Carbon Dioxide: An Integrated Review of Catalysis and Life Cycle Assessment. Chem. Rev. 2018, 118, 434–504. [Google Scholar] [CrossRef]
- He, M.; Sun, Y.; Han, B. Green Carbon Science: Scientific Basis for Integrating Carbon Resource Processing, Utilization and Recycling. Angew. Chem. Int. Ed. 2013, 52, 9620–9633. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.; Ma, X.; Gong, J. Recent Advances in Catalytic Hydrogenation of Carbon Dioxide. Chem. Soc. Rev. 2011, 40, 3703–3727. [Google Scholar] [CrossRef]
- Álvarez, A.; Bansode, A.; Urakawa, A.; Bavykina, A.V.; Wezendonk, T.A.; Makkee, M.; Gascon, J.; Kapteijn, F. Challenges in the Greener Production of Formates/Formic Acid, Methanol, and DME by Heterogeneously Catalyzed CO2 Hydrogenation Processes. Chem. Rev. 2017, 117, 9804–9838. [Google Scholar] [CrossRef]
- Wu, J.; Huang, Y.; Ye, W.; Li, Y. CO2 Reduction: From the Electrochemical to Photochemical Approach. Adv. Sci. 2017, 4, 1700194. [Google Scholar] [CrossRef]
- Pardakhti, M.; Jafari, T.; Tobin, Z.; Dutta, B.; Moharreri, E.; Shemshaki, N.S.; Suib, S.; Srivastava, R. Trends in Solid Adsorbent Materials Development for CO2 Capture. ACS Appl. Mater. Interfaces 2019, 11, 34533–34559. [Google Scholar] [CrossRef]
- Wang, X.; He, T.; Hu, J.; Liu, M. The Progress of Nanomaterials for Carbon Dioxide Capture via the Adsorption Process. Environ. Sci. Nano 2021, 8, 890–912. [Google Scholar] [CrossRef]
- Yuan, Y.; You, H.; Ricardez-Sandoval, L. Recent Advances on First-Principles Modeling for the Design of Materials in CO2 Capture Technologies. Chin. J. Chem. Eng. 2019, 27, 1554–1565. [Google Scholar] [CrossRef]
- Najafabadi, A.T. Emerging Applications of Graphene and Its Derivatives in Carbon Capture and Conversion: Current Status and Future Prospects. Renew. Sustain. Energy Rev. 2015, 41, 1515–1545. [Google Scholar] [CrossRef]
- Schaub, T. CO2-Based Hydrogen Storage: CO2 Hydrogenation to Formic Acid, Formaldehyde and Methanol. Phys. Sci. Rev. 2018, 3, 20170015–20170139. [Google Scholar]
- Baena-Moreno, F.M.; Rodríguez-Galán, M.; Vega, F.; Alonso-Fariñas, B.; Vilches Arenas, L.F.; Navarrete, B. Carbon Capture & Utilization Technologies: A Literature Review and Recent Advances. Energy Sources Part A Recovery Util. Environ. Eff. 2019, 41, 1403–1433. [Google Scholar]
- Leclaire, J.; Heldebrant, D.J. A Call to (Green) Arms: A Rallying Cry for Green Chemistry and Engineering for CO2 Capture, Utilization and Storage. Green Chem. 2018, 20, 5058–5081. [Google Scholar] [CrossRef]
- Rios, C.; Salcedo, R. CO2 Capture and a Route to Transform it in Formic Acid: A Theoretical Approach. J. Mol. Model. 2022, 28, 183. [Google Scholar] [CrossRef]
- Liu, X.; Li, B.Q.; Ni, B.; Wang, L.; Peng, H.J. A Perspective on the Electrocatalytic Conversion of Carbon Dioxide to Methanol with Metallomacrocyclic Catalysts. J. Energy Chem. 2022, 64, 263–275. [Google Scholar] [CrossRef]
- Piccirilli, L.; Lobo Justo Pinheiro, D.; Nielsen, M. Recent Progress with Pincer Transition Metal Catalysts for Sustainability. Catalysts 2020, 10, 773. [Google Scholar] [CrossRef]
- Leitner, W. The Coordination Chemistry of Carbon Dioxide and Its Relevance for Catalysis: A Critical Survey. Coord. Chem. Rev. 1996, 153, 257–284. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A.; Quaranta, E. Reaction Mechanisms in Carbon Dioxide Conversion; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Paparo, A.; Okuda, J. Carbon Dioxide Complexes: Bonding Modes & Synthetic Methods. Coord. Chem. Rev. 2017, 334, 136–149. [Google Scholar]
- Fischer, E.O.; Hafner, W. Di-benzol-chrom. Z. Nat. B 1955, 10, 665–668. [Google Scholar] [CrossRef]
- Seyfert, D. Bis(benzene)chromium. Organometallics 2002, 21, 1520–1530. [Google Scholar]
- Sola, M. Forty Years of Clar’s Aromatic π-Sextet Rule. Front. Chem. 2013, 1, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Foster, N.R.; Grieves, G.A.; Buchanan, J.W.; Flynn, N.D.; Duncan, M.A. Growth and Photodissociation of Crx-(Coronene)y Complexes. J. Phys. Chem. A 2000, 104, 11055–11062. [Google Scholar] [CrossRef]
- Philpott, M.R.; Kawazoe, Y. Chromium Aromatic Hydrocarbon Sandwich Molecules and the Eighteen-Electron Rule. J. Phys. Chem. A 2008, 112, 2034–2042. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Truhlar, D.G. The M06 Suite of Density Functionals for Main Group Thermochemistry, Thermochemical Kinetics, Noncovalent Interactions, Excited States, and Transition Elements: Two New Functionals and Systematic Testing of Four M06-class Functionals and 12 Other Functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 16, Revision, A.1; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Reed, A.E.; Weinstock, R.B.; Weinhold, F. Natural Population Analysis. J. Chem. Phys. 1985, 83, 735–746. [Google Scholar] [CrossRef]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular Interactions from a Natural Bond Orbital, Donor-Acceptor Viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Kruszewski, J.; Krygowski, T.M. Definition of Aromaticity Basing on the Harmonic Oscillator Model. Tetrahedron Lett. 1972, 13, 3839–3842. [Google Scholar] [CrossRef]
- Krygowski, T.M. Crystallographic Studies of Inter-and Intramolecular Interactions Reflected in Aromatic Character of π-Electron Systems. J. Chem. Inf. Model. 1993, 33, 70–78. [Google Scholar]
- Schleyer, P.V.R.; Maerker, C.; Dransfeld, A.; Jiao, H.; van Eikema Hommes, N.J. Nucleus-Independent Chemical Shifts: A Simple and Efficient Aromaticity Probe. J. Am. Chem. Soc. 1996, 118, 6317–6318. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wannere, C.S.; Corminboeuf, C.; Puchta, R.; Schleyer, P.V.R. Nucleus-Independent Chemical Shifts (NICS) as an Aromaticity Criterion. Chem. Rev. 2005, 105, 3842–3888. [Google Scholar] [CrossRef] [PubMed]
- Fallah-Bagher-Shaidaei, H.; Wannere, C.S.; Corminboeuf, C.; Puchta, R.; Schleyer, P.V.R. Which NICS Aromaticity Index for Planar π Rings Is Best? Org. Lett. 2006, 8, 863–866. [Google Scholar] [CrossRef] [PubMed]
- Kitaura, K.; Morokuma, K. A New Energy Decomposition Scheme for Molecular Interactions within the Hartree-Fock Approximation. Int. J. Quantum Chem. 1976, 10, 325–340. [Google Scholar] [CrossRef]
- Ziegler, T.; Rauk, A. On the Calculation of Bonding Energies by the Hartree Fock Slater Method. Theor. Chim. Acta 1977, 46, 1–10. [Google Scholar] [CrossRef]
- Rayón, V.M.; Frenking, G. Bis(Benzene)Chromium Is a δ-Bonded Molecule and Ferrocene Is a π-Bonded Molecule. Organometallics 2003, 22, 3304–3308. [Google Scholar] [CrossRef]
- Popov, I.A.; Boldyrev, A.I. Chemical Bonding in Coronene, Isocoronene, & Circumcoronene. Eur. J. Org. Chem. 2012, 2012, 3485–3491. [Google Scholar]
- Zubarev, D.Y.; Boldyrev, A.I. Revealing Intuitively Assessable Chemical Bonding Patterns in Organic Aromatic Molecules via AdNDP. J. Org. Chem. 2008, 73, 9251–9258. [Google Scholar] [CrossRef] [PubMed]
- Fedik, N.; Boldyrev, A.I. Insight into the Nature of Rim Bonds in Coronene. J. Phys. Chem. A 2018, 122, 8585–8590. [Google Scholar] [CrossRef] [PubMed]
- Karadakov, P.B. Magnetic Shielding Study of Bonding and Aromaticity in Corannulene and Coronene. Chemistry 2021, 3, 861–872. [Google Scholar] [CrossRef]
- Pandey, R.; Rao, B.K.; Jena, P.; Blanco, M.A. Electronic Structure and Properties of Transition Metal−Benzene Complexes. J. Am. Chem. Soc. 2001, 123, 3799–3808. [Google Scholar] [CrossRef] [PubMed]
- Janowski, T.; Ford, A.R.; Pulay, P. Accurate Correlated Calculation of the Intermolecular Potential Surface in the Coronene Dimer. Mol. Phys. 2010, 108, 249–257. [Google Scholar] [CrossRef]
- Podeszwa, R. Interactions of Graphene Sheets Deduced from Properties of Polycyclic Aromatic Hydrocarbons. J. Chem. Phys. 2010, 132, 044704. [Google Scholar] [CrossRef]
- Wang, R.; Liu, G.; Kim, S.K.; Bowen, K.H.; Zhang, X. Gas-Phase CO2 Activation with Single Electrons, Metal Atoms, Clusters, and Molecules. J. Energy Chem. 2021, 63, 130–137. [Google Scholar] [CrossRef]
- Liu, C.; Cundari, T.R.; Wilson, A.K. Periodic Trends in 3d Metal Mediated CO2 Activation. In Applications of Molecular Modeling to Challenges in Clean Energy; American Chemical Society: Washington, DC, USA, 2013; pp. 67–88. [Google Scholar]
- Sakaki, S. Theoretical and Computational Study of a Complex System Consisting of Transition Metal Element(s): How to Understand and Predict Its Geometry, Bonding Nature, Molecular Property, and Reaction Behavior. Bull. Chem. Soc. Jpn. 2015, 88, 889–938. [Google Scholar] [CrossRef]
- Fan, T.; Chen, X.; Lin, Z. Theoretical Studies of Reactions of Carbon Dioxide Mediated and Catalysed by Transition Metal Complexes. Chem. Commun. 2012, 48, 10808–10828. [Google Scholar] [CrossRef]
- Kégl, T.; Ponec, R.; Kollár, L. Theoretical Insights Into the Nature of Nickel-Carbon Dioxide Interactions in Ni(PH3)2 (η2-CO2). J. Phys. Chem. A 2011, 115, 12463–12473. [Google Scholar] [CrossRef]
- Li, S.; Zhao, X.; Shi, J.; Jia, Y.; Guo, Z.; Cho, J.-H.; Gao, Y.; Zhang, Z. Interplay between the Spin-Selection Rule and Frontier Orbital Theory in O2 Activation and CO Oxidation by Single-Atom-Sized Catalysts on TiO2 (110). Phys. Chem. Chem. Phys. 2016, 18, 24872–24879. [Google Scholar] [CrossRef]
- Park, J.; Cho, M.; Rhee, Y.M.; Jung, Y. Theoretical Study on the Degree of CO2 Activation in CO2-Coordinated Ni(0) Complexes. ACS Omega 2021, 6, 7646–7654. [Google Scholar] [CrossRef]
- Li, B.; Du, W.; Wu, Q.; Dai, Y.; Huang, B.; Ma, Y. Coronene-Based 2D Metal–Organic Frameworks: A New Family of Promising Single-Atom Catalysts for Nitrogen Reduction Reaction. J. Phys. Chem. C 2021, 125, 20870–20876. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, M.; Zhou, M. Infrared Spectra & Structures of the Neutral & Charged CrCO2 and Cr(CO2)2 Isomers in Solid Neon. J. Phys. Chem. A 2014, 118, 6009–6017. [Google Scholar]
- Guzmán-Angel, D.; Gutiérrez-Oliva, S.; Toro-Labbé, A. Hydrogenation and Hydration of Carbon Dioxide: A Detailed Characterization of the Reaction Mechanisms Based on the Reaction Force and Reaction Electronic Flux Analyses. J. Mol. Model. 2019, 25, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Hu, C.; Yang, H.; Su, Z. Theoretical Study on the Reaction Mechanism of the Gas-Phase H2/CO2/Ni(3D) System. J. Phys. Chem. A 2005, 109, 6498–6502. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; He, X.; Rong, C.; Zhong, A.; Liu, S.; Zhao, D. On the origin and nature of internal methyl rotation barriers: An information-theoretic approach study. Theor. Chem. Acc. 2022, 141, 68. [Google Scholar] [CrossRef]
- Cao, X.; Rong, C.; Zhong, A.; Lu, T.; Liu, S. Molecular acidity: An accurate description with information-theoretic approach in density functional reactivity theory. J. Comput. Chem. 2018, 39, 117–129. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, S.; Rong, C.; Zhong, A.; Liu, S. Toward understanding the isomeric stability of fullerenes with density functional theory and the information-theoretic approach. ACS Omega 2018, 3, 17986–17990. [Google Scholar] [CrossRef]
- Gao, Z.A.; Xian, J.Y.; Rong, L.R.; Qin, H.; Jie, L. Theoretical study of metal ion impact on geometric and electronic properties of terbutilamine compounds. Mon. Chem. 2019, 150, 1355–1364. [Google Scholar] [CrossRef]





| Complex | Cr–R (Å) | Cr–C (Å) | C–C′ (Å) |
|---|---|---|---|
| Cr3-Cor2 (This work) | 1.595 | 2.142 | 3.206 |
| Cr-Bz2 (theo) [37] | 1.616 | 2.152 | 3.232 |
| Cr-Bz2 (exp) [37] | 1.613 | 2.150 | 3.226 |
| Aromaticity Index | Coronene | Cr3-Cor2 Complex |
|---|---|---|
| HOMA | 0.701/0.791 | 0.672/0.281/0.213 |
| NICS(ZZ)0 | 19.2/−12.84 | −25.4/13.8/33.7 |
| NICS(ZZ)1 | −6.97/−31.37 | −33.8/−7.9/6.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guillén, L.G.; Fomina, L.; Salcedo, R. Capture and Reaction of CO2 and H2 Catalyzed by a Complex of Coronene: A Computational Study. Physchem 2023, 3, 342-354. https://doi.org/10.3390/physchem3030024
Guillén LG, Fomina L, Salcedo R. Capture and Reaction of CO2 and H2 Catalyzed by a Complex of Coronene: A Computational Study. Physchem. 2023; 3(3):342-354. https://doi.org/10.3390/physchem3030024
Chicago/Turabian StyleGuillén, Luis G., Lioudmila Fomina, and Roberto Salcedo. 2023. "Capture and Reaction of CO2 and H2 Catalyzed by a Complex of Coronene: A Computational Study" Physchem 3, no. 3: 342-354. https://doi.org/10.3390/physchem3030024





