Abstract
Biogels (hydrogels, oleogels, and bigels) are structured systems used as delivery vehicles for bioactive substances. The objective of this study was to provide an updated view of green materials used as biogels, discussing the different aspects related to their formulation. An overview of the application possibilities of these gels in different areas, such as food, cosmetics, pharmaceuticals, and medicine, is reported. Furthermore, an evaluation of the profile of studies using biogels was carried out in the last decades (1980–2023), showing the advances in knowledge about these materials in different application domains. Additionally, a consideration of future demands regarding studies involving biogels from a technological and process engineering point of view is highlighted.
1. Introduction
In recent years, the interest in environmentally compatible processes and the use of green products have increased the demand for research in the academic and industrial sectors. In regard to the world’s biodiversity, the need to preserve natural resources has driven society and governments to change their way of thinking. Today, biodiversity is no longer a “heritage of humanity” but a “concern of humanity” [1,2]. The sustainable use of biodiversity and awareness of its preservation is fundamental in industrial operations and in the preparation of new products today. The current scenario seeks the smart and sustainable use of products and processes from renewable sources (Figure 1), reducing the environmental impact while helping the global economy.
Figure 1.
Representation of green logistics.
In this context, green materials have been seen as a viable alternative for many diverse applications in the chemical, food, cosmetic, and pharmaceutical industries. In this class are biogels, which are materials constituted of bioactive compounds derived from food with great health benefits, such as antioxidant, anti-inflammatory, antifungal, antimicrobial, and anticancer properties, among others.
Gels are semi-solid systems consisting of a liquid (nonpolar or polar solvent) and a gelling agent (defined as gelator or organogelator). They have a three-dimensional network of gelator molecules, which allows for the entrapment of the liquid phase, resulting in a viscoelastic gel [3,4]. They are two-phase systems formed by means of covalent crosslinks of network constituents (chemical gels) or by means of non-covalent interactions, amongst them hydrogen bonds, hydrophobic interactions, and ionic interactions of network constituents (physical gels) [5,6,7]. According to IUPAC [8], a gel is a “non-fluid colloidal network or polymer network that is expanded throughout its whole volume by a fluid”.
In rheological terms, a gel is a material that does not flow and presents elastic attributes of solid and viscous attributes of a liquid. The gel point occurs in the so-called equilibrium modulus. The determination of this point is made through monitoring over time of the properties that characterize the elasticity of the material, called storage modulus-G′ (which represents the stored energy), and the property that characterizes the viscosity of the material, called loss modulus-G″ (which represents the strain energy). Generally, before gelation, G″ is greater than G′. The gelation point occurs when the values of G′ and G″ become equal. After a certain induction time, the value of G″ becomes negligible, and G′ increases rapidly until reaching a steady state level, with its value much larger than G″, indicating a more elastic response. The value of the G″/G′ ratio tends to be <0.1. The higher the value obtained for the G′ modulus, the stronger the gel formed [7].
A gel is called a hydrogel when the liquid constituent is a polar solvent (water), and it is called an organogel when the liquid constituent is an organic solvent, which includes alcohols or hexane and also oils. In this last case, the system formed is defined as an oleogel. Likewise, a subdivision can be made with organogelators, which may be high molecular weight (HMW) or low molecular weight (LMW) gelling agents (Figure 2). Polymers are the traditionally more common class of gelators. In this group, proteins, polysaccharides, carrageenan, alginate, and starches can also be cited as widely studied gelators. Moreover, among the biopolymers considered GRAS (generally regarded as safe) solvents, there are propylene glycol, pectin, ethyl cellulose, and hydroxypropylmethyl cellulose (HPMC), among others. In parallel, among LWN gelators, special attention has been given to triacylglycerols (TAG), monoacylglycerol (MAG), and fatty acids due to their bioactive characteristics [4,9]. In addition to the bioactive, biofunctional, and bionutritive characteristics, fatty acids are considered renewable sources of chemical products since several products can be obtained from reactions and formulations using these compounds [10,11,12]. Furthermore, expanding the list of possibilities, there are also the bigels, which are a class formulated from the junction of hydrogel and organogel, presenting particular characteristics compared to individual gel systems. The type of interaction between the constituents of the gel acts directly on the formation of the matrix of the gel obtained and, therefore, on the appearance and structure of the gel formed. According to the constituents present in the system, the formed gels will present specific and distinct viscoelastic and thermodynamic characteristics due to the three-dimensional structures resulting from each mixture [9]. Gels obtained through chemical interactions are generally resistant, as they are mainly formed by covalent bonds (primary forces), while gels resulting from physical interactions are less resistant, as they are maintained through different secondary forces, such as hydrogen bonds, hydrophobic interactions, or van der Waals forces. On the other hand, physical gels tend to be thermally reversible, a behavior not observed in chemical gels [13]. Although it is well accepted that physical and chemical forces act and control the structuring of the gel network, a precise description of the mechanism that permeates gel formation, as well as the influence of solvent-gelling agent interactions on gel formation and gel behavior remain poorly understood [14,15,16]. In the case of hydrogels, different mechanisms arising from stimuli-sensitive (physical, chemical, and biological/biochemical) to hydrogels can explain the formation of the gel [17]. However, in the case of organogels: (i) the full understanding of the organogelation mechanism; (ii) the knowledge of the behavior and interactions between the gelator and different solvents; (iii) the relationship between the chemical structure of the gelator and the gelation process; and (iv) the effect of process conditions on the physical behavior of the resulting gels are still unknown [14,15,16,17,18,19,20].
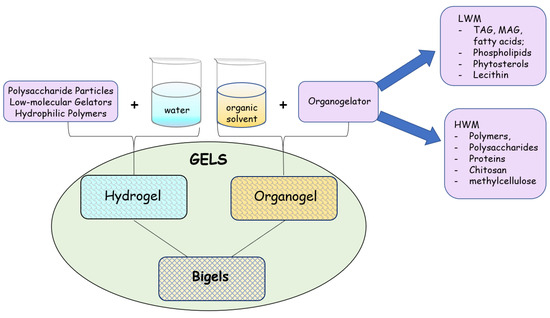
Figure 2.
Classification of types of biogels based on solvents and structuring agents used.
Regarding the physical–chemical aspects, the synergy between the gel components is an essential factor since the formation of these systems strictly depends on the particular condition that exists between the solubility vs. insolubility of the gelling molecule within the solvent and the resulting molecular interactions. Without this condition, there is no gel formation. In other words, this particular condition exists because of the possibility of two opposite situations occurring: (i) systems where there is high solubility between the constituents, with high interaction of the solvent with the gelator, resulting in the formation of a solution, instead of a desired gel system, and (ii) systems with low solubility between the constituents where there will be the formation of precipitates [21,22].
Extensive reviews have discussed the formulation of hydrogels/organogels and their applications. They are well reasoned and present relevant information for the sector [2,23,24,25,26,27]. Their contributions are mainly focused on the formulation of gels for drug delivery and specific active ingredients. On the other hand, the comprehensive review of biogels and notable advances for different application sectors from the perspective of green chemistry are still absent. When it comes to green chemistry, green materials are defined as materials derived from renewable sources or from the processing of agricultural crops, non-toxic, which degrade into harmless products, whose processing reduces or eliminates the use and production of hazardous substances [28,29,30]. In this sense, gels (whether hydrogels, organogels, or bigels) formed from biocompounds of natural or renewable origin, which are non-toxic and have a reduced impact on human health and the environment, can be considered green gels. In this scenario, there are biogels with effective bioactivity and biofunctionality.
When it comes to the industrial production of materials from the perspective of green chemistry, the environmental issue goes beyond reducing toxic waste. In this regard, keeping in mind the environmental impacts of the processes that involve the formulation of new materials and at the same time having the purpose of composing this extremely ordered and well-defined gel structure, the choice of the ideal gelator, in physical–chemical/rheological terms, which is sustainable and economical, thus becomes an item of fundamental importance. Sagiri and Rao [31] indicate six features that should be considered when choosing an ideal oleogelator (indicated schematically in Figure 3).
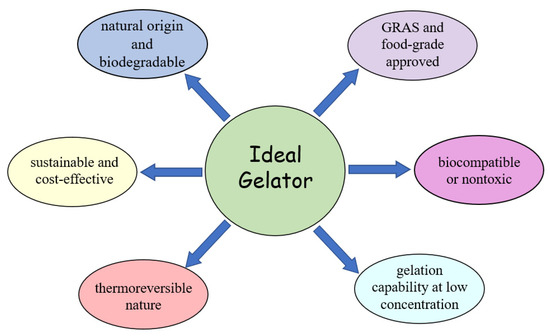
Figure 3.
Ideal gelator (according to [31]).
Although, up to now, little has been explored from this point of view, green gels have great applicability and a range of possibilities that go beyond the mixture of biocompounds. Investigations have been carried out to evaluate biocompatible and efficient materials capable of acting in specific circumstances. Studies have also revealed that biogels, whether originating from bio-sourced materials or renewable products, have great applicability at an industrial level. Being the object of study by several researchers, the formulation and discovery of new biogels have grown in recent years, presenting promising results in several areas of activity. In this sense, this review presents a comprehensive description of different classes of gels, their main applications, and recent discoveries, focusing on the sustainable use of materials and products in obtaining green gels. Technological challenges and issues that still exist in the formulation and production of these materials are also highlighted and discussed.
2. Hydrogels
Hydrogels are hydrophilic, three-dimensional frameworks which are capable of holding large amounts of water and biological fluids [32]. This characteristic makes them an excellent material to be used as drug delivery vectors, biosensors, and carriers or matrices for cells in tissue engineering [33]. Table 1 presents a summary of the main characteristics of hydrogels, a general comparison with organogels and bigels, and highlights the advantages, disadvantages, and molecules involved in each gel.

Table 1.
Main aspects, characteristics, and bioconstituents of hydrogels, organogels, and bigels.
The networks of hydrogels are composed of homo or copolymers and may present chemical crosslinks (tie-points, junctions), or physical crosslinks, such as entanglements or crystallites [32]. Due to existing interactions, physical hydrogels are not homogeneous systems, as the structures obtained by clusters of molecular tangles or hydrophobically or ionically associated domains create heterogeneous regions [75].
Hydrogels are responsive to various stimuli such as heating, pH, light, ionic strength, electromagnetic radiation, and chemical agents and may also show a swelling behavior dependent on the external environment and chemical composition [76,77,78,79,80,81,82,83,84]. Depending on the degree of ionization, chemical compound, or any other external stimulus, the behavior, osmotic balance, ionic strength, network structure, and swelling properties of the hydrogel can be changed, making these materials attractive gels to be applied as biosensors, controlled drug release systems, self-healing materials, superabsorbent materials, and hemostasis bandages [85,86,87,88,89,90,91].
There are numerous applications of hydrogels in the medical, pharmaceutical, and cosmetic sectors [92,93,94,95,96,97,98]. They have been used as drug delivery [99,100,101,102,103], scaffolds [104,105,106,107,108], actuators [109,110,111,112,113], biosensors [114,115,116,117,118], ophthalmic [119,120,121,122], tissue engineering [99,123,124,125], wound dressing [126,127,128], analytical separation, and detection [129,130] materials, among others. The great differential of these materials is their properties (water solubility, spreadability, miscibility) that make them a highly used gel. In addition to their porosity and soft consistency, the three-dimensional and hydrophilic structure of hydrogels enables the absorption of a large amount of water in their interstitial space, which provides physical characteristics similar to natural living tissue. Moreover, the fact that they are non-toxic, biocompatible, versatile, and easy to handle makes them interesting not only in the administration of nutrients/drugs and tissue engineering but also in food sectors and bioadsorbent systems [92,131,132].
A great variety of hydrophilic polymers have been used in the formulation of hydrogels. The main classes consist of natural polymers (polysaccharides and proteins) and synthetic polymers containing hydrophilic functional groups (such as –COOH, –OH, –CONH2, SO3H, amines, and R4N+ and ether) [75]. Depending on the intrinsic properties of the polymers, a synergistic effect of properties is observed in the hydrogel formed [133,134,135,136]. This characteristic can be addressed to modify or adjust the formulation of materials with specific needs, mainly with regards to the medical and pharmaceutical areas. Carvalho et al. [137] present an interesting review of the use of polysaccharides as biomaterials for tissue engineering, focusing on advances involving technologies and materials for the repair and regeneration of an injured brain. In contrast, the use of hydrogels is not limited to medical and pharmaceutical applications. Yang et al. [138] report the use of mixed polysaccharide–protein systems in the manufacture of multistructured food gels, including hydrogels. As noted in the examples cited, a wide variety of biopolymers have been employed in the formulation of hydrogels, some of which are illustrated in Figure 4.
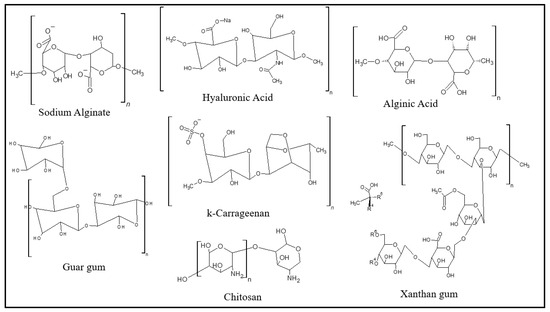
Figure 4.
Molecular structure of some biopolymers used for hydrogel preparation.
3. Organogels
Organogels are defined as semi-solid systems whose organic liquid phase is entrapped within a thermoreversible three-dimensional gel network. In these systems, gelator molecules are present in low concentrations (lower than 15 wt%), which makes them interesting from an industrial point of view [9,139,140].
Organogels have structuring bonds similar to those observed in hydrogels, including weak interactions, such as Van der Waals forces and hydrogen bonds [141,142,143]. Nonetheless, the intrinsic properties of organic solvents and gelators make them more stable systems in thermodynamic and kinetic terms. These characteristics are assigned to two events: (i) the interaction of opposing forces related to the partial solubility of the organogelator in the continuous phase [144] and (ii) the spontaneous formation of a fibrous structure that has a low energy state [145].
In practical terms, these systems appear to be quite multifunctional, with applicability in several sectors, such as the food industry, where organogelation is favorable for inhibiting the migration of oils and fats, trapping these components in the matrix, reducing their movement and thus decreasing the occurrence of the fat bloom phenomenon. In addition, because they exhibit behaviors that reproduce the texture of trans fat, they are of great importance in this replacement, as they can also act in the encapsulation of bioactive and functional lipophilic components such as carotenoids, flavorings and essential unsaturated fatty acids, with the aim of increasing their stability, since they are commonly susceptible to oxidation in foods [21]. Moreover, studies of organogels applied to food are the most varied [133,146,147,148,149] due to the large number of food-derived compounds that enable their use in the formulation of these gels and also due to the bioactivity and biofunctionality of these biocompounds, as is the case of TAG/MAG/fatty acids, proteins, carbohydrates, applied in the formulation of different matrices.
In the pharmaceutical industry, their use refers to the placement of drugs as delivery vehicles, which may occur through different routes of administration, such as topical, transdermal, parenteral, and oral [31]. Diverse literature can be found on the application of organogels in the pharmaceutical sector. Tankov et al. [150], for instance, developed systems containing two types of mesoporous silica particles incorporated in oleogel for dermal delivery of quercetin. Among the results obtained by the authors, the formulated oleogels presented excellent biocompatibility and a lack of hypersensitivity to quercetin. In another study, Wang et al. [151] formulated oleogels derived from glycerol monostearate for lipophilic bioactive delivery and observed that oleogels formed with 10% GMS showed denser network structures and high stability, preventing the degradation of astaxanthin. In another study, organogels formulated with lecithin were evaluated for the delivery of anti-inflammatory drugs against sprains, strains, and contusions [152]. In this study, the authors observed that the presence of lecithin in the new gel formulation promoted a faster and significantly more marked therapeutic effect compared to that of gel without lecithin.
At the same time, in the cosmetic industry, organogels play an important role both in the thickening and structuring of hydrophobic liquids in formulations to minimize syneresis on the surface (thus improving their rheological properties) and in the distribution of functional ingredients conveyed in moisturizers and coloring agents (improving the delivery profiles to the skin’s surface). In this application, organogels are presented precisely to accommodate and maximize the partition of the active ingredient in the skin tissue for the best delivery of bioactives due to better chemical stability since when using the same ingredients, the organogels promote skin permeation to a greater degree [21,153]. This type of application is quite common in anti-aging formulations or in creams intended for cellulite treatments, where permeation of the gel containing the active ingredient is desired and where a modulation of structures and rigidity more appropriate for each case is necessary [153].
This primordial structuring ability based on organic nature, chain symmetry, and molecular weight, as well as characteristics that take into account issues of saturation and molecular chain length, result in distinct microstructures, as well as different physicochemical characteristics. Some examples of well-known and widely used structuring agents are polyethylenes, polyalkylene glycols, polyesters, those of synthetic/mineral origin, and commonly those of natural origin, for example, fatty acids and alcohols such as 12-hydroxystearic acid, wax esters such as carnauba wax and candelilla wax, phospholipids such as lecithin, monoacylglycerols (glyceryl monopalmitate and monostearate), and phytosterols such as β-oryzanol and ceramides [154,155].
Table 2 shows organogel systems reported in the literature with examples of solvents and structuring agents used for their formation. This table also presents the use of some constituents that allow for the incorporation of water-insoluble bioactives, generating greater bioaccessibility and bioavailability of these assets, as is the case of the system explored by Yu et al. [156] who developed an oleogel with high-loading and bioavailability of curcuminoids for use in food grade.

Table 2.
Examples of organogel systems formed from different pairs of organic solvents and structuring agents.
The literature also describes that, like sugars, substances such as ethylene glycol, diethylene glycol, propylene glycol, 1,4-butane diol, and 1,6-hexane diol for example (group of polyols), have interesting properties such as resistance to tension, tearing, cutting, abrasion, adhesion, and dimensional stability [171]. Such mechanical properties can be explained by the greater compatibility of the flexible polar polyester segments with the rigid polar segments, which causes a slower phase separation, making them relevant in the composition and distribution of the polymeric matrix [171]. However, the disadvantages of this system configuration refer to the ester group being more sensitive to hydrolysis and microbial proliferation. An alternative is the use of glycols or longer chain diacids such as palmitic acid (C16) to increase resistance to hydrolysis, resulting in a greater hydrophobic portion of the polyester polyol, also leading to the formation of the desired oil gel.
In order to minimize the resistance to hydrolysis, additives, such as dispersions of vinyl polymers like polyvinylpyrrolidone in the polyester polyol mass, can be used in order to obtain greater hardness at the same density, more uniform cellular structure, and better dimensional stability [171]. An alternative has been proposed by Gandolfo et al. [172] to adjust the physical–chemical, rheological, thermal, and textural properties of oleogels. These authors mixed stearic acid with fatty alcohols of different chain length (C16 to C22) at a concentration of 5% (w/w) in sunflower oil, producing an oleogel of better quality than the oleogels formed by individual gelators. Another example of the use of gelator blends was studied by Kamali et al. [156], in which amaranth oil oleogel was formulated with a mixture of palmitic and stearic acid. In this work, the use of the mixture of fatty acids as a gelator promoted the creation of a structured gel richer in solid contents than the reference sample. Kim et al. [157] studied blends of candelilla wax (CDW) and glycerol monostearate (GMS) in the preparation of canola oil oleogels and verified that the ratio of 60:40 of CDW:GMS presented better results, producing oleogels with a harder texture and lower melting temperature.
4. Bigels
The idea of elaborating defined materials to obtain bigels is quite new compared to other gel structures. The literature mentions the work by Almeida et al. [72] as the first study on the elaboration of these materials with pharmaceutical applications. In this study, the authors evaluated formulations of the hydrogel/organogel mixture using different oleogels mixed with polyacrylic acid hydrogel, and found bigels with an improved moisturizing effect, making them promising candidates for topical formulations. Bigels are uniform semi-solid systems obtained by mixing hydrogel and organogel at a given temperature and appear as a single gel when visually observed [173,174]. The peculiarity of bigels comes from their ability to deliver hydrophilic and lipophilic active agents at the same time.
Bigels bring together in a single gel interesting properties of each original gel; that is, they have characteristics arising from both aqueous and oily phases and have better properties than gels in their original forms [68]. The great advantage of bigels is their greater stability, in addition to being easy to prepare and not requiring large amounts of surfactants to be formed [69]. The greater stability of the bigels is associated with the formation of extra-fine colloidal dispersions, which are the result of the immobilization of the original gel phases in a three-dimensional gel structure [72]. In addition, among other advantages of these gels, the following can be mentioned: they are easy to spread and absorb through the skin, they have refreshing, emollient, and moisturizing effects due to the enrichment of hydration in the stratum corneum, as well as easy washability after administration on the skin [38,60,70,71,72,74,175,176] (other qualities are listed in Table 1). Depending on the method of preparation, structural organization, characteristics of the original gel, and organogel/hydrogel ratios, the bigels can be presented as (i) organogel dispersed in a hydrogel system (O/W); (ii) hydrogel dispersed in an organogel system (W/O); or iii) a bi-continuous system [38] (Figure 5). The latter may have greater structural complexity depending on the organogel/hydrogel ratio and methodology for obtaining the bigel [69]. The preparation method has a strong influence on the type and structure of the bigels due to intrinsic factors in gel formation, such as oleogel content, homogenization temperature, shear level and time, and gelling state of each phase [177]. The balance between these factors will promote the most appropriate training for a given application.
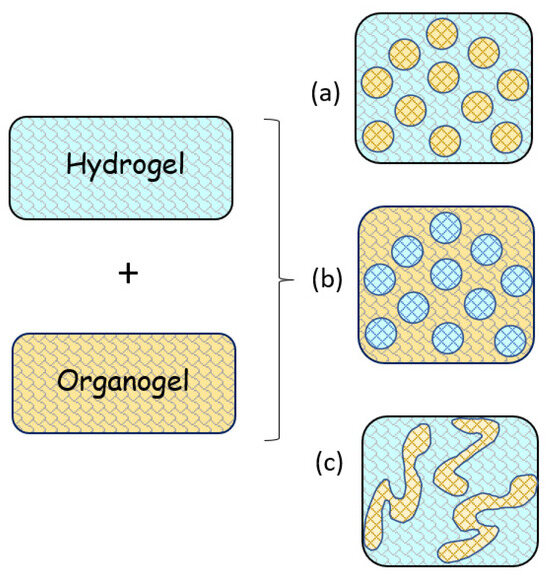
Figure 5.
Representative illustration of different types of bigels: (a) organogel-in-hydrogel, (b) hydrogel-in-organogel; (c) bi-continuous.
The literature cites several studies of the applications of bigels in the food, cosmetics, pharmaceutical, and medical sectors. Some of them are listed in Table 3. This is due to certain functional characteristics of bigels, such as greater thermal stability, firmness, electrical insulation, and greater ease of permeation of the bioactive due to its emollient properties [38,69,70,177].

Table 3.
Some bigel systems developed for the food, cosmetic, pharmaceutical, and medical sectors.
Interesting studies have been developed for the investigation of the physical–chemical characteristics of these systems. Loza-Rodríguez et al. [197] formulated a bigel consisting of oil-beeswax oleogel and hydrogel, with a high potential for drug delivery through the skin. In this work, differences in each type of gel produced were pointed out, with the evidence that the bigel, although visually presented as a homogeneous phase, in reality, is a heterogeneous system. Analyses performed by fluorescence microscopy indicated the presence of two phases in the bigel structure, with the hydrogel phase incorporated into a continuous matrix of oleogel (Figure 6).

Figure 6.
Hydrogel (1), oleogel (2), and bigel (3) obtained using hydrogenated soy phosphatidylcholine, olive oil, beeswax, and α-tocopherol as constituents. (A) Visual appearance of the materials, (B) Fluorescence micrographs (10 µm) [197], with permission from Elsevier Ltd., Copyright 2023 Elsevier.
Two of the questions involved in the formulation of all these gels (hydrogels, organogels, and bigels) are (i) knowing the proportion of the constituents and (ii) what structure one wants to obtain and for a given purpose of application. In this sense, the study of phase equilibrium is presented as an important tool in the analysis of the different possibilities of gel elaboration [9]. The importance of knowing the phase behavior of these systems lies in defining the application of the gel as a function of the amount of the constituent used, together with the functionality of the product obtained. Literature involving the study of the phase equilibrium of these systems is still very scarce. In a recent study, Corredor-Chaparro et al. [198] showed the formation of HPMC bigels formed by organogelator system (sorbitan monostearate:polysorbate 80), sesame oil, and hydrogel. In this work, the authors illustrate the region of existence of the bigel (Figure 7) and describe the bigel as a uniform and brilliant color system, without lumps, and smooth and creamy to the touch. In another study, Cortés et al. [199] evaluated the use of two non-ionic surfactants derived from castor oil (Kolliphor ELP and Kolliphor RH40) that are commonly used in pharmaceutical formulations in the evaluation of phase diagrams with water, sunflower oil, and ethanol as cosurfactant. In the study, the use of Kolliphor RH40 exhibited a larger microemulsion (ME) area than that formulated with Kolliphor ELP, along with other regions of emulsion (E) and phase separation (2P), with a region of gel-like behavior similar to lipogels and gel microemulsion, characterized by the region indicated as L9 in Figure 8. In both cases, without studying the phase's behavior for the entire range of compositions, knowledge of the limits and existing possibilities for a given mixture of solvent (s) + structuring agent (s) + bioactive (s) would be restricted to the range of pre-prepared compositions, and perhaps it would not be possible to visualize the biphasic regions so clearly.
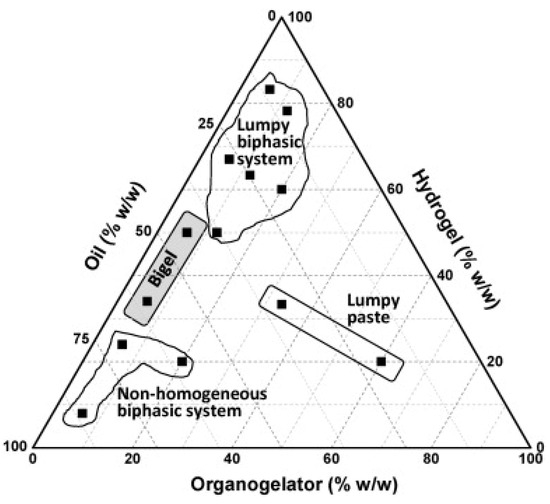
Figure 7.
Ternary diagram for the hydrogel system (constituted by HPMC 10% aqueous dispersion), organogelators (sorbitan monostearate/polysorbate 80), and sesame oil [198], with permission from Elsevier Ltd., Copyright 2023 Elsevier.
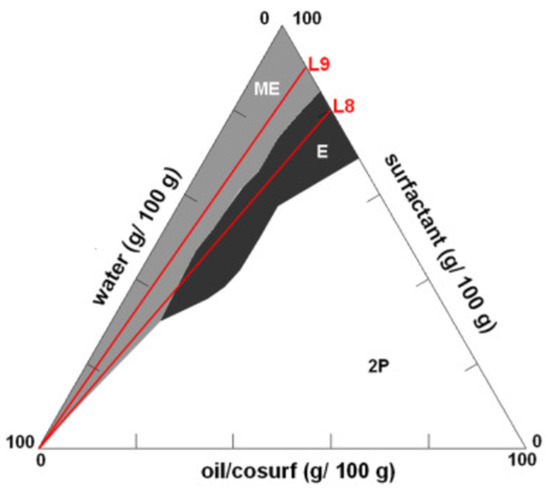
Figure 8.
Pseudo-ternary phase diagrams for systems Kolliphor RH40-water-oil, where the lines L9 and L8 are indications of the ratio 9:1 and 8:2 of surfactant: (oil + cosurfactant), respectively, and ME, E, and 2P correspond to microemulsion, E: emulsion, 2P: two phases [199], with permission from Elsevier Ltd., Copyright 2023 Elsevier.
5. Concluding Remarks and Future Challenges
In recent years, the concern and need to use biocompatible products have increased the search for new materials and processes. The use of clean technologies with green materials has been the basis of several research groups. Linked to this, there are biogels, made up of biomaterials with great applicability in the food, cosmetics, pharmaceutical, and medical sectors (some of which are listed in Table 1, Table 2 and Table 3. Bioactive raw materials play a crucial role in the design and synthesis of multifunctional gels for applications in the various production axes.
Research in different databases (Science Direct and SciELO) was performed to evaluate the advance in knowledge of these materials using the terms bigel, hydrogel, and organogel as keywords, considering the main sectors of application: food, pharmaceutical, cosmetic, and medical (simple and combined search). Figure 9 and Figure 10 illustrate the distribution of literature by application area and the annual evolution of scientific papers, respectively. A total of 20,066 scientific papers were found in the 1980–2023 period (until July), with great advances in the last decades.

Figure 9.
Distribution of scientific papers by application area between 1980 and 2023 (until July).
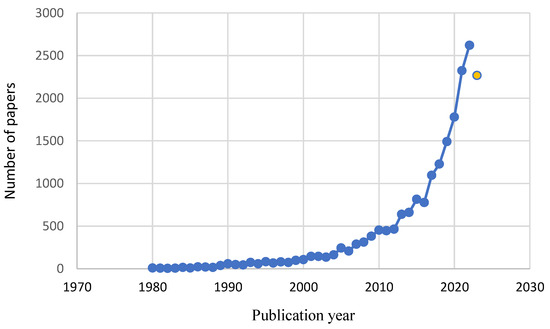
Figure 10.
Annual evolution of scientific papers on biogels between 1980 and 2023 (until July, last circle in yellow).
The vast majority of works were focused on the elaboration of hydrogels (96.5%), which is expected since the discovery of the others is more recent. Among the scientific papers evaluated, separated by area (Figure 9), most of the papers are related to the medical sector (12–37%), followed by the pharmaceutical (25–32%), food (25–33%) and cosmetic (8–30%) sectors. In recent years, research involving gels has increased exponentially, increasing from 815 in 2015 to 2267 papers published in the first seven months of 2023 (Figure 10). The increase in studies focused on the formulation of biogels broadens the possible applications, and the results obtained so far are quite encouraging.
Although the growth observed in studies with green gels is evident, knowledge of fundamental and specific points in the formulation of these materials is still the key to their implementation in the industrial sector. In general, studies are still needed in the design of new multifunctional molecules with specific applications, mainly regarding the issue of simultaneous delivery of bioactive substances with synergistic or non-synergistic effects.
In specific terms, while presenting numerous studies and reviews on hydrogels, due to the great importance and applicability of these materials, investigations still need to be carried out regarding the issue of stability and mechanical strength. New hydrogels that are more stable and have better mechanical strength are needed and remain an important direction for research. Furthermore, although a wide variety of polymers have been used for hydrogel formulations, among the constant concerns are the biocompatibility and biodegradability of the polymers used and that their networks make new systems effective and easy to obtain. In addition, special attention must also be paid to the synergistic behavior that may result from the combination of two polysaccharide networks, requiring fundamental studies to better understand the physicochemical, mechanical, and biological properties of each system. As far as organogels are concerned, one of the important points to be unraveled concerns the clear description of the mechanism of gel formations, as well as the effect of solvent–gelator interactions on the formation process and behavior of the gel formed. Furthermore, because there is a wide variety and structures of gelators that have been used in the formation of organogels, the complete and interconnected knowledge of the thermodynamic and kinetic factors that control the stability of gelling fibers continues to be a question that needs to be answered by researchers in the field.
Another important aspect to be highlighted is the characterization of biogels not only in rheological terms but also in physical–chemical and thermodynamic terms, including phase equilibrium (which is very scarce). This information is essential from the industrial point of view since knowledge of thermophysical and thermodynamic properties of materials plays an important role in simulating and designing new products and processes. Moreover, there is a great lack of studies involving the optimization and techno-economic analysis of the process, which may make the production of these materials on an industrial scale unfeasible. Nonetheless, the performance of engineering, science, and technology together can fill these gaps.
Author Contributions
Conceptualization, C.G.P.; investigation, M.E.B.C.S. and C.G.P.; writing—original draft preparation, M.E.B.C.S. and C.G.P.; writing—review and editing, C.G.P.; supervision, C.G.P.; project administration, C.G.P.; funding acquisition, C.G.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by CNPQ grant number PVG16653-2019/PIBIC And Federal University of Rio Grande do Norte.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Simões, C.M.O.; Schenkel, E.P.; Gosmann, G.; Auler Mentz, J.C.P.M.; Petrovick, P.R. Pharmacognosy: From Plant to Medicine (in Portuguese), 1st ed.; Editora da UFRGS/Ed. da UFSC: Florianópolis, Brazil; Editora da UFSC: Porto Alegre, Brazil, 1999. [Google Scholar]
- ABIFITO. Available online: http://www.abifito.org.br (accessed on 12 October 2022).
- Sagiri, S.S.; Behera, B.; Rafanan, R.R.; Bhattacharya, C.; Pal, K.; Banerjee, I.; Rousseau, D. Organogels as matrices for controlled drug delivery: A review on the current state. Soft Mater. 2014, 12, 47–72. [Google Scholar] [CrossRef]
- Murdan, S.; Andrysek, T.; Son, D. Novel gels and their dispersions—Oral drug delivery systems for ciclosporin. Int. J. Pharm. 2005, 300, 113–124. [Google Scholar] [CrossRef]
- Lewis, L.; Hatzikiriakos, S.G.; Hamad, W.Y.; MacLachlan, M.J. Freeze−Thaw Gelation of Cellulose Nanocrystals. ACS Macro Lett. 2019, 8, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Aleman, J.; Chadwick, A.V.; He, J.; Hess, M.; Horie, K.; Jones, R.G.; Kratochvil, P.; Meisel, I.; Mita, I.; Moad, G.; et al. Definitions of terms relating to the structure and processing of sols, gels, networks, and inorganic-organic hybrid materials (IUPAC Recommendations 2007). Pure Appl. Chem. 2007, 79, 1801–1827. [Google Scholar] [CrossRef]
- Banerjee, S.; Bhattacharya, S. Food gels: Gelling process and new applications Crit. Rev. Food Sci. Nutr. 2011, 52, 334–346. [Google Scholar]
- Jastram, A.; Claus, J.; Janmey, P.A.; Kragl, U. Rheological properties of hydrogels based on ionic liquids. Polym. Test. 2021, 93, 106943. [Google Scholar] [CrossRef]
- Ract, N.R.; Cruz, R.G.; Pereira, C.G. Chapter 14—Phase Equilibrium of Organogels. In Thermodynamics of Phase Equilibria in Food Engineering; Elsevier: London, UK, 2019; pp. 563–591. [Google Scholar]
- Liu, H.; Cheng, T.; Xian, M.; Cao, Y.; Fang, F.; Zou, H. Fatty acid from the renewable sources: A promising feedstock for the production of biofuels and biobased chemicals. Biotechnol. Adv. 2014, 32, 382–389. [Google Scholar] [CrossRef]
- Biermann, U.; Bornscheuer, U.; Meier, M.A.R.; Metzger, J.O.; Schäfer, H.J. Oils and Fats as Renewable Raw Materials in Chemistry. Angew. Chem. Int. Ed. 2011, 50, 3854–3871. [Google Scholar] [CrossRef]
- Tomás-Pejó, E.; González-Fernández, C.; Greses, S.; Kennes, C.; Otero-Logilde, N.; Veiga, M.C.; Bolzenella, D.; Muller, B.; Passoth, V. Production of short-chain fatty acids (SCFAs) as chemicals or substrates for microbes to obtain biochemicals. Biotechnol. Biofuels 2023, 16, 96. [Google Scholar] [CrossRef]
- Bohidar, H.B. Dynamics in thermoreversible polymer gels. Curr. Sci. 2001, 80, 1008–1017. [Google Scholar]
- Tamayo, M.R. Using Computational Methods to Rationalize Organogel Formation. Ph.D. Thesis, Sorbonne University, Paris, France, 2021. [Google Scholar]
- Zinic, M.; Vogtle, F.; Fages, F. Top. Cholesterol-based gelators. Curr. Chem. 2005, 256, 39–76. [Google Scholar]
- Tamaru, S.; Nakamura, M.; Takeuchi, M.; Shinkai, S. Rational Design of a Sugar-Appended Porphyrin Gelator that is Forced to Assemble into a One-Dimensional Aggregate. Org. Lett. 2001, 3, 3631–3634. [Google Scholar] [CrossRef]
- Kushwaha, S.K.S.; Saxena, P.; Rai, A.K. Stimuli sensitive hydrogels for ophthalmic drug delivery: A review. Int. J. Pharm. Investig. 2012, 2, 54–60. [Google Scholar] [CrossRef]
- Cerqueira, M.A.; Valoppi, F.; Pal, K. Oleogels and Organogels: A Promising Tool for New Functionalities. Gels 2022, 8, 349. [Google Scholar] [CrossRef]
- Murata, K.; Aoki, M.; Suzuki, T.; Harafa, T.; Kawabata, H.; Komori, T.; Ohseto, F.; Ueda, K.; Shinkai, S. Thermal and light control of the sol-gel phase transition in cholesterol-based organic gels. Novel helical aggregation modes as detected by circular dichroism and electron microscopic observation. J. Am. Chem. Soc. 1994, 116, 6664–6676. [Google Scholar] [CrossRef]
- Abdallah, D.J.; Weiss, R.G. Organogels and low molecular mass organic gelators. Adv. Mater. 2000, 12, 1237–1247. [Google Scholar] [CrossRef]
- John, G.; Zhu, G.; Li, J.; Dordick, J.S. Enzymatically Derived Sugar-Containing Self-Assembled Organogels with Nanostructured Morphologies. Angew. Chem. Int. Ed. 2006, 45, 4772–4775. [Google Scholar] [CrossRef]
- Co, E.D.; Marangoni, A.G. Organogels: An alternative edible oil-structuring method. J. Am. Chem. Soc. 2012, 89, 749–780. [Google Scholar]
- Barbucci, R. Hydrogels: Biological Properties and Applications; Springer-Verlag Italia: Milan, Italy, 2009; pp. 1–179. [Google Scholar]
- Rimmer, S. Biomedical Hydrogels–Biochemistry, Manufacture and Medical Applications; Woodhead Publishing: Sawston, UK, 2011. [Google Scholar]
- Bouten, P.J.M.; Zonjee, M.; Bender, J.; Yauw, S.T.K.; van Goor, H.; van Hest, J.C.M.; Hoogenboom, R. The chemistry of tissue adhesive materials. Prog. Polym. Sci. 2014, 39, 1375–1405. [Google Scholar] [CrossRef]
- Shakeel, A.; Lupi, F.R.; Gabriele, D.; Baldino, N.; Cindio, B. Bigels: A unique class of materials for drug delivery applications. Soft Mater. 2018, 16, 77–93. [Google Scholar] [CrossRef]
- Martín-Illana, A.; Notario-Pérez, F.; Cazorla-Luna, R.; Ruiz-Caro, R.; Bonferoni, M.C.; Tamayo, A.; Veiga, M.D. Bigels as drug delivery systems: From their components to their applications. Drug Discov. Today 2022, 27, 1008–1026. [Google Scholar] [CrossRef]
- Capello, C.; Fischer, U.; Hungerbühler, K. What is a green solvent? A comprehensive framework for the environmental assessment of solvents. Green Chem. 2007, 9, 927–934. [Google Scholar] [CrossRef]
- Clarke, C.J.; Tu, W.C.; Levers, O.; Bröhl, A.; Hallett, J.P. Green and Sustainable Solvents in Chemical Processes. Chem. Rev. 2018, 118, 747–800. [Google Scholar] [CrossRef]
- IUPAC—International Union of Pure and Applied Chemistry. Sustainable Chemistry. Available online: https://iupac.org/who-we-are/committees/sustainable-chemistry/ (accessed on 4 September 2023).
- Sagiri, S.S.; Rao, K.J. Chapter 22—Natural and bioderived molecular gelator-based oleogels and their applications. In Biopolymer-Based Formulations; Elsevier: Amsterdam, The Netherlands, 2020; pp. 513–559. [Google Scholar]
- Peppas, N.A.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for Biomedical Applications: Their Characteristics and the Mechanisms behind Them. Gels 2017, 3, 6. [Google Scholar] [CrossRef]
- Ahmed, A.A.K.; Naik, H.S.B.; Sherigara, B.S. Synthesis and characterization of chitosan-based pH-sensitive semi-interpenetrating network microspheres for controlled release of diclofenac sodium. Carbohydr. Res. 2009, 344, 699–706. [Google Scholar] [CrossRef]
- Peppas, N.A. Physiologically Responsive Hydrogels. J. Bioact. Compat. Polym. 1991, 6, 241–246. [Google Scholar] [CrossRef]
- Mura, P.; Faucci, M.T.; Bramanti, G.; Corti, P. Evaluation of transcutol as a clonazepam transdermal permeation enhancer from hydrophilic gel formulations. Eur. J. Pharm. Sci. 2000, 9, 365–372. [Google Scholar] [CrossRef]
- Du, X.; Zhou, J.; Shi, J.; Xu, B. Supramolecular Hydrogelators and Hydrogels: From Soft Matter to Molecular Biomaterials. Chem. Rev. 2015, 115, 13165–13307. [Google Scholar] [CrossRef] [PubMed]
- Lupi, F.R.; Gentile, L.; Gabriele, D.; Mazzulla, S.; Baldino, N.; Cindio, B. Olive oil and hyperthermal water bigels for cosmetic uses. J. Colloid Interface Sci. 2015, 459, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Bot, A.; Adel, R.D.; Roijers, E.C. Fibrils of γ-oryzanol + β-sitosterol in edible oil organogels. J. Am. Oil Chem. Soc. 2008, 85, 1127–1134. [Google Scholar] [CrossRef]
- Hamachi, I.; Kiyonaka, S.; Shinkai, S. Solid-phase lipid synthesis (SPLS)-2: Incidental discovery of organogelators based on artificial glycolipids. Tetrahedron Lett. 2001, 42, 6141–6145. [Google Scholar] [CrossRef]
- Behera, B.; Sagiri, S.S.; Pal, K.; Srivastava, A. Modulating the physical properties of sunflower oil and sorbitan monopalmitate-based organogels. J. Appl. Polym. Sci. 2003, 127, 4910–4917. [Google Scholar] [CrossRef]
- Hwang, H.S.; Kim, S.; Singh, M.; Winkler-Moser, J.K.; Liu, S.X. Organogel formation of soybean oil with waxes. J. Am. Oil Chem. Soc. 2011, 89, 639–647. [Google Scholar] [CrossRef]
- Rocha, J.C.B.; Lopes, J.D.; Mascarenhas, M.C.N.; Arellano, D.B.; Guerreiro, L.M.R.; Cunha, R.L.D. Thermal and rheological properties of organogels formed by sugarcane or candelilla wax in soybean oil. Food Res. Int. 2013, 50, 318–323. [Google Scholar] [CrossRef]
- Teramoto, N.; Shibata, M.; Synthesis and properties of pullulan acetate. Thermal properties, biodegradability, and a semi-clear gel formation in organic solvents. Carbohydr. Polym. 2006, 63, 476–481. [Google Scholar] [CrossRef]
- Mitra, A.; Sarkar, V.; Mukhopadhyay, B. Simple carbohydrate-derived multifunctional gels. ChemistrySelect 2017, 2, 9958–9961. [Google Scholar] [CrossRef]
- Patel, A.R.; Babaahmadi, M.; Lesaffer, A.; Dewettinck, K. Rheological profiling of organogels prepared at critical gelling concentrations of natural waxes in a triacylglycerol solvent. J. Agric. Food Chem. 2015, 63, 4862–4869. [Google Scholar] [CrossRef] [PubMed]
- Mandua, C.; Barrera-Arellano, D.; Santana, M.; Fernandes, G. Waxes used as structuring agents for food organogels: A review. Grasas Aceites 2000, 71, e344. [Google Scholar] [CrossRef]
- Cui, J.; Zheng, Y.; Shen, Z.; Wan, X. Alkoxy tail length dependence of gelation ability and supramolecular chirality of sugar-appended organogelators. Langmuir 2010, 26, 15508–15515. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Liu, A.; Guan, Y.; Zheng, J.; Shen, Z.; Wan, X. Tuning the helicity of self-assembled structure of a sugar-based organogelator by the proper choice of cooling rate. Langmuir 2010, 26, 3615–3622. [Google Scholar] [CrossRef] [PubMed]
- Prathap, A.; Sureshan, K.M. Sugar-based organogelators for various applications. Langmuir 2019, 35, 6005–6014. [Google Scholar] [CrossRef] [PubMed]
- Wyne, A.; Whitefield, M.; Dixon, A.; Anderson, S. An effective, cosmetically acceptable, novel hydro-gel emollient for the management of dry skin conditions. J. Dermatol. Treat. 2002, 13, 61–66. [Google Scholar] [CrossRef]
- Matheson, A.B.; Koutsos, V.; Dalkas, G.; Euston, S.; Clegg, P. Microstructure of β-sitosterol:γ-oryzanol edible organogels. Langmuir 2017, 33, 4537–4542. [Google Scholar] [CrossRef]
- Truong, T.; Prakash, S.; Bhandari, B. Effects of crystallisation of native phytosterols and monoacylglycerols on foaming properties of whipped oleogels. Food Chem. 2019, 285, 86–93. [Google Scholar] [CrossRef]
- Tempestini, E.; Bucci, M.; Mastromartino, V.; Gori, M.; Tanini, D.; Ambrosi, M.; Fratini, E.; Capperucci, A.; Lo Nostro, P. Organogels from double-chained vitamin C amphiphilic derivatives. ChemPhysChem 2017, 18, 1400–1406. [Google Scholar] [CrossRef]
- Nostro, P.L.; Ramsch, R.; Fratini, E.; Lagi, M.; Ridi, F.; Carretti, E.; Ambrosi, M.; Ninham, B.W.; Baglioni, P. Organogels from a vitamin C-based surfactant. J. Phys. Chem. B 2007, 111, 11714–11721. [Google Scholar] [CrossRef]
- Biswas, G.; Moon, H.J.; Boraty´nski, P.; Jeong, B.; Kwon, Y.U. Structural sensitivity of peptoid-based low molecular mass organogelator. Mater. Des. 2016, 108, 659–665. [Google Scholar] [CrossRef]
- Schaink, H.; Van Malssen, K.; Morgado-Alves, S.; Kalnin, D.; Van der Linden, E. Crystal network for edible oil organogels: Possibilities and limitations of the fatty acid and fatty alcohol systems. Food Res. Int. 2007, 40, 1185–1193. [Google Scholar] [CrossRef]
- Sawalha, H.; Venema, P.; Bot, A.; Flöter, E.; van der Linden, E. The Influence of Concentration and Temperature on the Formation of ¿-Oryzanol + ß-Sitosterol Tubules in Edible Oil Organogels. Food Biophys. 2011, 6, 20–25. [Google Scholar] [CrossRef]
- Rehman, K.; Amin, M.C.I.M.; Zulfakar, M.H. Development and physical characterization of polymer-fish oil bigel (hydrogel/oleogel) system as a transdermal drug delivery vehicle. J. Oleo Sci. 2014, 63, 961–970. [Google Scholar] [CrossRef]
- Behera, B.; Sagiri, S.S.; Pal, K.; Pramanik, K.; Rana, U.A.; Shakir, I.; Anis, A. Sunflower Oil and Protein-based Novel Bigels as Matrices for Drug Delivery Applications-Characterization and in vitro Antimicrobial Efficiency. Polym. Plast. Technol. Eng. 2015, 54, 837–850. [Google Scholar] [CrossRef]
- Leal-Calderon, F.; Schmitt, V. Solid-stabilized emulsions. Curr. Opin. Colloid Interface Sci. 2008, 13, 217–227. [Google Scholar] [CrossRef]
- Charyulu, R.N.; Muaralidharan, A.; Sandeep, D.S. Design and evaluation of bigels containing flurbiprofen. Res. J. Pharm. Technol. 2018, 11, 143–152. [Google Scholar] [CrossRef]
- Behera, B.; Sagiri, S.S.; Singh, V.K.; Pal, K.; Anis, A. Mechanical properties and delivery of drug/probiotics from starch and non-starch based novel bigels: A comparative study. Starch Starke 2014, 66, 865–879. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Hafez, S.A.; Mahdy, M.M. Organogels, hydrogels and bigels as transdermal delivery systems for diltiazem hydrochloride. Asian J. Pharm. Sci. 2013, 8, 48–57. [Google Scholar] [CrossRef]
- Shakeel, A.; Farooq, U.; Iqbal, T.; Yasin, S.; Lupi, F.R.; Gabriele, D. Key Characteristics and Modelling of Bigels Systems: A Review. Mater. Sci. Eng. C 2019, 97, 932–953. [Google Scholar] [CrossRef]
- Andonova, V.; Peneva, P.; Georgiev, G.S.; Toncheva, V.T.; Apostolova, E.; Peychev, Z.; Dimitrova, S.; Katsarova, M.; Petrova, N.; Kassarova, M. Ketoprofenloaded polymer carriers in bigel formulation: An approach to enhancing drug photostability in topical application forms. Int. J. Nanomed. 2017, 12, 6221–6238. [Google Scholar] [CrossRef]
- Singh, V.K.; Banerjee, I.; Agarwal, T.; Pramanik, K.; Bhattacharya, M.K.; Pal, K. Guar gum and sesame oil based novel bigels for controlled drug delivery. Colloids Surf. B 2014, 123, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Andonova, V.Y.; Peneva, P.T.; Apostolova, E.G.; Dimcgeva, T.D.; Peychev, Z.L.; Kassarova, M.I. Carbopol hydrogel/sorbitan monostearate-almond oil based organogel biphasic formulations: Preparation and characterization of the bigels. Trop. J. Pharm. Res. 2017, 16, 1455–1463. [Google Scholar] [CrossRef]
- Lupi, F.R.; Shakeel, A.; Greco, V.; Oliviero Rossi, C.; Baldino, N.; Gabriele, D. A Rheological and Microstructural Characterisation of Bigels for Cosmetic and Pharmaceutical Uses. Mater. Sci. Eng. C 2016, 69, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Rehman, K.; Zulfakar, M.H. Recent advances in gel technologies for topical and transdermal drug delivery. Drug Dev. Ind. Pharm. 2014, 40, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Di Michele, L.; Fiocco, D.; Varrato, F.; Sastry, S.; Eiser, E.; Foffi, G. Aggregation dynamics, structure, and mechanical properties of bigels. Soft Matter 2014, 10, 3633–3648. [Google Scholar] [CrossRef] [PubMed]
- Almeida, I.F.; Fernandes, A.R.; Fernandes, L.; Ferreira, M.R.; Costa, P.C.; Bahia, M.F. Moisturizing effect of oleogel/hydrogel mixtures. Pharm. Dev. Technol. 2008, 13, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Blumlein, A.; McManus, J.J. Bigels formed via spinodal decomposition of unfolded protein. J. Mater. Chem. B 2015, 3, 3429–3435. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Anis, A.; Banerjee, I.; Pramanik, K.; Bhattacharya, M.K.; Pal, K. Preparation and characterization of novel carbopol based bigels for topical delivery of metronidazole for the treatment of bacterial vaginosis. Mater. Sci. Eng. C 2014, 44, 151–158. [Google Scholar] [CrossRef]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2002, 43, 3–12. [Google Scholar] [CrossRef]
- Zhu, Z.; Guan, Z.; Jia, S.; Lei, Z.; Lin, S.; Zhang, H.; Ma, Y.; Tian, Z.Q.; Yang, C.J. Au@Pt nanoparticle encapsulated target-responsive hydrogel with volumetric bar-chart chip readout for quantitative point-of-care testing. Angew. Chem. Int. Ed. 2014, 53, 12503–12507. [Google Scholar] [CrossRef]
- Jin, Z.; Liu, X.; Duan, S.; Yu, X.; Huang, Y.; Hayat, T.; Li, J. The adsorption of Eu(III) on carbonaceous nanofibers: Batch experiments and modeling study. J. Mol. Liq. 2016, 222, 456–462. [Google Scholar] [CrossRef]
- Pan, G.; Guo, Q.; Ma, Y.; Yang, H.; Li, B. Thermo-responsive hydrogel layers imprinted with RGDS peptide: A system for harvesting cell sheets. Angew. Chem. Int. Ed. 2013, 52, 6907–6911. [Google Scholar] [CrossRef]
- Ishihara, K.; Kobayashi, M.; Ishimaru, N.; Sinohara, I. Glucose Induced Permeation Control of Insulin through a Complex Membrane Consisting of Immobilized Glucose Oxidase and a Poly(amine). Polym. J. 1984, 16, 625–631. [Google Scholar] [CrossRef]
- Hoffman, A.S. Applications of thermally reversible polymers and hydrogels in therapeutics and diagnostics. J. Control. Release 1987, 6, 297–305. [Google Scholar] [CrossRef]
- Tanaka, T. Phase transitions in gels and a single polymer. Polymer 1979, 20, 1404–1412. [Google Scholar] [CrossRef]
- Dong, L.C.; Hoffman, A.S. Reversible Polymer Gels and Related Systems; Russo, P.S., Ed.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1987; Volume 350, pp. 236–244. [Google Scholar]
- Monji, N.; Hoffman, A.S. A novel immuno assay system and bioseparation process based on thermal phase separating polymers. Appl. Biochem. Biotechnol. 1987, 14, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Park, T.G.; Hoffman, A.S. Immobilization and characterization of beta-galactosidase in thermally reversible hydrogel beads. J. Biomed. Mater. Res. 1990, 24, 21–38. [Google Scholar] [CrossRef] [PubMed]
- Brannon-Peppas, L.; Peppas, N.A. Solute and penetrant diffusion in swellable polymers. IX. The mechanisms of drug release from ph-sensitive swelling-controlled systems. J. Control. Release 1989, 8, 267–274. [Google Scholar] [CrossRef]
- Ding, R.; Yu, X.; Wang, P.; Zhang, J.; Zhou, Y.; Cao, X.; Tang, H.; Ayres, N.; Zhang, P. Hybrid photosensitizer based on amphiphilic block copolymer stabilized silver nanoparticles for highly efficient photodynamic inactivation of bacteria. RSC Adv. 2016, 6, 20392–20398. [Google Scholar] [CrossRef]
- Tirrell, D.A. Macromolecular switches for bilayer membranes. J. Control. Release 1987, 6, 15–21. [Google Scholar] [CrossRef]
- Pan, L.; Yu, G.; Zhai, D.; Lee, H.R.; Zhao, W.; Liu, N.; Wang, H.; Tee, B.C.K.; Shi, Y.; Cui, Y.; et al. Hierarchical nanostructured conducting polymer hydrogel with high electrochemical activity. Proc. Natl. Acad. Sci. USA 2012, 109, 9287–9292. [Google Scholar] [CrossRef]
- Zhai, D.; Liu, B.; Shi, Y.; Pan, L.; Wang, Y.; Li, W.; Zhang, R.; Yu, G. Highly sensitive glucose sensor based on Pt nanoparticle/polyaniline hydrogel heterostructures. ACS Nano 2013, 7, 3540–3546. [Google Scholar] [CrossRef]
- Chen, C.; Vassallo, J.C.; Chatterjee, P.K. Absorbency; Chatterjee, P.K., Ed.; Elsevier: New York, NY, USA, 1985; pp. 257–281. [Google Scholar]
- Li, L.; Wang, Y.; Pan, L.; Shi, Y.; Cheng, W.; Shi, Y.; Yu, G. A nanostructured conductive hydrogels-based biosensor platform for human metabolite detection. Nano Lett. 2015, 15, 1146–1151. [Google Scholar] [CrossRef] [PubMed]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Esposito, C.L.; Kirilov, P.; Roullin, V.G. Organogels, promising drug delivery systems: An update of state-of-the-art and recent applications. J. Control. Release 2018, 271, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chi, J.; Xu, P.; Dong, X.; Le, A.T.; Shi, K.; Liu, Y.; Xiao, J. Supramolecular G-quadruplex hydrogels: Bridging fabrication to biomedical application. J. Mater. Sci. Technol. 2023, 155, 238–252. [Google Scholar] [CrossRef]
- Ren, X.; Wang, N.; Zhou, Y.; Song, A.; Jin, G.; Li, Z.; Luan, Y. An injectable hydrogel using an immunomodulating gelator for amplified tumor immunotherapy by blocking the arginase pathway. Acta Biomater. 2021, 124, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.Y.; Moon, S.E.; Kim, J.H.; Kang, S.M. Ultrasensitive and Highly Stretchable Multiple-Crosslinked Ionic Hydrogel Sensors with Long-Term Stability. Nano-Micro Lett. 2023, 15, 51. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Jiang, Z.; Lin, Y.; Yu, C.; Nie, X.; Xu, G.; Xu, W.; Jiang, Y.; Luan, Y. Tumor lysates-constructed hydrogel to potentiate tumor immunotherapy. J. Control. Release 2023, 358, 345–357. [Google Scholar] [CrossRef]
- Mitura, S.; Sionkowska, A.; Jaiswal, A. Biopolymers for hydrogels in cosmetics: Review. J. Mater. Sci. Mater. Med. 2020, 31, 50. [Google Scholar] [CrossRef]
- Matricardi, P.; Di Meo, C.; Coviello, T.; Hennink, W.; Alhaique, F. Interpenetrating Polymer Networks polysaccharide hydrogels for drug delivery and tissue engineering. Adv. Drug Deliv. Rev. 2013, 65, 1172–1187. [Google Scholar] [CrossRef]
- Dattilo, M.; Patitucci, F.; Prete, S.; Parisi, O.I.; Puoci, F. Polysaccharide-Based Hydrogels and Their Application as Drug Delivery Systems in Cancer Treatment: A Review. J. Funct. Biomater. 2023, 14, 55. [Google Scholar] [CrossRef]
- Singh, B.; Chauhan, N. Dietary fiber psyllium based hydrogels for use in insulin delivery. Int. J. Diab. Mellitus. 2010, 2, 32–37. [Google Scholar] [CrossRef][Green Version]
- Schmaljohann, D. Thermo- and pH-responsive polymers in drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1655–1670. [Google Scholar] [CrossRef] [PubMed]
- Schoener, C.A.; Hutson, H.N.; Peppas, N.A. pH-responsive hydrogels with dispersed hydrophobic nanoparticles for the oral delivery of chemotherapeutics. J. Biomed. Mater. Res. A 2013, 101A, 2229–2236. [Google Scholar] [CrossRef] [PubMed]
- Niemczyk-Soczynska, B.; Gradys, A.; Kolbuk, D.; Krzton-Maziopa, A.; Rogujski, P.; Stanaszek, L.; Lukomska, B.; Sajkiewicz, P. A methylcellulose/agarose hydrogel as an innovative scaffold for tissue engineering. RSC Adv. 2022, 12, 26882–26894. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Xie, W.; Cui, Z.; Huang, J.; Cao, H.; Li, Y. 3D printed alginate/gelatin-based porous hydrogel scaffolds to improve diabetic wound healing. Giant 2023, 16, 10018. [Google Scholar] [CrossRef]
- Zhao, M.; Xu, R.; Yang, Y.; Tong, L.; Liang, J.; Jiang, Q.; Fan, Y.; Zhang, X.; Sun, Y. Bioabsorbable nano-micelle hybridized hydrogel scaffold prevents postoperative melanoma recurrence. J. Control. Release 2023, 356, 219–231. [Google Scholar] [CrossRef]
- Aldhaher, A.; Shahabipour, F.; Shaito, A.; Al-Assaf, S.; Elnour, A.A.M.; Sallam, E.E.; Teimourtash, S.; Elfadil, A.A. 3D hydrogel/ bioactive glass scaffolds in bone tissue engineering: Status and future opportunities. Heliyon 2023, 9, e17050. [Google Scholar] [CrossRef]
- Yan, M.; Wang, L.; Wu, Y.; Wang, L.; Lu, Y. Three-dimensional highly porous hydrogel scaffold for neural circuit dissection and modulation. Acta Biomater. 2023, 157, 252–262. [Google Scholar] [CrossRef]
- Ismail, Y.A.; Shabeeba, A.K.; Sidheekha, M.P.; Rajan, L. Chapter 9: Conducting polymer/hydrogel systems as soft actuators. In Actuators: Fundamentals, Principles, Materials and Applications; Inamuddin, I., Boddula, R., Asiri, A.M., Eds.; Scrivener Publishing LLC: Beverly, MA, USA, 2020; pp. 211–252. [Google Scholar]
- Liu, J.; He, S.; Liu, Z.; Wu, X.; Liu, J.; Shao, W. Novel multi-responsive soft actuator assembled with a graphene oxide nanoribbons doped strain hydrogel sensor with high sensitive and NIR-triggered performances. Sens. Actuators B Chem. 2023, 393, 15–134217. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, H.; Cao, Y.; Chen, Y.; Akkus, O.; Liu, H.; Cao, C. Bio-inspired anisotropic hydrogels and their applications in soft actuators and robots. Matter 2023, 6, 3803–3837. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, X.; Hou, Y.; Cheng, Y.; Zhang, J.; Xiao, L.; Zhao, J.; Li, W. Bio-inspired hydrogel actuator with rapid self-strengthening behavior. Eur. Polym. J. 2023, 188, 111941. [Google Scholar] [CrossRef]
- Li, W.; Guan, Q.; Li, M.; Saiz, E.; Hou, X. Nature-inspired strategies for the synthesis of hydrogel actuators and their applications. Prog. Polym. Sci. 2023, 140, 101665. [Google Scholar] [CrossRef]
- Jing, Y.; Wang, A.; Li, J.; Li, Q.; Han, Q.; Zheng, X.; Cao, H.; Bai, S. Preparation of conductive and transparent dipeptide hydrogels for wearable biosensor. Bio-Des. Manuf. 2022, 5, 153–162. [Google Scholar] [CrossRef]
- Barhoum, A.; Sadak, O.; Ramirez, I.A.; Iverson, N. Stimuli-bioresponsive hydrogels as new generation materials for implantable, wearable, and disposable biosensors for medical diagnostics: Principles, opportunities, and challenges. Adv. Colloid Interface Sci. 2023, 317, 102920. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wang, W.; Lei, Z.; Wang, W.; Yu, D. A transparent, anti-fatigue, flexible multifunctional hydrogel with self-adhesion and conductivity for biosensors. Polymer 2023, 281, 126121. [Google Scholar] [CrossRef]
- Wang, Q.; Jiao, C.; Wang, X.; Wang, Y.; Sun, K.; Li, L.; Fan, Y.; Hu, L. A hydrogel-based biosensor for stable detection of glucose. Biosens. Bioelectr. 2023, 221, 114908. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Kong, W.; Lu, T.; Irudayaraj, J. Soft hydrogel-shell confinement systems as bacteria-based bioactuators and biosensors. Biosens. Bioelectr. 2023, 219, 114809. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, X.; Serpe, M.J. Stimuli-responsive microgel-based etalons for optical sensing. RSC Adv. 2015, 5, 44074–44087. [Google Scholar] [CrossRef]
- Narayanaswamy, R.; Torchilin, V.P. Hydrogels and their applications in targeted drug delivery. Molecules 2019, 24, 603. [Google Scholar] [CrossRef]
- Gong, Q.; Zhao, Y.; Qian, T.; Wang, H.; Li, Z. Functionalized hydrogels in ophthalmic applications: Ocular inflammation, corneal injuries, vitreous substitutes and intravitreal injection. Mater. Des. 2022, 224, 111277. [Google Scholar] [CrossRef]
- Long, L.; Ge, Z.; Zhang, F.; Dong, R.; Yang, L.; Chen, Z.; Tang, S.; Wang, Y. Development of injectable hyaluronic acid-based hydrogels with antioxidant activity for the treatment of corneal neovascularization. Chem. Eng. J. 2023, 478, 147147. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Y.; Shi, X.; Zhang, R.; He, Y.; Zhang, H.; Wang, W. Application of polyvinyl alcohol/chitosan copolymer hydrogels in biomedicine: A review. Int. J. Biol. Macrom. 2023, 242, 125192. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.; Ajazuddin; Khan, J.; Saraf, A.; Saraf, S. Polyethylene glycol (PEG)-Poly(N-isopropylacrylamide) (PNIPAAm) based thermosensitive injectable hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2014, 88, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Aminabhavi, T.M.; Nadagouda, M.N.; More, U.A.; Joshi, S.D.; Kularni, V.H.; Noolvi, M.N.; Kulkarni, P.V. Controlled release of therapeutics using interpenetrating polymeric network. Expert Opin. Drug Deliv. 2015, 12, 669–688. [Google Scholar] [CrossRef]
- Singh, B.; Kumar, R.; Kumar, S.; Thakur, N.; Ram, K. Design of polysaccharide gum based network copolymeric hydrogels for drug delivery and wound dressing applications. Med. Nov. Technol. Devices 2023, 20, 100262. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.; Sharma, V.; Sharma, P.; Kumar, R. Functionalization of bioactive moringa gum for designing hydrogel wound dressings. Hybrid Adv. 2023, 4, 100096. [Google Scholar] [CrossRef]
- Zong, Q.; Peng, X.; Ding, Y.; Wu, H.; Lu, C.; Ye, J.; Sun, W.; Zhang, J.; Zhai, Y. Multifunctional hydrogel wound dressing with rapid on-demand degradation property based on aliphatic polycarbonate and chitosan. Int. J. Biol. Macromol. 2023, 244, 125138. [Google Scholar] [CrossRef]
- Meng, L.; Meng, P.; Tang, B.; Zhang, Q.; Wang, Y. Water-compatible molecularly imprinted photonic hydrogels for fast screening of morphine in urine. Chin. J. Anal. Chem. 2015, 43, 490–496. [Google Scholar]
- Lorenzo, R.A.; Carro, A.M.; Concheiro, A.; Alvarez-Lorenzo, C. Stimuli-responsive materials in analytical separation. Anal. Bioanal. Chem. 2015, 407, 4927–4948. [Google Scholar] [CrossRef]
- Kamath, K.R.; Park, K. Biodegradable hydrogels in drug delivery. Adv. Drug Deliv. Rev. 1993, 11, 59–84. [Google Scholar] [CrossRef]
- Li, J.; Jia, X.; Yin, L. Hydrogel: Diversity of Structures and Applications in Food Science. Food Rev. Int. 2021, 37, 313–372. [Google Scholar] [CrossRef]
- Pernetti, M.; Malssen, K.F.; Floten, E.; Bot, A. Structuring of edible oils by alternatives to crystalline fat. Curr. Opin. Colloid Interface Sci. 2007, 12, 221–231. [Google Scholar] [CrossRef]
- Weng, L.; Gouldstone, A.; Wu, Y.; Chen, W. Mechanically strong double network photo cross linked hydrogels from N, N-dimethylacrylamide and glycidyl methacrylated hyaluronan. Biomaterial 2008, 29, 2153–2163. [Google Scholar] [CrossRef] [PubMed]
- Moura, M.R.; Aouada, F.A.; Guilherme, M.R.; Radovanovic, E.; Rubira, A.F.; Muniz, E.C. Thermo-sensitive IPN hydrogels composed of PNIPAAm gels supported on alginate-Ca2+ with LCST tailored close to human body temperature. Polym. Test. 2006, 25, 961–969. [Google Scholar] [CrossRef]
- Moura, M.R.; Guilherme, M.R.; Campese, G.M.; Radovanovic, E.; Rubira, A.F.; Muniz, E.C. Porous alginate-Ca2+ hydrogels interpenetrated with PNIPAAm networks: Interrelationship between compressive stress and pore morphology. Eur. Polym. J. 2005, 41, 2845–2852. [Google Scholar] [CrossRef]
- Carvalho, I.C.; Mansur, H.S.; Leonel, A.G.; Mansur, A.A.P.; Lobato, Z.I.P. Soft matter polysaccharide-based hydrogels as versatile bioengineered platforms for brain tissue repair and regeneration. Int. J. Biol. Macromol. 2021, 182, 1091–1111. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, A.; Li, D.; Guo, Y.; Sun, L. Applications of mixed polysaccharide-protein systems in fabricating multi-structures of binary food gels—A review. Trends Food Sci. Technol. 2021, 109, 197–210. [Google Scholar] [CrossRef]
- Gronwald, O.; Shinkai, S. Sugar-Integrated Gelators of Organic Solvents. Chem. Eur. J. 2001, 7, 4328–4334. [Google Scholar] [CrossRef]
- Alsaab, H.; Bonam, S.P.; Bahl, D.; Chowdhury, P.; Alexander, K.; Boddu, S.H.S. Organogels in Drug Delivery: A Special Emphasis on Pluronic Lecithin Organogels. J. Pharm. Pharm. Sci. 2016, 19, 252–273. [Google Scholar] [CrossRef]
- Terech, P.; Weiss, R.G. Low molecular mass gelators of organic liquids and properties of their gels. Chem. Rev. 1997, 97, 3133–3159. [Google Scholar] [CrossRef]
- Van, E.J.H.; Feringa, B.L. New functional materials based on self assembling organogels: From serendipity towards design. Angew. Chem. Int. Ed. 2000, 39, 2263–2266. [Google Scholar]
- Abdallah, D.J.; Weiss, R.G. n-Alkanes gel n-alkanes (and many other organic liquids). Langmuir 2000, 16, 352–355. [Google Scholar] [CrossRef]
- Vintiloiu, A.; Leroux, J.C. Organogels and their use in drug delivery—A review. J. Control. Release 2008, 125, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Kumar, N.; Bhattacharya, C.; Sagiri, S.S.; Jain, K.; Pal, K.; Ray, S.S.; Nayak, B. Organogels: Properties and Applications in Drug Delivery. Des. Monomers Polym. 2011, 14, 95–108. [Google Scholar] [CrossRef]
- Ogutcu, M.; Yilmaz, E. Oleogels of virgin olive oil with carnauba wax and monoglyceride as spreadable products. Grasas Aceites 2014, 65, e040. [Google Scholar]
- Bemer, H.L.; Limbaugh, M.; Cramer, E.D.; Harper, W.J.; Maleky, F. Vegetable organogels incorporation in cream cheese products. Food Res. Int. 2016, 85, 67–75. [Google Scholar] [CrossRef]
- Han, L.; Li, L.; Li, L.; Zhao, L.; Liu, G.Q.; Liu, X.; Wang, X. Structure and physical properties of organogels developed by sitosterol and lecithin with sunflower oil. J. Am. Oil Chem. Soc. 2014, 91, 1783–1792. [Google Scholar] [CrossRef]
- Patel, A.R.; Rajarethinem, P.S.; Gredowska, A.; Turhan, O.; Lesaffer, A.; De Vos, W.H.; Van de Walle, D.; Dewettinck, K. Edible applications of shellac oleogels: Spreads, chocolate paste and cakes. Food Funct. 2014, 5, 645–652. [Google Scholar] [CrossRef]
- Tzankov, B.; Voycheva, C.; Tosheva, A.; Stefanova, D.; Tzankova, V.; Spassova, I.; Kovacheva, D.; Avramova, K.; Tzankova, D.; Yoncheva, K. Novel oleogels for topical delivery of quercetin based on mesoporous silica MCM-41 and HMS particles. J. Drug Deliv. Sci. Technol. 2023, 86, 104727. [Google Scholar] [CrossRef]
- Wang, S.; Chen, K.; Liu, G. Monoglyceride oleogels for lipophilic bioactive delivery—Influence of self-assembled structures on stability and in vitro bioaccessibility of astaxanthin. Food Chem. 2022, 375, 131880. [Google Scholar] [CrossRef]
- Mahler, P.; Mahler, F.; Duruz, H.; Ramazzina, M.; Liguori, V.; Mautone, G. Double-blind, randomized, controlled study on the efficacy and safety of a novel diclofenac epolamine gel formulated with lecithin for the treatment of sprains, strains and contusions. Drugs Exp. Clin. Res. 2003, 29, 45–52. [Google Scholar] [PubMed]
- Martinez, R.M.; Rosado, C.; Velasco, M.V.R.; Lannes, S.C.S.; Baby, A.R. Main features and applications of organogels in cosmetics. Int. J. Cosmetic Sci. 2019, 41, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Sansón, M.D.S. Development of Oils Based on High Oleic Sunflower Oil Structured by Sorbitan Monostearate and Candelilla Wax. Master’s Thesis, University of São Paulo, São Paulo, Brazil, 2019. (In Portuguese). [Google Scholar]
- Botega, D.C.Z. Development of Organogels for Application in Cold Emulsions for Cosmetic Products. Ph.D. Thesis, Estadual Univserity of Campinas, Campinas, Brazil, 2018. (In Portuguese). [Google Scholar]
- Yu, H.; Shi, K.; Liu, D.; Huang, Q. Development of a food-grade organogel with high bioaccessibility and loading of curcuminoids. Food Chem. 2012, 131, 48–54. [Google Scholar] [CrossRef]
- Kamali, E.; Sahari, M.A.; Barzegar, M.; Gavlighi, H.A. Novel oleogel formulation based on amaranth oil: Physicochemical characterization. Food Sci. Nutr. 2019, 7, 1986–1996. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Hwang, H.S.; Jeong, S.; Lee, S. Utilization of oleogels with binary oleogelator blends for filling creams low in saturated fat. LWT 2022, 155, 112972. [Google Scholar] [CrossRef]
- O’Sullivan, C.M.; Davidovich-Pinhas, M.; Wright, A.J.; Barbut, S.; Marangoni, A.G. Ethylcellulose oleogels for lipophilic bioactive delivery—effect of oleogelation on in vitro bioaccessibility and stability of beta-carotene. Food Funct. 2017, 8, 1438–1451. [Google Scholar] [CrossRef] [PubMed]
- Oh, I.; Lee, J.; Lee, H.G.; Lee, S. Feasibility of hydroxypropyl methylcellulose oleogel as an animal fat replacer for meat patties. Food Res. Int. 2019, 122, 566–572. [Google Scholar] [CrossRef]
- Wei, G.; Xiao-lu, G.; Hai-bo, H.; Xiang, L.; Yu, X.; Jiang-Ning, H. Structural characterization of modified whey protein isolates using cold plasma treatment and its applications in emulsion oleogels. Food Chem. 2021, 356, 129703. [Google Scholar]
- Al-Saedi, Z.H.F.; Salih, Z.T.; Ahmed, K.K.; Ahmed, R.A.; Jasim, S.A. Formulation and Characterization of Oleogel as a Topical Carrier of Azithromycin. AAPS Pharm. Sci. Technol. 2023, 24, 17. [Google Scholar] [CrossRef]
- Shuai, X.; McClements, D.J.; Geng, Q.; Dai, T.; Ruan, R.; Du, L.; Liu, Y.; Chen, J. Macadamia oil-based oleogels as cocoa butter alternatives: Physical properties, oxidative stability, lipolysis, and application. Food Res. Int. 2023, 172, 113098. [Google Scholar] [CrossRef]
- Suriaini, N.; Arpi, N.; Syamsuddin, Y.; Supardan, M.D. Characteristics of palm oil-based oleogel and its potency as a shortening replacer. S. Afr. J. Chem. Eng. 2023, 43, 197–203. [Google Scholar] [CrossRef]
- Silva-Avellaneda, E.; Bauer-Estrada, K.; Prieto-Correa, R.E.; Quintanilla-Carvajal, M.X. The effect of composition, microfluidization and process parameters on formation of oleogels for ice cream applications. Sci. Rep. 2021, 11, 7161. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Zhang, Q.; Vriesekoop, F.; Yuan, Q.; Liang, H. Preparation of Organogel with Tea Polyphenols Complex for Enhancing the Antioxidation Properties of Edible Oil. J. Agric. Food Chem. 2014, 62, 8379–8384. [Google Scholar] [CrossRef] [PubMed]
- Thakur, D.; Singh, A.; Prabhakar, P.K.; Meghwal, M.; Upadhyay, A. Optimization and characterization of soybean oil-carnauba wax oleogel. LWT 2022, 157, 113108. [Google Scholar] [CrossRef]
- Gravelle, A.J.; Davidovich-Pinhas, M.; Zetzl, A.K.; Barbut, S.; Marangoni, A.G. Influence of solvent quality on the mechanical strength of ethylcellulose oleogels. Carbohydr. Polym. 2016, 135, 169–179. [Google Scholar] [CrossRef]
- Nikiforidis, C.V.; Scholten, E. Self-assemblies of lecithin and a-tocopherol as gelators of lipid material. RSC Adv. 2014, 4, 2466–2473. [Google Scholar] [CrossRef]
- Silva, S.L.; Amaral, J.T.; Ribeiro, M.; Sebastiao, E.E.; Vargas, C.; Franzen, F.D.; Schneider, G.; Lorenzo, J.M.; Fries, L.L.M.; Cichoski, A.J.; et al. Fat replacement by oleogel rich in oleic acid and its impact on the technological, nutritional, oxidative, and sensory properties of Bologna-type sausages. Meat Sci. 2019, 149, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Vilar, W.D. Química e Tecnologia dos Poliuretanos, 3rd ed.; Vilar Consultoria: Rio de Janeiro, Brazil, 2004. [Google Scholar]
- Gandolfo, F.; Bot, A.; Floöer, E. Structuring of edible oils by long-chain FA, fatty alcohols, and their mixtures. J. Am. Oil Chem. Soc. 2004, 81, 1–6. [Google Scholar] [CrossRef]
- Varrato, F. Routes to Novel Colloidal Gels. Ph.D. Thesis, École Polythechnique Fédérale de Lausanne, Lausanne, Switzerland, 2012. [Google Scholar]
- Zampouni, K.; Mouzakitis, C.K.; Lazaridou, A.; Moschakis, T.; Katsanidis, E. Physicochemical properties and microstructure of bigels formed with gelatin and k-carrageenan hydrogels and monoglycerides in olive oil oleogels. Food Hydrocolloids 2023, 140, 108636. [Google Scholar] [CrossRef]
- Mazurkeviciute, A.; Ramanauskiene, K.; Ivaskiene, M.; Grigonis, A.; Briedis, V. Topical antifungal bigels: Formulation, characterization and evaluation. Acta Pharm. 2018, 68, 223–233. [Google Scholar] [CrossRef]
- Rhee, G.J.; Woo, J.S.; Hwang, S.J.; Al, E. Topical oleo-hydrogel preparation of ketoprofen with enhanced skin permeability. Drug Dev. Ind. Pharm. 1999, 25, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Bollom, M.A.; Clark, S.; Acevedo, N.C. Development and characterization of a novel soy lecithin-stearic acid and whey protein concentrate bigel system for potential edible applications. Food Hydrocolloids 2020, 101, 105570. [Google Scholar] [CrossRef]
- Hamed, R.; AbuRezeq, A.; Tarawneh, O. Development of Hydrogels, Oleogels and Bigels as Local Drug Delivery Systems for Periodontitis. Drug Dev. Ind. Pharm. 2018, 44, 1488–1497. [Google Scholar] [CrossRef] [PubMed]
- Tomczykowa, M.; Wróblewska, M.; Winnicka, K.; Wieczorek, P.; Majewski, P.; Celínska-Janowicz, K.; Sawczuk, R.; Miltyk, W.; Tryniszewska, E.; Tomczyk, M. Novel Gel Formulations as Topical Carriers for the Essential Oil of Bidens Tripartita for the Treatment of Candidiasis. Molecule 2018, 23, 2517. [Google Scholar] [CrossRef]
- Zheng, H.; Mao, L.; Cui, M.; Liu, J.; Gao, Y. Development of food-grade bigels based on κ-carrageenan hydrogel and monoglyceride oleogels as carriers for β-carotene: Roles of oleogel fraction. Food Hydrocolloids 2020, 105, 105855. [Google Scholar] [CrossRef]
- Zulfakar, M.H.; Chan, L.M.; Rehman, K.; Wai, L.K.; Heard, C.M. Coenzyme Q10-Loaded Fish Oil-Based Bigel System: Probing the Delivery Across Porcine Skin and Possible Interaction with Fish Oil Fatty Acids. AAPS Pharm. Sci. Technol. 2018, 19, 1116–1123. [Google Scholar] [CrossRef]
- Rehman, K.; Zulfakar, M.H. Novel Fish Oil-Based Bigel System for Controlled Drug Delivery and Its Influence on Immunomodulatory Activity of Imiquimod Against Skin Cancer. Pharm. Res. 2017, 34, 36–48. [Google Scholar] [CrossRef]
- Wróblewska, M.; Szyma´nska, E.; Szekalska, M.; Winnicka, K. Different Types of Gel Carriers as Metronidazole Delivery Systems to the Oral Mucosa. Polymers 2020, 12, 680. [Google Scholar] [CrossRef]
- Vergara, D.; Loza-Rodríguez, N.; Acevedo, F.; Bustamante, M.; López, O. Povidone-Iodine Loaded Bigels: Characterization and Effect as a Hand Antiseptic Agent. J. Drug Deliv. Sci. Technol. 2022, 72, 103427. [Google Scholar] [CrossRef]
- Liu, C.; Zheng, Z.; Shi, Y.; Zhang, Y.; Liu, Y. Development of low-oil emulsion gel by solidifying oil droplets: Roles of internal beeswax concentration. Food Chem. 2021, 345, 128811. [Google Scholar] [CrossRef]
- Paul, S.R.; Qureshi, D.; Yogalakshmi, Y.; Nayak, S.K.; Singh, V.K.; Syed, I.; Sarkar, P.; Pal, K. Development of Bigels Based on Stearic Acid–Rice Bran Oil Oleogels and Tamarind Gum Hydrogels for Controlled Delivery Applications. J. Surfactants Deterg. 2018, 21, 17–29. [Google Scholar] [CrossRef]
- Kodela, S.P.; Pandey, P.M.; Nayak, S.K.; Uvanesh, K.; Anis, A.; Pal, K. Novel agar-stearyl alcohol oleogel-based bigels as structured delivery vehicles. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 669–678. [Google Scholar] [CrossRef]
- Singh, V.K.; Anis, A.; Al-Zahrani, S.M.; Pradhan, D.K.; Pal, K. Molecular and Electrochemical Impedance Spectroscopic Characterization of the Carbopol Based Bigel and Its Application in Iontophoretic Delivery of Antimicrobials. Int. J. Electrochem. Sci. 2014, 9, 5049–5060. [Google Scholar] [CrossRef]
- Satapathy, S.; Singh, V.K.; Sagiri, S.S.; Agarwal, T.; Banerjee, I.; Bhattacharya, M.K.; Kumar, N.; Pal, K. Development and characterization of gelatin-based hydrogels, emulsion hydrogels, and bigels: A comparative study. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Wakhet, S.; Singh, V.K.; Sahoo, S.; Sagiri, S.S.; Kulanthaivel, S.; Bhattacharya, M.K.; Kumar, N.; Banerjee, I.; Pal, K. Characterization of gelatin-agar based phase separated hydrogel, emulgel and bigel: A comparative study. J. Mater. Sci. Mater. Med. 2015, 26, 118. [Google Scholar] [CrossRef]
- Zhu, Q.; Gao, J.; Han, L.; Han, K.; Wei, W.; Wu, T.; Li, J.; Zhang, M. Development and characterization of novel bigels based on monoglyceride-beeswax oleogel and high acyl gellan gum hydrogel for lycopene delivery. Food Chem. 2021, 365, 130419. [Google Scholar] [CrossRef]
- Behera, B.; Dey, S.; Sharma, V.; Pal, K. Rheological and Viscoelastic Properties of Novel Sunflower Oil-Span 40-Biopolymer–Based Bigels and Their Role as a Functional Material in the Delivery of Antimicrobial Agents. Adv. Polym. Technol. 2015, 34, 21488. [Google Scholar] [CrossRef]
- Sahoo, S.; Singh, V.K.; Uvanesh, K.; Biswal, D.; Anis, A.; Rana, U.A.; Al-Zahrani, S.M.; Pal, K. Development of ionic and non-ionic natural gum-based bigels: Prospects for drug delivery application. J. Appl. Polym. Sci. 2015, 132, 42561. [Google Scholar] [CrossRef]
- Behera, B.; Singh, V.K.; Kulanthaivel, S.; Bhattacharya, M.K.; Paramanik, K.; Banerjee, I.; Pal, K. Physical and mechanical properties of sunflower oil and synthetic polymers based bigels for the delivery of nitroimidazole antibiotic—A therapeutic approach for controlled drug delivery. Eur. Polym. J. 2015, 64, 253–264. [Google Scholar] [CrossRef]
- Martinez, R.M.; Magalhães, W.V.; Silva Sufi, B.; Padovani, G.; Nazato, L.I.S.; Velasco, M.V.R.; Lannes, S.C.S.; Baby, A.R. Vitamin E-loaded bigels and emulsions: Physicochemical characterization and potential biological application. Colloid Surf. B 2021, 201, 111651. [Google Scholar] [CrossRef]
- Khelifi, I.; Saada, M.; Hayouni, E.A.; Tourette, A.; Bouajila, J.; Ksouri, R. Development and characterization of novel bi-gel-based 1, 4-naphthoquinones for topical application with antioxidant potential. Arab. J. Sci. Eng. 2020, 45, 53–61. [Google Scholar] [CrossRef]
- Loza-Rodríguez, N.; Millan-Sanchez, A.; Lopez, O. A biocompatible lipid-based bigel for topical applications. Eur. J. Pharm. Biopharm. 2023, 190, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Corredor-Chaparro, M.Y.; Vargas-Riveros, D.; Mora-Huertas, C.E. Hypromellose—Collagen hydrogels/sesame oil organogel based bigels as controlled drug delivery systems. J. Drug Deliv. Sci. Technol. 2022, 75, 103637. [Google Scholar] [CrossRef]
- Cortés, N.M.; Lorenzo, G.; Califano, A.N. Food grade microemulsion systems: Sunflower oil/castor oil derivative-ethanol/water. Rheological and physicochemical analysis. Food Res. Int. 2018, 107, 41–47. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).