Production of the Macroalgae Ulva lactuca Integrated with the Shrimp Penaeus vannamei in a Biofloc System: Effect of Total Suspended Solids and Nutrient Concentrations
Abstract
:1. Introduction
2. Materials and Methods
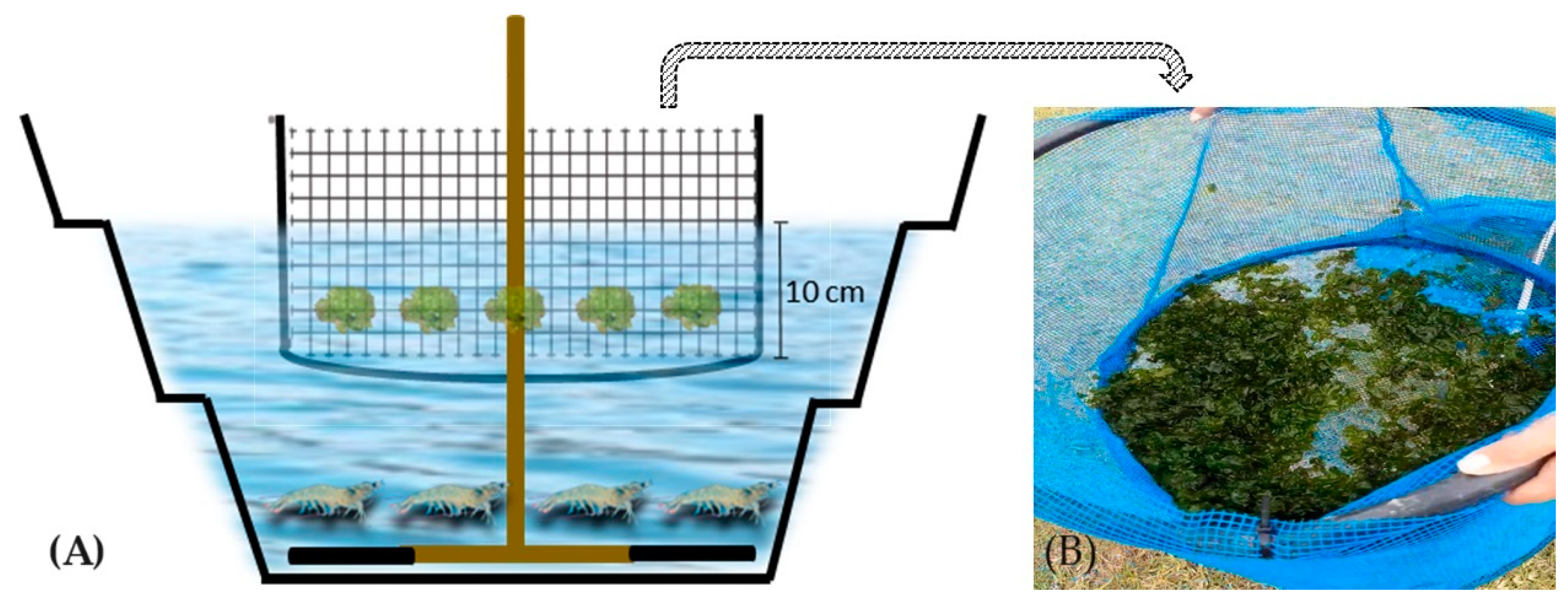
2.1. Experimental Design and Facilities
2.2. Physical and Chemical Parameters
2.3. Growth and Nutrient Absorption by Macroalgae
2.4. Proximal Composition and Biocompounds of Macroalgae
2.5. Shrimp Performance
- Mean final weight (g): final biomass of live animals (g)/total number of animals;
- Weekly weight gain (g week-1): weight gain (g)/number of weeks.
- Final biomass (g): ∑ final weight of all live animals (g);
- Survival (%) = (final number of animals/initial number of animals) × 100;
- Feed conversion factor (FCR) = ∑ration offered (g)/(biomass gains (g);
- Productivity (kg m−3): (final biomass (kg)/tank volume (m3);
2.6. Statistical Analysis
3. Results
3.1. Physical and Chemical Parameters
3.2. Macroalgae Performance
3.3. Proximal Composition and Biocompounds of Macroalgae
3.4. Shrimp Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. State of the World Fisheries and Aquaculture—2022 (SOFIA); FAO: Rome, Italy, 2022; ISBN 9789251072257. [Google Scholar]
- de Alencar, J.R.; Junior, P.A.H.; Celino, J.J. Cultivo de Camarão Branco Litopenaeus vannamei (Boone, 1931) Com a Macroalga Ulva lactuca Linneaus (Chlorophyta) No Tratamento de Efluentes Em Sistema Fechado de Recirculação. Rev. Biol. Ciências Terra 2010, 10, 117–137. [Google Scholar]
- Copertino, M.D.S.; Tormena, T.; Seeliger, U. Biofiltering Efficiency, Uptake and Assimilation Rates of Ulva clathrata (Roth) J. Agardh (Clorophyceae) Cultivated in Shrimp Aquaculture Waste Water. J. Appl. Phycol. 2009, 21, 31–45. [Google Scholar] [CrossRef]
- Chopin, T. Marine Aquaculture in Canada: Well-Established Monocultures of Finfish and Shellfish and an Emerging Integrated Multi-Trophic Aquaculture (IMTA) Approach Including Seaweeds, Other Invertebrates, and Microbial Communities. Fisheries 2015, 40, 28–31. [Google Scholar] [CrossRef]
- Chopin, T.; Buschmann, A.H.; Halling, C.; Troell, M.; Kautsky, N.; Neori, A.; Kraemer, G.P.; Zertuche-González, J.A.; Yarish, C.; Neefus, C. Integrating Seaweeds into Marine Aquaculture Systems: A Key toward Sustainability. J. Phycol. 2001, 37, 975–986. [Google Scholar] [CrossRef]
- Troell, M.; Joyce, A.; Chopin, T.; Neori, A.; Buschmann, A.H.; Fang, J.G. Ecological Engineering in Aquaculture—Potential for Integrated Multi-Trophic Aquaculture (IMTA) in Marine Offshore Systems. Aquaculture 2009, 297, 1–9. [Google Scholar] [CrossRef]
- Nobre, A.M.; Robertson-Andersson, D.; Neori, A.; Sankar, K. Ecological-Economic Assessment of Aquaculture Options: Comparison between Abalone Monoculture and Integrated Multi-Trophic Aquaculture of Abalone and Seaweeds. Aquaculture 2010, 306, 116–126. [Google Scholar] [CrossRef]
- Holanda, M.; Wasielesky, W.; de Lara, G.R.; Poersch, L.H. Production of Marine Shrimp Integrated with Tilapia at High Densities and in a Biofloc System: Choosing the Best Spatial Configuration. Fishes 2022, 7, 283. [Google Scholar] [CrossRef]
- Verdian, A.H.; Effendi, I.; Budidardi, T.; Diatin, I. Production Performance Improvement of White Shrimp (Litopenaeus vannamei) Culture with Integrated Multi Trophic Aquaculture System in Seribu Islands, Jakarta, Indonesia. Iran. J. Fish. Sci. 2020, 19, 1415–1427. [Google Scholar] [CrossRef]
- Poli, M.A.; Legarda, E.C.; de Lorenzo, M.A.; Pinheiro, I.; Martins, M.A.; Seiffert, W.Q.; do Nascimento Vieira, F. Integrated Multitrophic Aquaculture Applied to Shrimp Rearing in a Biofloc System. Aquaculture 2019, 511, 734274. [Google Scholar] [CrossRef]
- Ferreira, G.S.; Santos, D.; Schmachtl, F.; Machado, C.; Fernandes, V.; Bögner, M.; Schleder, D.D.; Seiffert, W.Q.; Vieira, F.N. Heterotrophic, Chemoautotrophic and Mature Approaches in Biofloc System for Pacific White Shrimp. Aquaculture 2021, 533, 736099. [Google Scholar] [CrossRef]
- Gaona, C.A.P.; de Almeida, M.S.; Viau, V.; Poersch, L.H.; Wasielesky, W. Effect of Different Total Suspended Solids Levels on a Litopenaeus vannamei (Boone, 1931) BFT Culture System during Biofloc Formation. Aquac. Res. 2017, 48, 1070–1079. [Google Scholar] [CrossRef]
- Fleckenstein, L.J.; Tierney, T.W.; Fisk, J.C.; Ray, A.J. The Effects of Different Solids and Biological Filters in Intensive Pacific White Shrimp (Litopenaeus vannamei) Production Systems. Aquac. Eng. 2020, 91, 102120. [Google Scholar] [CrossRef]
- Queiroz, H.M.; Ferreira, T.O.; Taniguchi, C.A.K.; Barcellos, D.; do Nascimento, J.C.; Nóbrega, G.N.; Otero, X.L.; Artur, A.G. Nitrogen Mineralization and Eutrophication Risks in Mangroves Receiving Shrimp Farming Effluents. Environ. Sci. Pollut. Res. 2020, 27, 34941–34950. [Google Scholar] [CrossRef] [PubMed]
- Legarda, E.C.; da Silva, D.; Miranda, C.S.; Pereira, P.K.M.; Martins, M.A.; Machado, C.; de Lorenzo, M.A.; Hayashi, L.; do Nascimento Vieira, F. Sea Lettuce Integrated with Pacific White Shrimp and Mullet Cultivation in Biofloc Impact System Performance and the Sea Lettuce Nutritional Composition. Aquaculture 2021, 534, 736265. [Google Scholar] [CrossRef]
- Brito, L.O.; Arantes, R.; Magnotti, C.; Derner, R.; Pchara, F.; Olivera, A.; Vinatea, L. Water Quality and Growth of Pacific White Shrimp Litopenaeus vannamei (Boone) in Co-Culture with Green Seaweed Ulva lactuca (Linaeus) in Intensive System. Aquac. Int. 2014, 22, 497–508. [Google Scholar] [CrossRef]
- Reis, W.G.; Wasielesky, W.; Abreu, P.C.; Brandão, H.; Krummenauer, D. Rearing of the Pacific White Shrimp Litopenaeus vannamei (Boone, 1931) in BFT System with Different Photoperiods: Effects on the Microbial Community, Water Quality and Zootechnical Performance. Aquaculture 2019, 508, 19–29. [Google Scholar] [CrossRef]
- Duke, C.S.; Litaker, W.; Ramus, J. Effects of the Temperature, Nitrogen Supply and Tissue Nitrogen on Ammonium Uptake Rates of the Chlorophyte Seaweeds Ulva curvata and Codium decorticatum. J. Phycol. 1989, 25, 113–120. [Google Scholar] [CrossRef]
- Carvalho, A.; Costa, L.C.d.O.; Holanda, M.; Poersch, L.H.; Turan, G. Influence of Total Suspended Solids on the Growth of the Sea Lettuce Ulva lactuca Integrated with the Pacific White Shrimp Litopenaeus vannamei in a Biofloc System. Fishes 2023, 8, 163. [Google Scholar] [CrossRef]
- He, J.; Xu, Y.; Chen, H.; Sun, P. Extraction, Structural Characterization, and Potential Antioxidant Activity of the Polysaccharides from Four Seaweeds. Int. J. Mol. Sci. 2016, 17, 1988. [Google Scholar] [CrossRef]
- Jory, D.E.; Cabrera, T.R.; Dugger, D.M.; Fegan, D.; Lee, P.G.; Lawrence, L.; Jackson, C.J.; Mcintosh, R.P.; Castañeda, J.I.; McIntosh, R.; et al. A Global Review of Shrimp Feed Management: Status and Perspectives. Aquaculture 2011, 318, 104–152. [Google Scholar]
- Wasielesky, W.; Krummenauer, D.; Lara, G.; Fóes, G. Cultivo de Camarões Em Sistema de Bioflocos: Realidades e Perspectivas. Rev. ABCC 2013, 15, 16–26. [Google Scholar]
- Unesco. Chemical Methods for Use in Marine Environmental Monitoring; Intergovernmental Oceanographic Commission: Paris, France, 1983. [Google Scholar]
- Bendschneider, K.; Robinson, R.J. New Spectrophotometric Method for the Determination of Nitrite in Water. Fresenius Environ. Bull. 1952, 10, 781–784. [Google Scholar]
- Aminot, A.; Chaussepied, M. Manuel Des Analyses Chimiques En Milieu Marin; Centre National pour l’exploitation des océans: Paris, France, 1983. [Google Scholar]
- Bogue, J.P.; Smith, L.F.; Lipsett, L. A Practical Handbook. J. High. Educ. 1957, 28, 405. [Google Scholar] [CrossRef]
- American Public Health Association APHA; American Water Works Association; Water Pollution Control Association. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Furtado, P.S.; Poersch, L.H.; Wasielesky, W. Effect of Calcium Hydroxide, Carbonate and Sodium Bicarbonate on Water Quality and Zootechnical Performance of Shrimp Litopenaeus vannamei Reared in Biofloc Technology (BFT) Systems. Aquaculture 2011, 321, 130–135. [Google Scholar] [CrossRef]
- Loureiro, R.R.; Reis, R.P.; Critchley, A.T. In Vitro Cultivation of Three Kappaphycus alvarezii (Rhodophyta, Areschougiaceae) Variants (Green, Red and Brown) Exposed to a Commercial Extract of the Brown Alga Ascophyllum nodosum (Fucaceae, Ochrophyta). J. Appl. Phycol. 2010, 22, 101–104. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis; Association of Official Analytical Chemists: Arlington, VA, USA, 2005; p. 245. [Google Scholar]
- Baethgen, W.E.; Alley, M. A Manual Colorimetric Procedure for Measuring Ammonium Nitrogen in Soil and Plant Kjeldahl Digests. Commun. Soil Sci. Plant Anal. 1989, 20, 961–969. [Google Scholar] [CrossRef]
- Barbarino, E.; Lourenço, S.O. An Evaluation of Methods for Extraction and Quantification of Protein from Marine Macro and Microalgae. J. Appl. Phycol. 2005, 17, 447–460. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of Total Carotenoids and Chlorophylls a and b of Leaf Extracts in Different Solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Schiavon, M.; Moro, I.; Pilon-Smits, E.A.H.; Matozzo, V.; Malagoli, M.; Dalla Vecchia, F. Accumulation of Selenium in Ulva Sp. and Effects on Morphology, Ultrastructure and Antioxidant Enzymes and Metabolites. Aquat. Toxicol. 2012, 122–123, 222–231. [Google Scholar] [CrossRef]
- Farasat, M.; Khavari-Nejad, R.-A.; Nabavi, S.M.B.; Namjooyan, F. Antioxidant Properties of Two Edible Green Seaweeds from Northern Coasts of the Persian Gulf. Jundishapur J. Nat. Pharm. Prod. 2013, 8, 47–52. [Google Scholar] [CrossRef]
- Decamp, O.; Cody, J.; Conquest, L.; Delanoy, G.; Tacon, A.G.J. Effect of Salinity on Natural Community and Production of Litopenaeus vannamei (Boone), within Experimental Zero-Water Exchange Culture Systems. Aquac. Res. 2003, 34, 345–355. [Google Scholar] [CrossRef]
- Van Wyk, P.; Davis-Hodgkins, M.; Laramore, R.; Main, K.L.; Mountain, J.; Scarpa, J. Farming Marine Shrimp in Recirculating Freshwater Systems Harbor Branch Oceanographic Institution. Farming Mar. Shrimp. Recirc. Freshw. Syst. 1999, 220, 125–140. [Google Scholar]
- Krummenauer, D.; Peixoto, S.; Cavalli, R.O.; Poersch, L.H.; Wasielesky, W. Superintensive Culture of White Shrimp, Litopenaeus vannamei, in a Biofloc Technology System in Southern Brazil at Different Stocking Densities. J. World Aquac. Soc. 2011, 42, 726–733. [Google Scholar] [CrossRef]
- Wyban, J.; Walsh, W.A.; Godin, D.M. Temperature Effects on Growth, Feeding Rate and Feed Conversion of the Pacific White Shrimp (Penaeus vannamei). Aquaculture 1995, 138, 267–279. [Google Scholar] [CrossRef]
- Samocha, T.M.; Patnaik, S.; Speed, M.; Ali, A.M.; Burger, J.M.; Almeida, R.V.; Ayub, Z.; Harisanto, M.; Horowitz, A.; Brock, D.L. Use of Molasses as Carbon Source in Limited Discharge Nursery and Grow-out Systems for Litopenaeus vannamei. Aquac. Eng. 2007, 36, 184–191. [Google Scholar] [CrossRef]
- de Morais, A.P.M.; Santos, I.L.; Carneiro, R.F.S.; Routledge, E.A.B.; Hayashi, L.; de Lorenzo, M.A.; do Nascimento Vieira, F. Integrated Multitrophic Aquaculture System Applied to Shrimp, Tilapia, and Seaweed (Ulva ohnoi) Using Biofloc Technology. Aquaculture 2023, 572, 739492. [Google Scholar] [CrossRef]
- Bews, E.; Booher, L.; Polizzi, T.; Long, C.; Kim, J.H.; Edwards, M.S. Effects of Salinity and Nutrients on Metabolism and Growth of Ulva lactuca: Implications for Bioremediation of Coastal Watersheds. Mar. Pollut. Bull. 2021, 166, 112199. [Google Scholar] [CrossRef]
- Ebeling, J.M.; Timmons, M.B.; Bisogni, J.J. Engineering Analysis of the Stoichiometry of Photoautotrophic, Autotrophic, and Heterotrophic Removal of Ammonia-Nitrogen in Aquaculture Systems. Aquaculture 2006, 257, 346–358. [Google Scholar] [CrossRef]
- Da Silva, K.R.; Wasielesky, W.; Abreu, P.C. Nitrogen and Phosphorus Dynamics in the Biofloc Production of the Pacific White Shrimp, Litopenaeus vannamei. J. World Aquac. Soc. 2013, 44, 30–41. [Google Scholar] [CrossRef]
- Lin, Y.C.; Chen, J.C. Acute Toxicity of Ammonia on Litopenaeus vannamei Boone Juveniles at Different Salinity Levels. J. Exp. Mar. Biol. Ecol. 2001, 259, 109–119. [Google Scholar] [CrossRef]
- Castelar, B.; Reis, R.P.; dos Santos Calheiros, A.C. Ulva lactuca and U. flexuosa (Chlorophyta, Ulvophyceae) Cultivation in Brazilian Tropical Waters: Recruitment, Growth, and Ulvan Yield. J. Appl. Phycol. 2014, 26, 1989–1999. [Google Scholar] [CrossRef]
- Macedo, C.F.; Sipaúba-Tavares, L. Eutrofização E Qualidade Da Água Na Piscicultura: Consequências E Recomendações Eutrophication and Water Quality in Pisciculture: Consequences and Recommendations. Bol. Inst. Pesca 2010, 36, 149–163. [Google Scholar]
- Farahdiba, A.U.; Hidayah, E.N.; Asmar, G.A.; Myint, Y.W. Growth and Removal of Nitrogen and Phosphorus by a Macroalgae Cladophora glomerata under Different Nitrate Concentrations. Nat. Environ. Pollut. Technol. 2020, 19, 809–813. [Google Scholar] [CrossRef]
- Ramos, R.; Vinatea, L.; Santos, J.; Da Costa, R. Tratamiento de Efluentes Del Cultivo de Litopenaeus vannamei Mediante Procesos de Sedimentación, Filtración y Absorción. Lat. Am. J. Aquat. Res. 2010, 38, 188–200. [Google Scholar] [CrossRef]
- Zirino, A.; Elwany, H.; Facca, C.; Maicu’, F.; Neira, C.; Mendoza, G. Nitrogen to Phosphorus Ratio in the Venice (Italy) Lagoon (2001–2010) and Its Relation to Macroalgae. Mar. Chem. 2016, 180, 33–41. [Google Scholar] [CrossRef]
- Carvalho, A.; Costa, L.C.d.O.; Holanda, M.; Gonçalves, M.; Santos, J.; Costa, C.S.B.; Turan, G.; Poersch, L.H. Growth of the Macroalgae Ulva lactuca Cultivated at Different Depths in a Biofloc Integrated System with Shrimp and Fish. Phycology 2023, 3, 280–293. [Google Scholar] [CrossRef]
- Carl, C.; De Nys, R.; Lawton, R.J.; Paul, N.A. Methods for the Induction of Reproduction in a Tropical Species of Filamentous Ulva. PLoS ONE 2014, 9, 2–11. [Google Scholar] [CrossRef]
- Martins, M.A.; da SILVA, V.F.; Tarapuez, P.R.; Hayashi, L.; Vieira, F.D.N. Cultivation of the Seaweed Ulva Spp. with Effluent from a Shrimp Biofloc Rearing System: Different Species and Stocking Density. Bol. Inst. Pesca 2020, 46. [Google Scholar] [CrossRef]
- Msuya, F.E.; Neori, A. Effect of Water Aeration and Nutrient Load Level on Biomass Yield, N Uptake and Protein Content of the Seaweed Ulva lactuca Cultured in Seawater Tanks. J. Appl. Phycol. 2008, 20, 1021–1031. [Google Scholar] [CrossRef]
- Tabarsa, M.; Rezaei, M.; Ramezanpour, Z.; Waaland, J.R. Chemical Compositions of the Marine Algae Gracilaria salicornia (Rhodophyta) and Ulva lactuca (Chlorophyta) as a Potential Food Source. J. Sci. Food Agric. 2012, 92, 2500–2506. [Google Scholar] [CrossRef]
- Queirós, A.S.; Circuncisão, A.R.; Pereira, E.; Válega, M.; Abreu, M.H.; Silva, A.M.S.; Cardoso, S.M. Valuable Nutrients from Ulva rigida: Modulation by Seasonal and Cultivation Factors. Appl. Sci. 2021, 11, 6137. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive Compounds in Seaweed: Functional Food Applications and Legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Silva, A.F.R.; Abreu, H.; Silva, A.M.S.; Cardoso, S.M. Effect of Oven-Drying on the Recovery of Valuable Compounds from Ulva rigida, Gracilaria Sp. and Fucus vesiculosus. Mar. Drugs 2019, 17, 90. [Google Scholar] [CrossRef] [PubMed]
- Levavasseur, G. Analyse Comparée Des Complexes Pigment-Protéines de Chlorophycophytes Marines Benthiques. Phycologia 1989, 28, 1–14. [Google Scholar] [CrossRef]
- Fillit, M. Seasonal Changes in the Photosynthetic Capacities and Pigment Content of Ulva rigida in a Mediterranean Coastal Lagoon. Bot. Mar. 1995, 38, 271–280. [Google Scholar] [CrossRef]
- Astorg, P. Food Carotenoids and Cancer Prevention: An Overview of Current Research. Trends Food Sci. Technol. 1997, 8, 406–413. [Google Scholar] [CrossRef]
- Yildiz, G.; Celikler, S.; Vatan, O.; Dere, S. Determination of the Anti-Oxidative Capacity and Bioactive Compounds in Green Seaweed Ulva rigida C. Agardh. Int. J. Food Prop. 2012, 15, 1182–1189. [Google Scholar] [CrossRef]
- Eismann, A.I.; Perpetuo Reis, R.; Ferreira da Silva, A.; Negrão Cavalcanti, D. Ulva Spp. Carotenoids: Responses to Environmental Conditions. Algal Res. 2020, 48, 101916. [Google Scholar] [CrossRef]
- Sampath-Wiley, P.; Neefus, C.D.; Jahnke, L.S. Seasonal Effects of Sun Exposure and Emersion on Intertidal Seaweed Physiology: Fluctuations in Antioxidant Contents, Photosynthetic Pigments and Photosynthetic Efficiency in the Red Alga Porphyra umbilicalis Kützing (Rhodophyta, Bangiales). J. Exp. Mar. Biol. Ecol. 2008, 361, 83–91. [Google Scholar] [CrossRef]
- Mabeau, S.; Fleurence, J. Seaweed in Food Products: Biochemical and Nutritional Aspects. Trends Food Sci. Technol. 1993, 4, 103–107. [Google Scholar] [CrossRef]
| Treatments | |||||
|---|---|---|---|---|---|
| Parameters | CONT | T100 | T200 | T250 | T300 |
| Total ammonia N–NH3 (mg L−1) | 0.02 ± 0.00 | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.05 ± 0.03 | 0.09 ± 0.01 |
| Nitrite NO2—N (mg L−1) | 0.01 ± 0.01 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.01 ± 0.03 | 0.01 ± 0.03 |
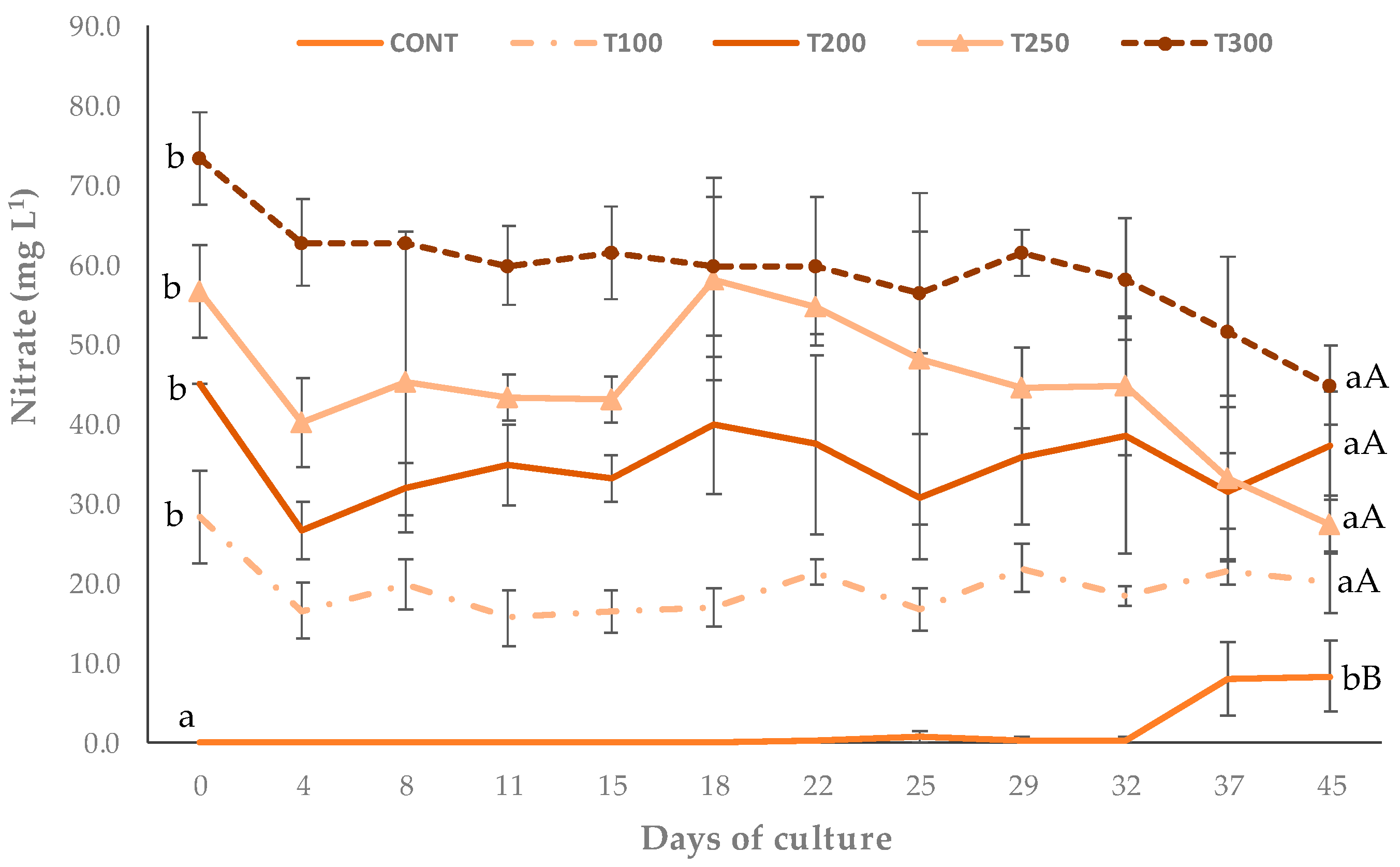
| Nitrate NO3—N (mg L−1) | 0.05 ± 0.04 a | 28.33 ± 5.77 b | 45.00 ± 0.00 c | 56.67 ± 5.77 d | 73.33 ± 5.77 e |
| Phosphate P–PO4−3 (mg L−1) | 0.14 ± 0.03 a | 0.81 ± 0.12 b | 1.25 ± 0.05 c | 1.55 ± 0.14 d | 1.60 ± 0.00 e |
| SS (mL L−1) | 0.00 ± 0.00 a | 1.97 ± 1.38 b | 8.00 ± 1.00 c | 11.67 ± 0.58 d | 19.67 ± 0.58 e |
| TSS (mg L−1) | 11.67 ± 2.89 a | 111.67 ± 63.31 b | 213.33 ± 50.08 c | 246.67 ± 2.89 d | 325.00 ± 21.21 e |
| Turbidity (NTU) | 2.57 ± 0.37 a | 26.23 ± 18.01 b | 74.63 ± 62.35 c | 176.10 ± 18.36 d | 304.10 ± 12.77 e |
| Treatments | |||||
|---|---|---|---|---|---|
| Parameters | CONT | T100 | T200 | T250 | T300 |
| Temperature (°C) | 26.92 ± 2.30 | 26.55 ± 2.18 | 26.56 ± 2.18 | 26.79 ± 2.46 | 26.56 ± 2.19 |
| DO (mg L−1) | 5.89 ± 0.70 | 6.03 ± 0.71 | 6.00 ± 0.70 | 5.90 ± 0.74 | 5.98 ± 0.71 |
| pH | 8.15 ± 0.09 c | 8.18 ± 0.07 c | 8.16 ± 0.08 b | 8.23 ± 0.07 a | 8.22 ± 0.07 a |
| Salinity (‰) | 30.43 ± 1.81 a | 30.00 ± 0.51 a | 29.43 ± 1.57 a | 29.19 ± 1.40 ab | 27.90 ± 0.66 b |
| Alkalinity (mg CaCO3 L−1) | 173.33 ± 23.93 cb | 174.36 ± 21.40 cb | 175.66 ± 24.69 cb | 205.92 ± 34.15 a | 183.08 ± 27.05 b |
| Total ammonia N–NH3 (mg L−1) | 0.12 ± 0.07 | 0.11 ± 0.03 | 0.09 ± 0.03 | 0.10 ± 0.02 | 0.10 ± 0.03 |
| Nitrite NO2—N (mg L−1) | 0.26 ± 0.55 a | 1.06 ± 1.36 b | 1.30 ± 1.96 b | 1.40 ± 2.43 b | 0.35 ± 0.40 b |
| Nitrate NO3—N (mg L−1) | 1.56 ± 3.13 a | 19.53 ± 3.53 b | 32.25 ± 4.85 c | 44.97 ± 9.01 d | 59.38 ± 6.80 e |
| Phosphate P-PO4− 3 (mg L−1) | 0.56 ± 0.36 a | 0.79 ± 0.38 ab | 1.21 ± 0.39 b | 1.16 ± 0.30 b | 1.67 ± 0.26 c |
| SS (mL L−1) | 2.65 ± 2.34 a | 5.93 ± 3.33 b | 11.15 ± 3.17 c | 15.08 ± 4.39 d | 21.71 ± 4.98 e |
| TSS (mg L−1) | 134.05 ± 81.36 a | 232.62 ± 92.76 b | 290.48 ± 65.36 b | 354.05 ± 88.69 c | 403.69 ± 79.03 c |
| Turbidity (NTU) | 80.37 ± 62.71 a | 106.26 ± 79.79 a | 167.31 ± 95.65 ab | 232.91 ± 101.9 b | 287.48 ± 104.59 b |
| Calcium hydroxide (g L−1) # | 0.13 ± 0.03 | 0.10 ± 0.00 | 0.13 ± 0.06 | 0.07 ± 0.06 | 0.08 ± 0.03 |
| Removal rate | |||||
| Nitrate (%) | 00.00 ± 00.00 c | 28.0 ± 13.0 b | 17.0 ± 15.0 bc | 55.0 ± 4.0 a | 38.0 ± 11.0 ab |
| Phosphate (%) | 00.00 ± 00.00 b | 00.00 ± 00.00 b | 00.00 ± 00.00 b | 31.0 ± 10.0 a | 00.00 ± 00.00 b |
| Treatments | |||||
|---|---|---|---|---|---|
| CONT | T100 | T200 | T250 | T300 | |
| Initial mean weight (g—FW) | 250.31 ± 0.31 | 250.05 ± 0.02 | 250.0 ± 0.00 | 250.51 ± 0.41 | 250.06 ± 0.04 |
| Final mean weight (g—FW) | 387.33 ± 115.09 | 317.67 ± 60.86 | 289.0 ± 73.75 | 304.33 ± 76.20 | 275.0 ± 36.69 |
| RGR (% dia−1) | 0.89 ± 0.61 | 0.49 ± 0.41 | 0.27 ± 0.52 | 0.38 ± 0.52 | 0.19 ± 0.30 |
| Treatments | ||
|---|---|---|
| Proximal Composition | CONT | T250 |
| Moisture (%) # | 77.42 ± 0.09 b | 75.50 ± 0.23 a |
| Protein content (%) | 22.85 ± 0.37 b | 24.26 ± 0.89 a |
| Lipids (%) | 0.53 ± 0.19 | 0.48 ± 0.19 |
| Ash (%) | 30.17 ± 1.80 b | 28.30 ± 0.64 a |
| Fiber (%) | 10.65 ± 0.78 | 11.59 ± 3.41 |
| Non-nitrogenous extract (%) | 35.77 ± 1.30 | 35.58 ± 1.44 |
| Biochemical Analysis | ||
| Protein (%) | 23.30 ± 1.59 | 25.85 ± 2.81 |
| DPPH (%) | 90.31± 5.38 | 92.97 ± 5.80 |
| Chlorophyll-a (mg g−1) | 2.18 ± 0.03 b | 2.27 ± 0.03 a |
| Chlorophyll-b (mg g−1) | 2.99 ± 0.28 | 3.31 ± 0.10 |
| Carotenoids (mg g−1) | 0.19 ± 0.10 | 0.10 ± 0.03 |
| Total Polyphenols (mg GAE g−1) | 0.38 ± 0.12 | 0.33 ± 0.09 |
| Treatments | |||||
|---|---|---|---|---|---|
| Shrimp | CONT | T100 | T200 | T250 | T300 |
| Final mean weight (g) | 6.47 ± 0.27 | 6.60 ± 0.80 | 5.89 ± 0.25 | 6.72 ± 0.26 | 6.31 ± 0.16 |
| WGW (g week−1) | 0.38 ± 0.04 | 0.40 ± 0.12 | 0.30 ± 0.04 | 0.42 ± 0.04 | 0.36 ± 0.03 |
| Final biomass (g) | 232.78 ± 9.84 | 227.72 ± 12.62 | 211.97 ± 8.96 | 235.07 ± 4.38 | 216.45 ± 17.65 |
| Survival (%) | 96.30 ± 6.41 | 96.30 ± 6.41 | 100.0 ± 0.0 | 97.22 ± 4.81 | 95.37 ± 8.02 |
| FCR | 1.90 ± 0.19 | 2.13 ± 0.30 | 2.18 ± 0.27 | 2.03 ± 0.09 | 2.41 ± 0.59 |
| Productivity (kg m−3) | 1.28 ± 0.04 | 1.26 ± 0.07 | 1.18 ± 0.05 | 1.31 ± 0.02 | 1.20 ± 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, A.; Braga, Í.; Chaar, F.; Cardozo, A.P.; Monserrat, J.M.; Ramírez, J.R.B.; Wasielesky, W., Jr.; Poersch, L.H. Production of the Macroalgae Ulva lactuca Integrated with the Shrimp Penaeus vannamei in a Biofloc System: Effect of Total Suspended Solids and Nutrient Concentrations. Phycology 2024, 4, 37-52. https://doi.org/10.3390/phycology4010002
Carvalho A, Braga Í, Chaar F, Cardozo AP, Monserrat JM, Ramírez JRB, Wasielesky W Jr., Poersch LH. Production of the Macroalgae Ulva lactuca Integrated with the Shrimp Penaeus vannamei in a Biofloc System: Effect of Total Suspended Solids and Nutrient Concentrations. Phycology. 2024; 4(1):37-52. https://doi.org/10.3390/phycology4010002
Chicago/Turabian StyleCarvalho, Andrezza, Ítalo Braga, Florencia Chaar, Alessandro Pereira Cardozo, José María Monserrat, Juan Rafael Buitrago Ramírez, Wilson Wasielesky, Jr., and Luís H. Poersch. 2024. "Production of the Macroalgae Ulva lactuca Integrated with the Shrimp Penaeus vannamei in a Biofloc System: Effect of Total Suspended Solids and Nutrient Concentrations" Phycology 4, no. 1: 37-52. https://doi.org/10.3390/phycology4010002









