Quantitative Analysis of Formate Production from Plasma-Assisted Electrochemical Reduction of CO2 on Pd-Based Catalysts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
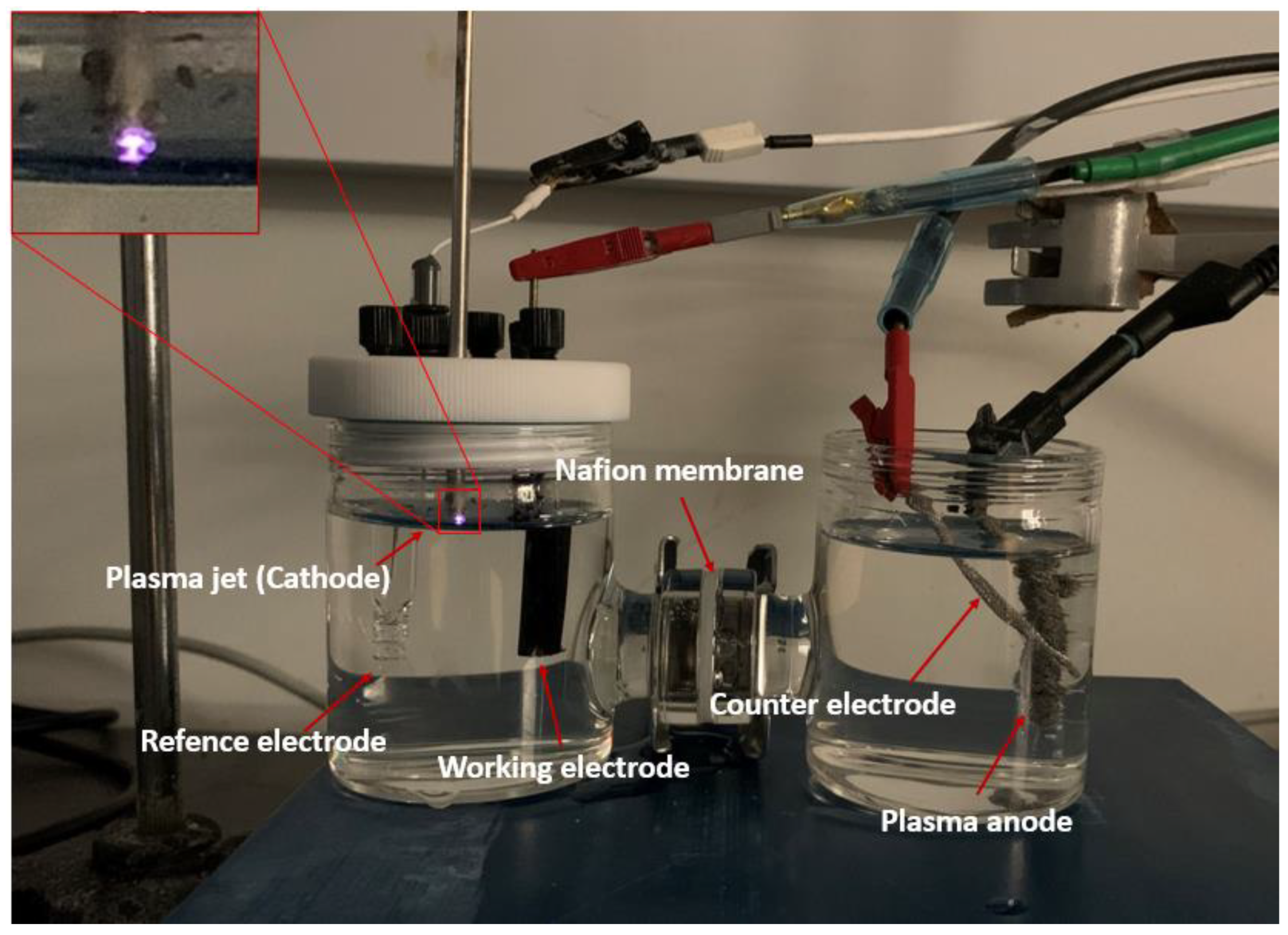
2.2. Preparation of Pd/C Electrodes
2.3. Electrochemical Measurements and Product Analysis
2.4. Quantitative Analysis of Formate
2.5. Faradic Efficiency and Production Rate Calculation
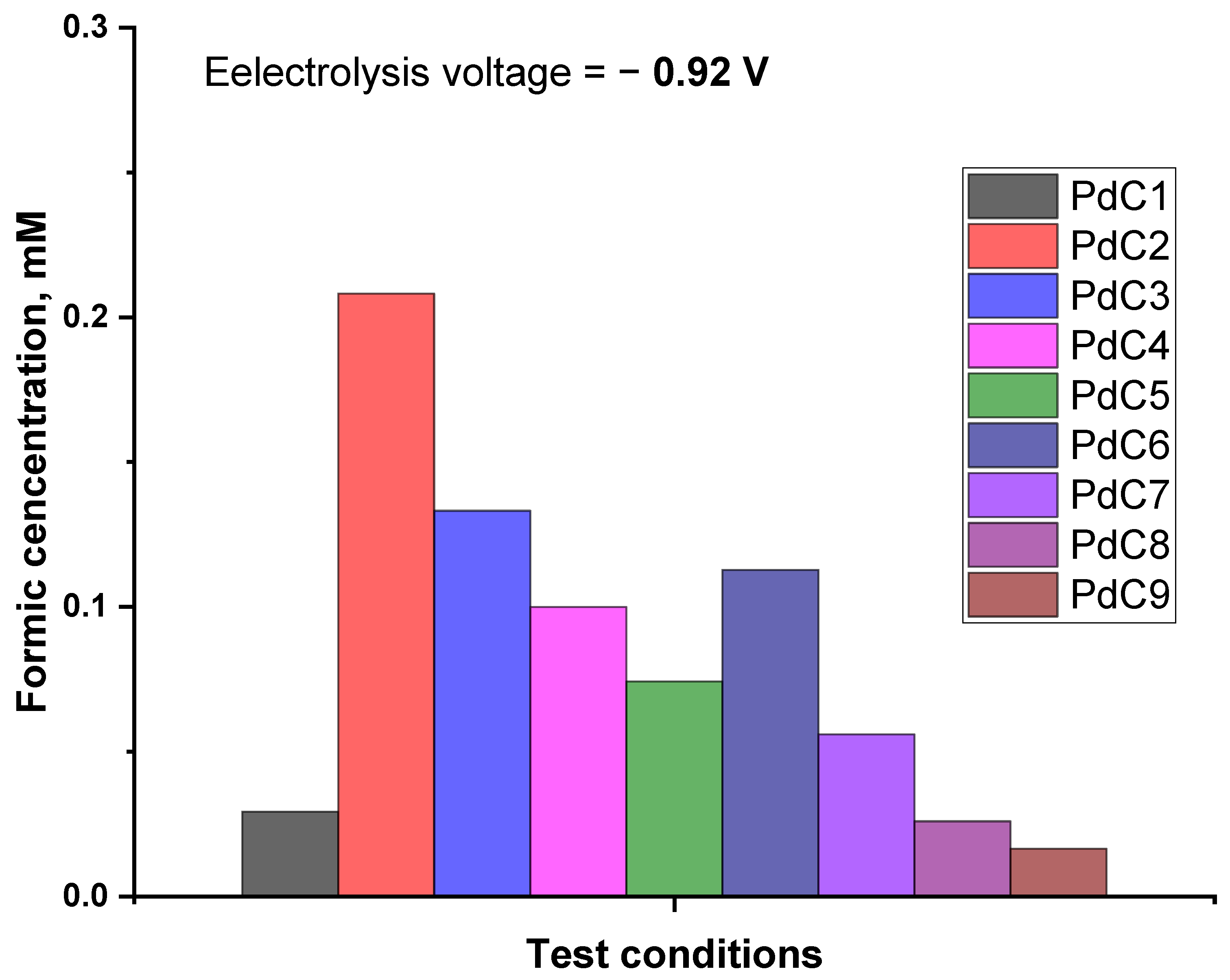
3. Results and Discussions
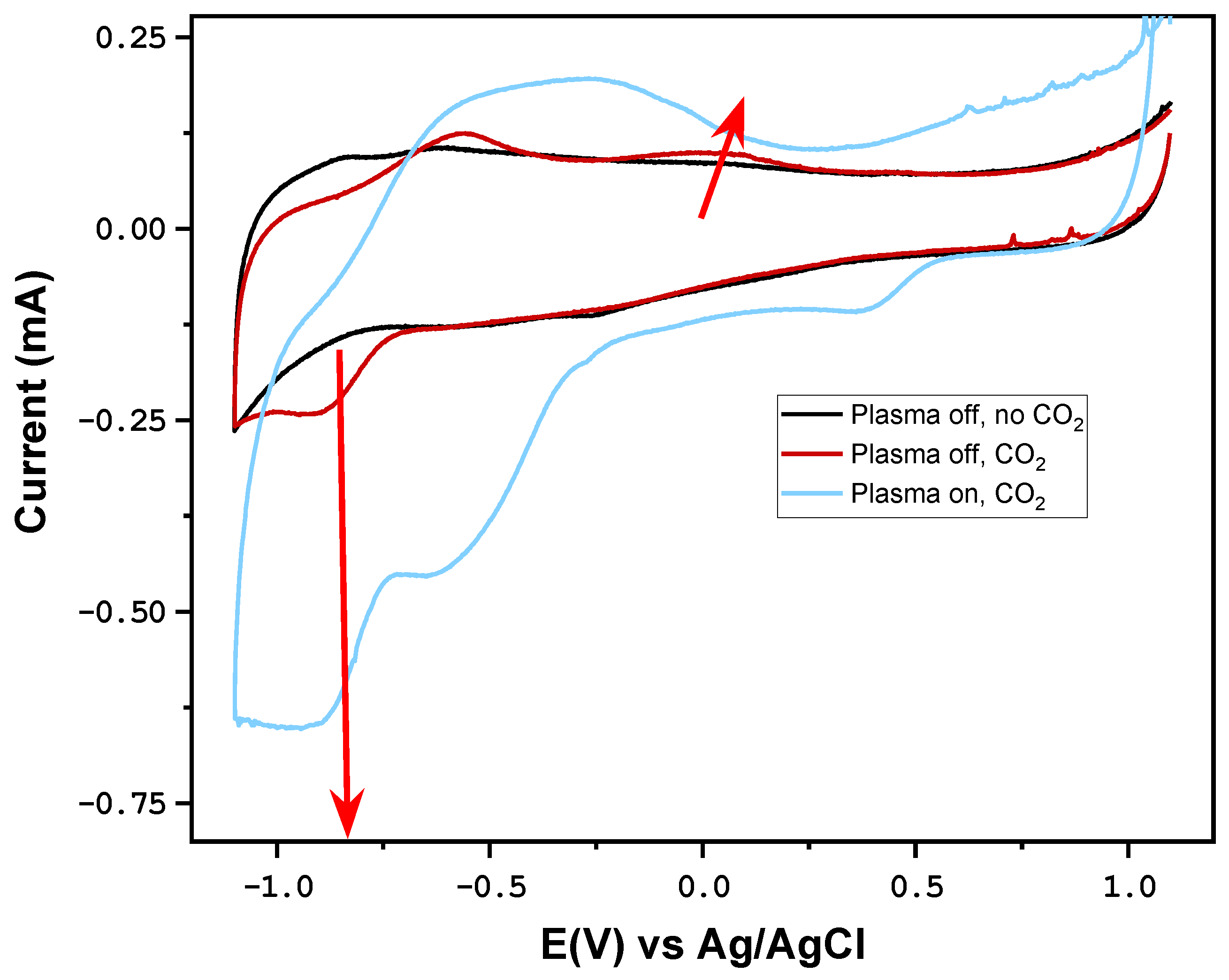
3.1. Impact of Plasma on CO2RR
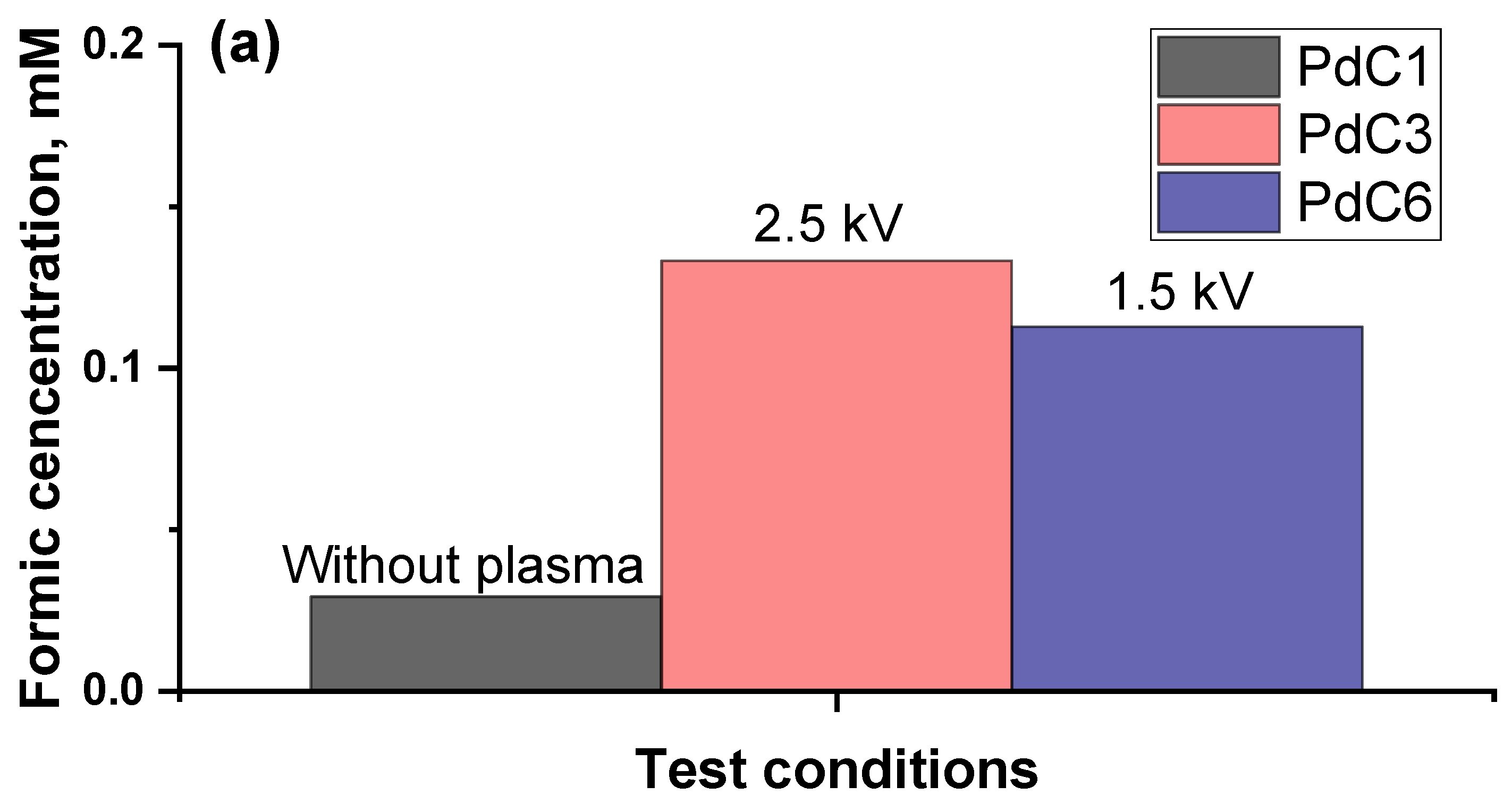
3.2. Impact of Plasma Discharging Voltage
3.3. Impact of Plasma Carrier Gas
3.4. Impact of Plasma Discharging Mode
3.5. Impact of Plasma Type
3.6. Impact of Switching Plasma Polarity
3.7. Faradic Efficiency and Production Rate
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, D.; Xu, X.; Qin, Y.; Jiang, S.P.; Shao, Z. Rational Design of Ag-Based Catalysts for the Electrochemical CO2 Reduction to CO: A Review. ChemSusChem 2020, 13, 39–58. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Zhang, X.; Chen, L.; Deng, Z.; Han, S.; Chen, Y.; Zhong, J.; Sun, H.; Lian, Y.; Yang, B. Geometric Modulation of Local CO Flux in Ag@Cu2O Nanoreactors for Steering the CO2RR Pathway toward High-Efficacy Methane Production. Adv. Mater. 2021, 33, 2101741. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.L.; Zhang, M.D.; Si, D.H.; Mao, M.J.; Hou, Y.; Huang, Y.B.; Cao, R. Highly Selective Tandem Electroreduction of CO2 to Ethylene over Atomically Isolated Nickel–Nitrogen Site/Copper Nanoparticle Catalysts. Angew. Chem. 2021, 133, 25689–25696. [Google Scholar] [CrossRef]
- Leverick, G.; Bernhardt, E.M.; Ismail, A.I.; Law, J.H.; Arifutzzaman, A.; Aroua, M.K.; Gallant, B.M. Uncovering the Active Species in Amine-Mediated CO2 Reduction to CO on Ag. ACS Catal. 2023, 13, 12322–12337. [Google Scholar] [CrossRef]
- Fernández-Caso, K.; Díaz-Sainz, G.; Alvarez-Guerra, M.; Irabien, A. Electroreduction of CO2: Advances in the Continuous Production of Formic Acid and Formate. ACS Energy Lett. 2023, 8, 1992–2024. [Google Scholar] [CrossRef]
- Yang, H.; Wu, Y.; Li, G.; Lin, Q.; Hu, Q.; Zhang, Q.; Liu, J.; He, C. Scalable production of efficient single-atom copper decorated carbon membranes for CO2 electroreduction to methanol. J. Am. Chem. Soc. 2019, 141, 12717–12723. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Jin, H.; Li, H.; Wang, H.; Duan, J.; Jiao, Y.; Qiao, S.-Z. Acidic CO2-to-HCOOH electrolysis with industrial-level current on phase engineered tin sulfide. Nat. Commun. 2023, 14, 2843. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Zhou, H.; Cai, F.; Wang, J.; Wang, G.; Bao, X. Pd-containing nanostructures for electrochemical CO2 reduction reaction. ACS Catal. 2018, 8, 1510–1519. [Google Scholar] [CrossRef]
- Hori, Y.; Wakebe, H.; Tsukamoto, T.; Koga, O. Electrocatalytic process of CO selectivity in electrochemical reduction of CO2 at metal electrodes in aqueous media. Electrochim. Acta 1994, 39, 1833–1839. [Google Scholar] [CrossRef]
- Zhao, C.; Yin, Z.; Wang, J. Efficient Electrochemical Conversion of CO2 to HCOOH Using Pd-polyaniline/CNT Nanohybrids Prepared in Situ. ChemElectroChem 2015, 2, 1974–1982. [Google Scholar] [CrossRef]
- Min, X.; Kanan, M.W. Pd-catalyzed electrohydrogenation of carbon dioxide to formate: High mass activity at low overpotential and identification of the deactivation pathway. J. Am. Chem. Soc. 2015, 137, 4701–4708. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Zhou, H.; Cai, F.; Wang, D.; Hu, Y.; Jiang, B.; Cai, W.-B.; Chen, X.; Si, R.; Yang, F. Switchable CO2 electroreduction via engineering active phases of Pd nanoparticles. J. Nano Res. 2017, 10, 2181–2191. [Google Scholar] [CrossRef]
- Hu, J.; Lin, G.; Liu, F. Plasma-Assisted Catalyst Active Phase Regeneration in Formate-Selective Carbon Dioxide Electroreduction. In Proceedings of the Electrochemical Society Meeting Abstracts 237, Montreal, QC, Canada, 10–14 May 2020; p. 1116. [Google Scholar]
- Hu, J. Non-Thermal Plasma Assisted Pd Catalyst Deactivation in Electrochemical CO2 Reduction Reaction. Ph.D. Dissertation, Mechanical Engineering, University of Massachusetts Lowell, Lowell, MA, USA, 2022. [Google Scholar]
- Locke, B.R.; Shih, K.-Y. Review of the methods to form hydrogen peroxide in electrical discharge plasma with liquid water. Plasma Sources Sci. Technol. 2011, 20, 034006. [Google Scholar] [CrossRef]
- Bruggeman, P.; Kushner, M.J.; Locke, B.R.; Gardeniers, J.G.; Graham, W.; Graves, D.B.; Hofman-Caris, R.; Maric, D.; Reid, J.P.; Ceriani, E. Plasma–liquid interactions: A review and roadmap. Plasma Sources Sci. Technol. 2016, 25, 053002. [Google Scholar] [CrossRef]
- Tian, W.; Kushner, M.J. Atmospheric pressure dielectric barrier discharges interacting with liquid covered tissue. J. Phys. D Appl. Phys. 2014, 47, 165201. [Google Scholar] [CrossRef]
- Li, J.; Wu, F.; Nie, L.; Lu, X. The effect of phase shift on the plasma driven by an AC voltage and a pulsed DC voltage. IEEE Trans. Plasma Sci. 2019, 47, 4818–4826. [Google Scholar] [CrossRef]
- Pang, Y.; Hammer, T.; Müller, D.; Karl, J. Investigation on the influence of non-thermal plasma on reaction degree of wood gasification in a drop tube reactor. Fuel 2019, 253, 95–105. [Google Scholar] [CrossRef]
- Pędziwiatr, P. Decomposition of hydrogen peroxide-kinetics and review of chosen catalysts. Acta Innov. 2018, 45–52. [Google Scholar] [CrossRef]
- Bahuguna, A.; Sasson, Y. Formate-Bicarbonate Cycle as a Vehicle for Hydrogen and Energy Storage. ChemSusChem 2021, 14, 1258–1283. [Google Scholar] [CrossRef]
- Rumbach, P.; Xu, R.; Go, D.B. Electrochemical production of oxalate and formate from CO2 by solvated electrons produced using an atmospheric-pressure plasma. J. Electrochem. Soc. 2016, 163, F1157. [Google Scholar] [CrossRef]
- Lee, H.; Lee, C.-H.; Choi, J.-W.; Song, H.K. The effect of the electric pulse polarity on CO2 reforming of CH4 using dielectric barrier discharge. Energy Fuels 2007, 21, 23–29. [Google Scholar] [CrossRef]
- Scialdone, O.; Galia, A.; Nero, G.L.; Proietto, F.; Sabatino, S.; Schiavo, B. Electrochemical reduction of carbon dioxide to formic acid at a tin cathode in divided and undivided cells: Effect of carbon dioxide pressure and other operating parameters. Electrochim. Acta 2016, 199, 332–341. [Google Scholar] [CrossRef]
- Natsui, K.; Iwakawa, H.; Ikemiya, N.; Nakata, K.; Einaga, Y. Stable and Highly Efficient Electrochemical Production of Formic Acid from Carbon Dioxide Using Diamond Electrodes. Angew. Chem. Int. Ed. 2018, 57, 2639–2643. [Google Scholar] [CrossRef] [PubMed]
- Kas, R.; Kortlever, R.; Yılmaz, H.; Koper, M.T.M.; Mul, G. Manipulating the Hydrocarbon Selectivity of Copper Nanoparticles in CO2 Electroreduction by Process Conditions. ChemElectroChem 2015, 2, 354–358. [Google Scholar] [CrossRef]

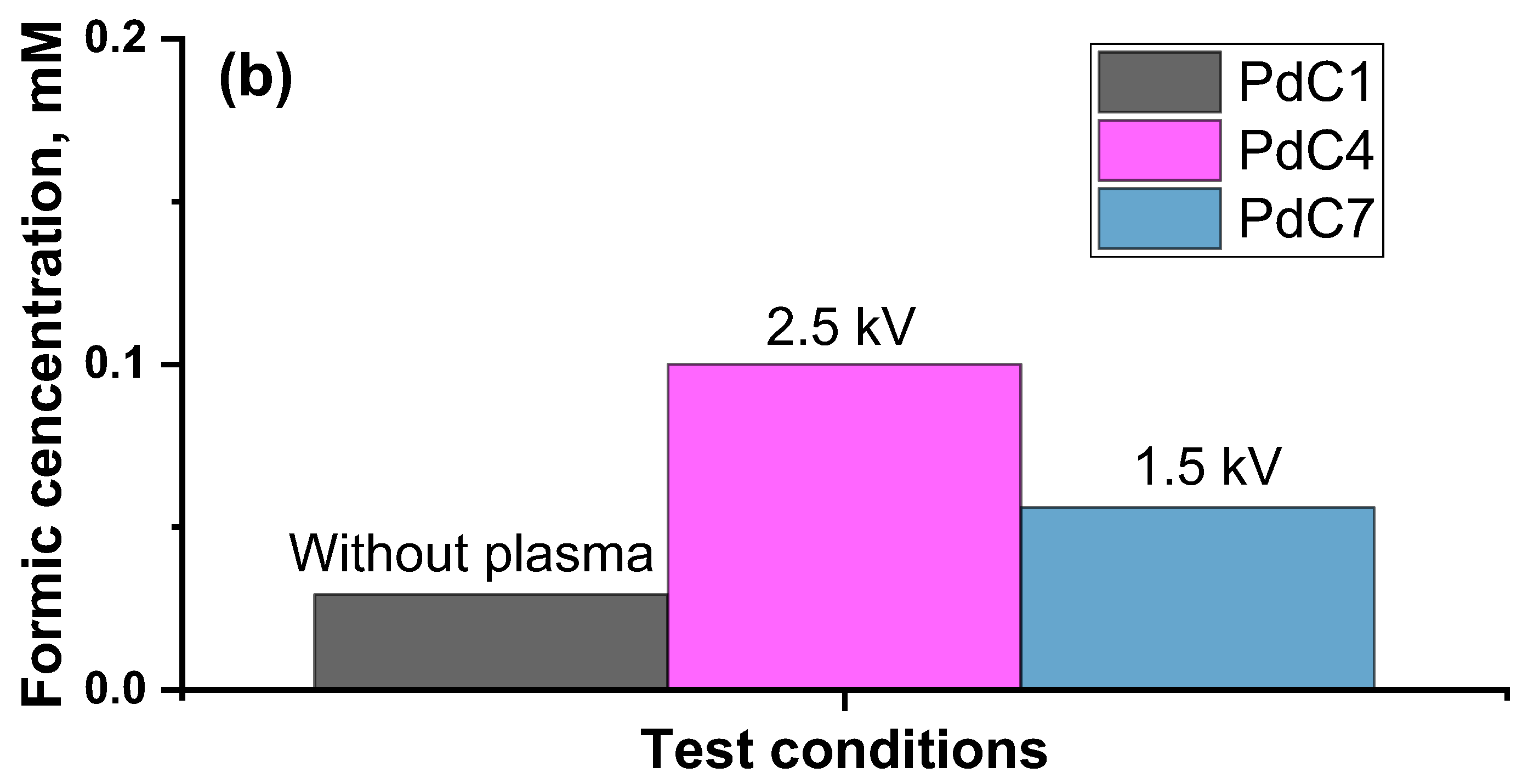


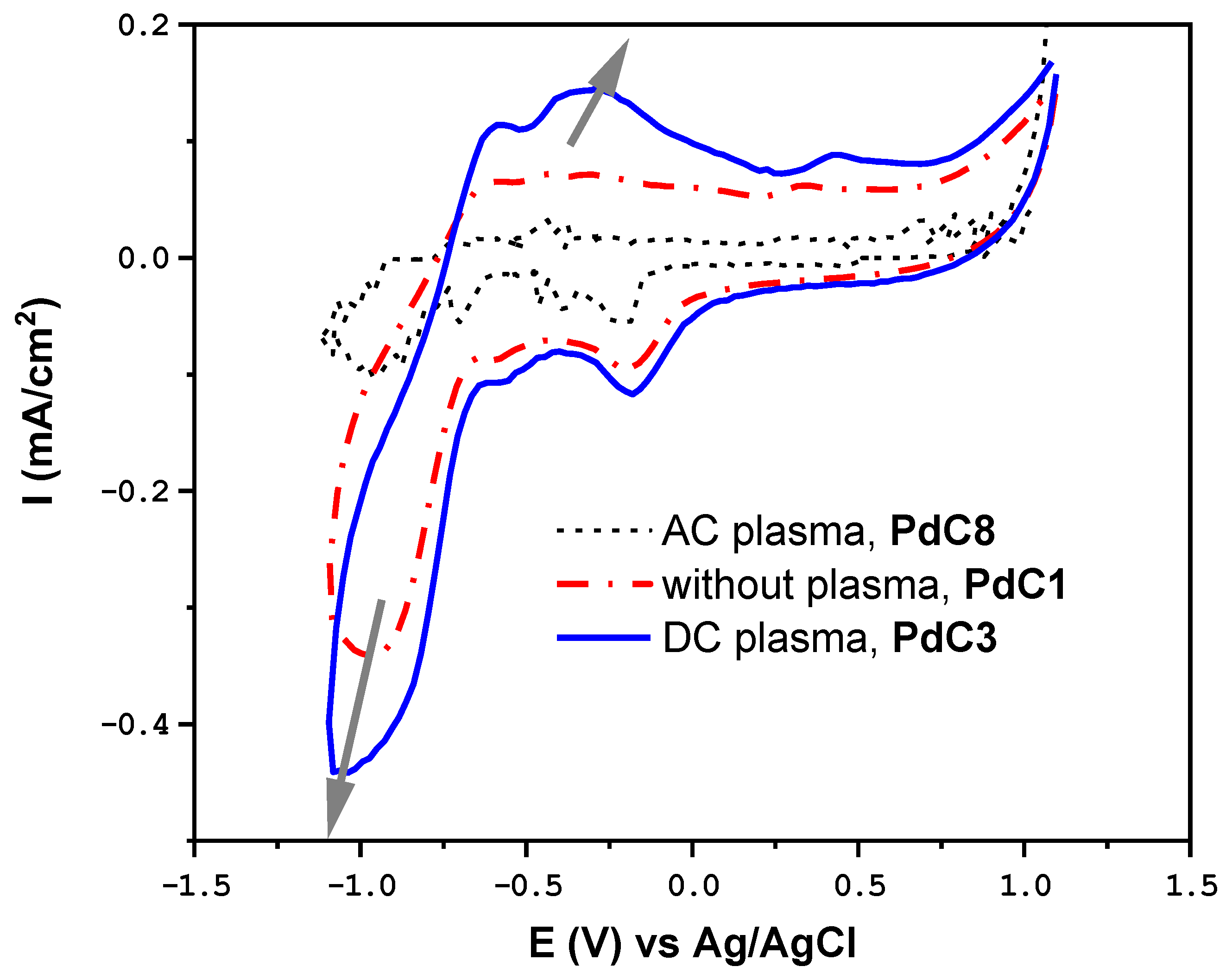

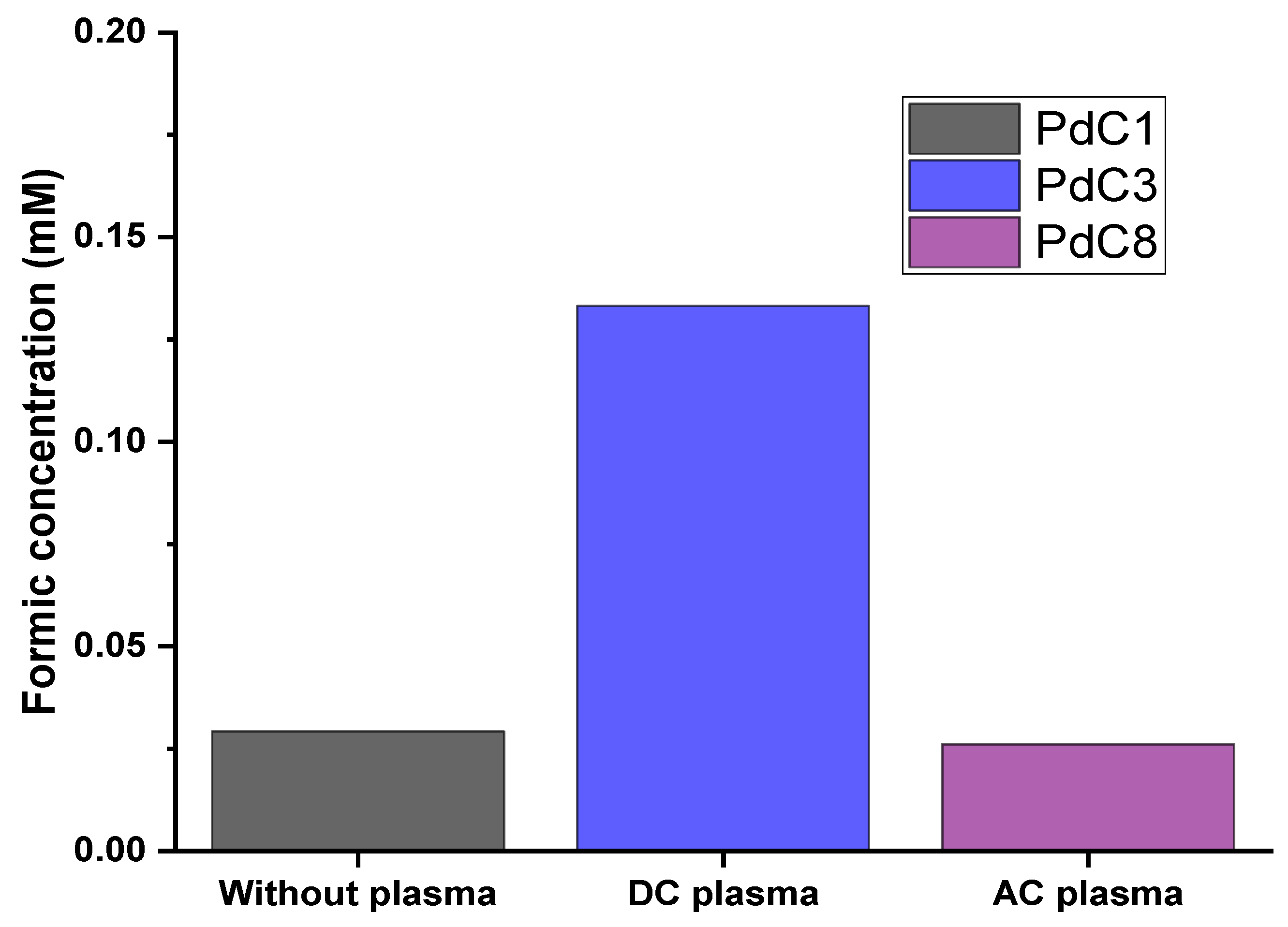


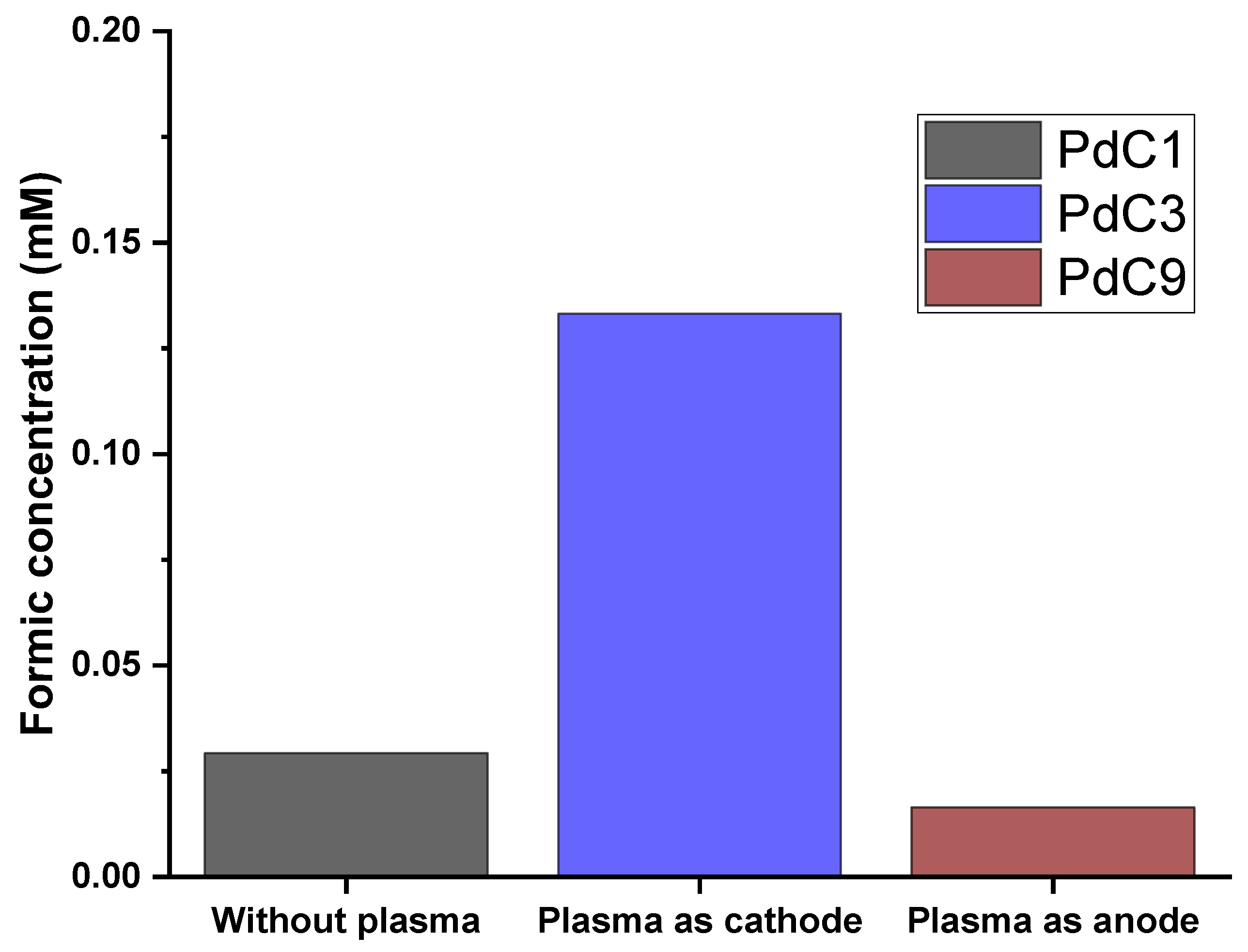
| Plasma Off | Plasma Discharging Voltage | Plasma Operating Gas | Plasma Discharging Mode | Electrolysis Voltage | ||||
|---|---|---|---|---|---|---|---|---|
| 1.5 kV | 2.5 kV | Ar | CO2 | SIM a | SEP b | −0.92 V | ||
| PdC1 | × | × | ||||||
| PdC2 | × | × | × | × | ||||
| PdC3 | × | × | × | × | ||||
| PdC4 | × | × | × | × | ||||
| PdC5 | × | × | × | × | ||||
| PdC6 | × | × | × | × | ||||
| PdC7 | × | × | × | × | ||||
| Without Plasma | AC Plasma | DC Plasma | |
|---|---|---|---|
| PdC1 | PdC8 | PdC3 | |
| Catalyst | Pd/C | Pd/C | Pd/C |
| Plasma discharging voltage | N/A | 2.5 kV | 2.5 kV |
| Plasma carrier gas | N/A | Ar | Ar |
| Plasma discharging mode | N/A | Simultaneously | Simultaneously |
| Anode | N/A | Plasma jet | Plasma jet |
| Cathode | N/A | Pt mesh | Pt mesh |
| Current type | N/A | AC | DC |
| Without Plasma | Plasma as Anode | Plasma as Cathode | |
|---|---|---|---|
| PdC1 | PdC9 | PdC3 | |
| Catalyst | Pd/C | Pd/C | Pd/C |
| Plasma discharging voltage | N/A | 2.5 kV | 2.5 kV |
| Plasma carrier gas | N/A | Ar | Ar |
| Plasma discharging mode | N/A | Simultaneously | Simultaneously |
| Anode | N/A | Plasma jet | Pt mesh |
| Cathode | N/A | Pt mesh | Plasma jet |
| Current type | N/A | DC | DC |
| Experiment Conditions | FEformate | Production Rate (mole/h) |
|---|---|---|
| PdC1 | 2.87% | 7.034 × 10−9 |
| PdC2 | 4.87% | 1.697 × 10−8 |
| PdC3 | 8.10% | 2.975 × 10−8 |
| PdC4 | 23.52% | 3.925 × 10−8 |
| PdC5 | 17.60% | 5.571 × 10−8 |
| PdC6 | 11.21% | 6.389 × 10−8 |
| PdC7 | 8.60% | 1.443 × 10−8 |
| PdC8 | 7.88% | 1.300 × 10−8 |
| PdC9 | 0.016% | 8.195 × 10−9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Liu, F. Quantitative Analysis of Formate Production from Plasma-Assisted Electrochemical Reduction of CO2 on Pd-Based Catalysts. AppliedChem 2024, 4, 174-191. https://doi.org/10.3390/appliedchem4020012
Hu J, Liu F. Quantitative Analysis of Formate Production from Plasma-Assisted Electrochemical Reduction of CO2 on Pd-Based Catalysts. AppliedChem. 2024; 4(2):174-191. https://doi.org/10.3390/appliedchem4020012
Chicago/Turabian StyleHu, Jie, and Fuqiang Liu. 2024. "Quantitative Analysis of Formate Production from Plasma-Assisted Electrochemical Reduction of CO2 on Pd-Based Catalysts" AppliedChem 4, no. 2: 174-191. https://doi.org/10.3390/appliedchem4020012





