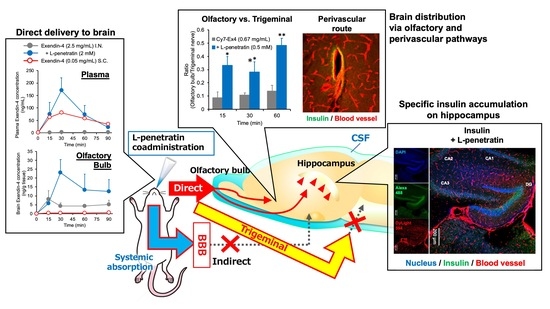
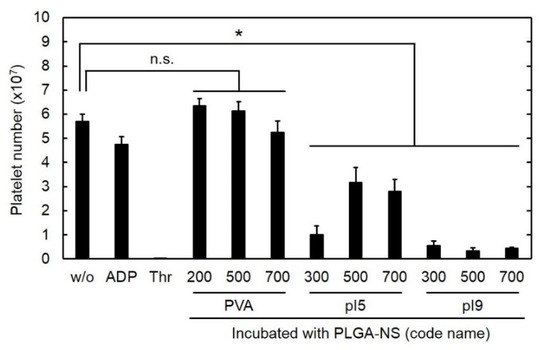
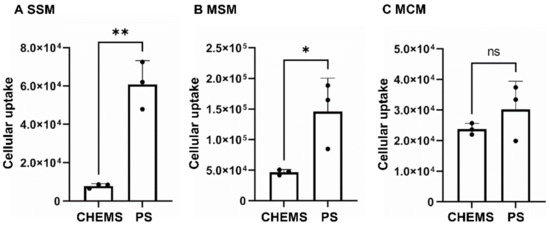
Advanced Pharmaceutical Science and Technology in Japan
A topical collection in Pharmaceutics (ISSN 1999-4923). This collection belongs to the section "Drug Delivery and Controlled Release".
Viewed by 30733Editors
Interests: drug delivery system; lipid nanoparticle; lipid synthesis; microfluidics; short interfering RNA; mRNA; genome editing
Topical Collection Information
Dear Colleagues,
Japanese researchers are dedicated to promoting advancements in pharmaceutical sciences and technology. Hence, Japan continues to have significant impact on the research field. In addition, recent notable progress on pharmaceutical applications of nanomaterials and nanotechnology have been made, in particular, the drug delivery of macromolecules, including RNAs (e.g., siRNAs, miRNAs, antisense oligonucleotides, and mRNAs), DNAs, proteins, and peptides as well as small molecule drugs. This topical collection will highlight the research within pharmaceutical sciences and technology that is currently ongoing in Japan, both within academic and industrial institutions, especially in the following pioneering topics: novel formulation design, drug delivery, polymer science, material science, pharmacokinetics, drug metabolism, drug transport, manufacturing science, and biotechnology. In doing so, we wish to share and extend upon the pharmaceutical expertise and advancements in pharmaceutical sciences in Japan.
This will be achieved by presenting a topical collection of research papers and review articles covering the recent progress and achievements in high-end pharmaceutical science.
Dr. Yusuke Sato
Prof. Dr. Yoshinobu Takakura
Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Pharmaceutics is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2900 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- formulation design
- drug delivery
- polymer science
- materials science
- pharmacokinetics
- drug metabolism and drug transport
- manufacturing science
- biotechnology
Related Special Issue
- Advanced Pharmaceutical Science and Technology in Pharmaceutics (3 articles)