Advanced Pharmaceutical Science and Technology in Portugal
Share This Topical Collection
Editors
 Dr. João Sousa
Dr. João Sousa
 Dr. João Sousa
Dr. João Sousa
E-Mail
Website
Guest Editor
1. Faculty of Pharmacy, University of Coimbra, 3000-548 Coimbra, Portugal
2. Coimbra Chemistry Center, Department of Chemistry, University of Coimbra, 3004-535 Coimbra, Portugal
Interests: oral sustained release formulations; skin delivery; nanotechnology; aseptic technology; regulatory affairs
 Dr. Carla Vitorino
Dr. Carla Vitorino
 Dr. Carla Vitorino
Dr. Carla Vitorino
E-Mail
Website
Guest Editor
1. Faculty of Pharmacy, University of Coimbra, 3000-548 Coimbra, Portugal
2. Coimbra Chemistry Centre, Institute of Molecular Sciences (IMS), Faculty of Sciences and Technology, University of Coimbra, 3004-535 Coimbra, Portugal
Interests: nanotechnology; drug permeation enhancement; transdermal and oral drug delivery; brain targeting
Special Issues, Collections and Topics in MDPI journals
 Dr. Alberto A. C. C. Pais
Dr. Alberto A. C. C. Pais
 Dr. Alberto A. C. C. Pais
Dr. Alberto A. C. C. Pais
E-Mail
Guest Editor
Coimbra Chemistry Center, Department of Chemistry, University of Coimbra, 3004-535 Coimbra, Portugal
Interests: nanoparticles; nucleic acids; pharmaceutical technology; supramolecular chemistry; molecular simulation; chemometrics and data science
Topical Collection Information
Dear Colleagues,
Portugal has significantly contributed to the scientific advance in the field of Pharmaceutical Sciences, increasingly assuming a pioneer role in tackling biomedical challenges, including those addressed by COVID-19 pandemic. This topical collection aims at emphasizing the pharmaceutical sciences research currently ongoing in Portugal, both within academic and industrial institutions, in areas including but not limited to drug discovery and development, drug delivery, drug repurposing, pharmacokinetics, drug metabolism and drug transport, pharmacogenomics and personalized medicine, pharmacy practice research, pharmacoepidemiology, pharmaceutical natural products, pharmacometrics, and artificial intelligence.
Dr. João Sousa
Dr. Carla Vitorino
Dr. Alberto A. C. C. Pais
Guest Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Pharmaceutics is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript.
The Article Processing Charge (APC) for publication in this open access journal is 2900 CHF (Swiss Francs).
Submitted papers should be well formatted and use good English. Authors may use MDPI's
English editing service prior to publication or during author revisions.
Related Special Issue
Published Papers (3 papers)
Open AccessReview
A Regulatory Perspective on Biosimilar Medicines
by
Marta Agostinho Cordeiro, Carla Vitorino, Carlos Sinogas and João J. Sousa
Cited by 2 | Viewed by 2308
Abstract
By definition, biosimilar medicinal products are biological medicinal products that are similar to other biological medicinal products that are already on the market—the reference medicinal products. Access to biosimilar medicines is a current reality. However, to achieve this goal, it is extremely important
[...] Read more.
By definition, biosimilar medicinal products are biological medicinal products that are similar to other biological medicinal products that are already on the market—the reference medicinal products. Access to biosimilar medicines is a current reality. However, to achieve this goal, it is extremely important to consistently and scientifically substantiate the regulatory requirements necessary for biosimilar medicines when accessing the market. Based on an analysis of the raw materials and the type of methods used in the manufacturing processes of biological medicines, it is known that this tends to be more complex for the quality of the finished product than the manufacture of molecules obtained through a chemical process. It is then relevant to highlight the main differences between both products: biological medicines manufactured using biotechnology and the current generics containing active pharmaceutical ingredients (APIs) obtained from synthetic processes. Once arriving at the approval process of these medicinal products, it is imperative to analyse the guidance documents and the regulatory framework that create the rules that allow these biosimilar medicinal products to come to the market. The present review aimed at documenting comparatively the specific provisions of European legislation, through the European Medicines Agency (EMA), as well as the legislation of the United States of America, through the Food and Drug Administration (FDA). This was then translated into a critical appraisal of what concerns the specific criteria that determine the favourable evaluation of a biosimilar when an application for marketing authorisation is submitted to different regulatory agencies. The gathered evidence suggests that the key to the success of biosimilar medicines lies in a more rigorous and universal regulation as well as a greater knowledge, acceptance, and awareness of health professionals to enable more patients to be treated with biological strategies at an earlier stage of the disease and with more affordable medicines, ensuring always the safety and efficacy of those medicines.
Full article
►▼
Show Figures
Open AccessArticle
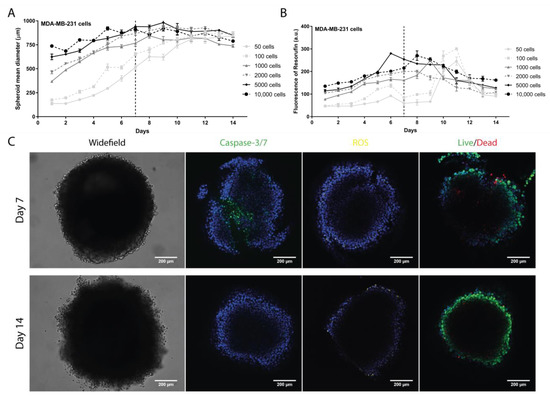
Development of Breast Cancer Spheroids to Evaluate Cytotoxic Response to an Anticancer Peptide
by
Marco Cavaco, Patrícia Fraga, Javier Valle, David Andreu, Miguel A. R. B. Castanho and Vera Neves
Cited by 16 | Viewed by 3078
Abstract
Breast cancer (BC) is the most commonly diagnosed cancer in women and one of the most common causes of cancer-related deaths. Despite intense research efforts, BC treatment still remains challenging. Improved drug development strategies are needed for impactful benefit to patients. Current preclinical
[...] Read more.
Breast cancer (BC) is the most commonly diagnosed cancer in women and one of the most common causes of cancer-related deaths. Despite intense research efforts, BC treatment still remains challenging. Improved drug development strategies are needed for impactful benefit to patients. Current preclinical studies rely mostly on cell-based screenings, using two-dimensional (2D) cell monolayers that do not mimic in vivo tumors properly. Herein, we explored the development and characterization of three-dimensional (3D) models, named spheroids, of the most aggressive BC subtypes (triple-negative breast cancer-TNBC; and human-epidermal growth receptor-2-HER2+), using the liquid overlay technique with several selected cell lines. In these cell line-derived spheroids, we studied cell density, proliferation, ultrastructure, apoptosis, reactive oxygen species (ROS) production, and cell permeabilization (live/dead). The results showed a formation of compact and homogeneous spheroids on day 7 after seeding 2000 cells/well for MDA-MB-231 and 5000 cells/well for BT-20 and BT-474. Next, we compared the efficacy of a model anticancer peptide (ACP) in cell monolayers and spheroids. Overall, the results demonstrated spheroids to be less sensitive to treatment than cell monolayers, revealing the need for more robust models in drug development.
Full article
►▼
Show Figures
Open AccessArticle
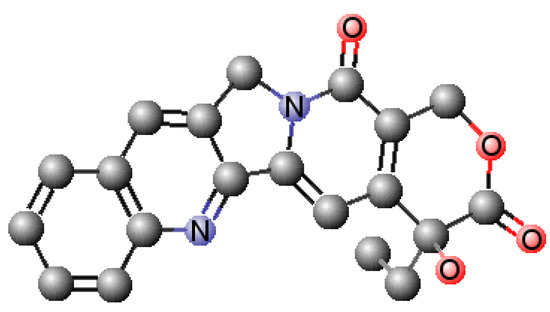
A Biophysical Insight of Camptothecin Biodistribution: Towards a Molecular Understanding of Its Pharmacokinetic Issues
by
Andreia Almeida, Eduarda Fernandes, Bruno Sarmento and Marlene Lúcio
Cited by 8 | Viewed by 2945
Abstract
Camptothecin (CPT) is a potent anticancer drug, and its putative oral administration is envisioned although difficult due to physiological barriers that must be overcome. A comprehensive biophysical analysis of CPT interaction with biointerface models can be used to predict some pharmacokinetic issues after
[...] Read more.
Camptothecin (CPT) is a potent anticancer drug, and its putative oral administration is envisioned although difficult due to physiological barriers that must be overcome. A comprehensive biophysical analysis of CPT interaction with biointerface models can be used to predict some pharmacokinetic issues after oral administration of this or other drugs. To that end, different models were used to mimic the phospholipid composition of normal, cancer, and blood–brain barrier endothelial cell membranes. The logD values obtained indicate that the drug is well distributed across membranes. CPT-membrane interaction studies also confirm the drug’s location at the membrane cooperative and interfacial regions. The drug can also permeate membranes at more ordered phases by altering phospholipid packing. The similar logD values obtained in membrane models mimicking cancer or normal cells imply that CPT has limited selectivity to its target. Furthermore, CPT binds strongly to serum albumin, leaving only 8.05% of free drug available to be distributed to the tissues. The strong interaction with plasma proteins, allied to the large distribution (VD
SS = 5.75 ± 0.932 L·Kg
−1) and tendency to bioaccumulate in off-target tissues, were predicted to be pharmacokinetic issues of CPT, implying the need to develop drug delivery systems to improve its biodistribution.
Full article
►▼
Show Figures