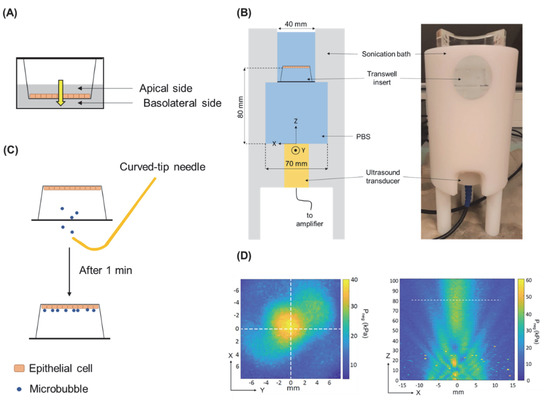
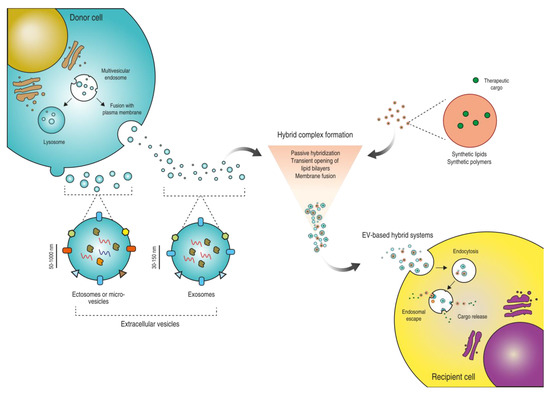
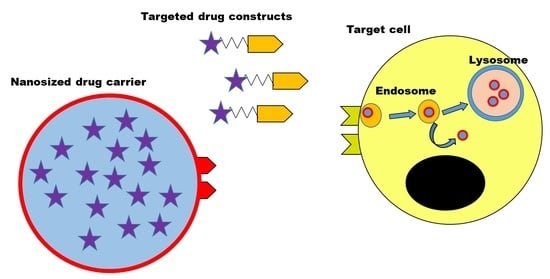
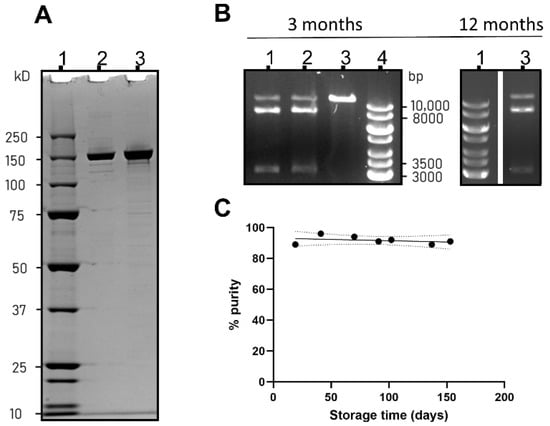
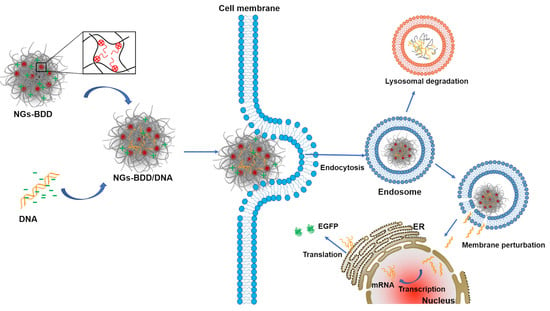
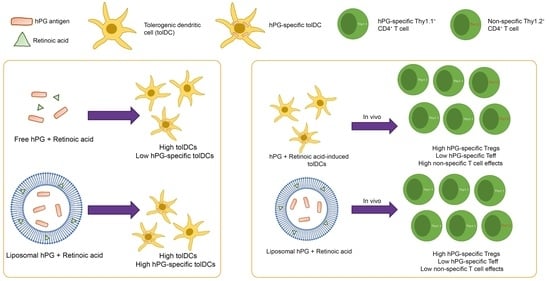
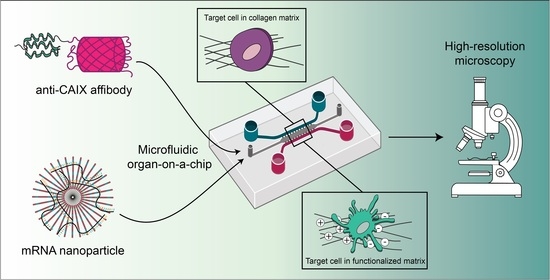
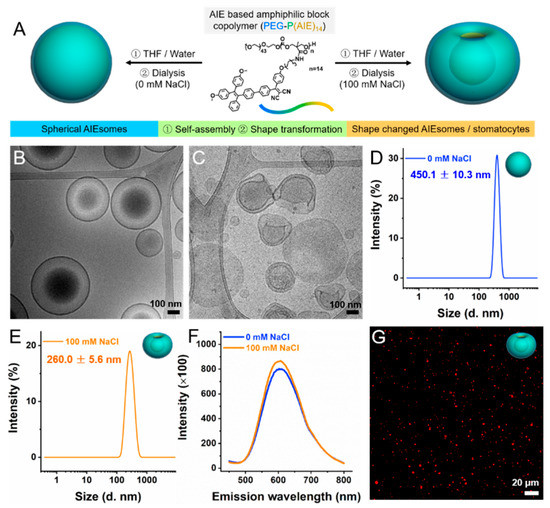
Drug Delivery in The Netherlands (Closed)
A topical collection in Pharmaceutics (ISSN 1999-4923). This collection belongs to the section "Drug Delivery and Controlled Release".
Viewed by 67594Editors
Interests: drug delivery; nanomedicines; gene therapy; CRISPR-Cas; vaccines; biomimetics
Interests: drug delivery; nonviral gene delivery; nanoparticles; nanocarrier–cell interactions; endocytosis; endosomal escape; blood–brain barrier; transcytosis
Topical Collection Information
Dear Colleagues,
Many drug substances, particularly biomacromolecular drugs, rely on the development of innovative drug delivery systems to enable the safe and effective use of these substances as medicines for human use. Drug delivery research spans from basic science to clinical trials, i.e., it is a truly multidisciplinary effort which builds on the knowledge innovation of academia and industry in concert with (university) hospitals.
Dutch universities have a longstanding international reputation in biomaterials science, including the testing of materials for their physicochemical properties, biomechanical behavior, in vitro/in vivo biocompatibility, and drug delivery applications. Moreover, many of the currently existing Dutch biotech companies evolved from universities through commercialization of know-how and technological advancement. This Special Issue provides exciting insight into the ongoing innovation in drug delivery research and development in the Netherlands in both academia and industry.
About the Guest Editors:
Enrico Mastrobattista and Inge Zuhorn both carried out their PhDs on Liposomal Drug Delivery, and in that time, jointly followed courses at the Groningen Utrecht Institute for Drug Exploration (GUIDE), a joint effort of the University of Utrecht and University of Groningen to strengthen Drug Delivery research. Later in their careers, they collaborated within the Top Institute Pharma project ‘Nanoscience as a tool to improve the bioavailability and blood−brain barrier penetration of CNS drugs’. For the Special Issue ‘Drug Delivery in the Netherlands’, Mastrobattista and Zuhorn have invited research groups in the Netherlands that are key players in the Drug Delivery field to contribute.
Prof. Dr. Enrico Mastrobattista
Prof. Dr. Inge S. Zuhorn
Guest Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Pharmaceutics is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2900 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- drug delivery
- gene delivery
- nanoparticles/nanocarriers
- extracellular vesicles
- targeting
- nanomedicine
- clinical translation
- theranostics
- imaging
- diagnosis
- treatment
- cancer
- tumor microenvironment
- personalized medicine
- immune response
- in vitro (cell) models
- microfluidics
Related Special Issue
- Advanced Pharmaceutical Science and Technology in Pharmaceutics (3 articles)