Noninvasive and Convenient Screening of Metabolic Syndrome Using the Controlled Attenuation Parameter Technology: An Evaluation Based on Self-Paid Health Examination Participants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Setting
2.3. Population and Data
2.4. Outcome
2.5. Statistical Analysis
2.6. Ethics
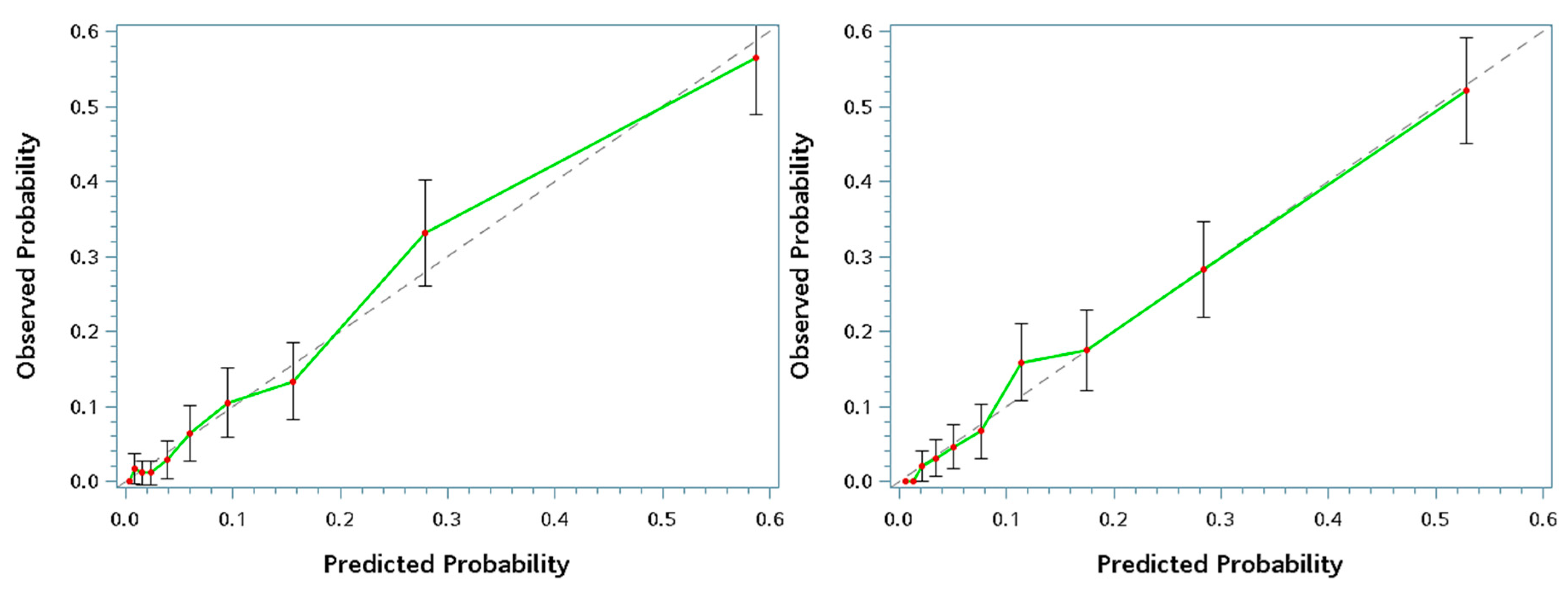
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kassi, E.; Pervanidou, P.; Kaltsas, G.; Chrousos, G. Metabolic syndrome: definitions and controversies. BMC medicine 2011, 9, 48. [Google Scholar] [CrossRef]
- Ding, C.; Yang, Z.; Wang, S.; Sun, F.; Zhan, S. The associations of metabolic syndrome with incident hypertension, type 2 diabetes mellitus and chronic kidney disease: A cohort study. Endocrine 2018, 60, 282–291. [Google Scholar] [CrossRef]
- Grundy, S.M. Metabolic Syndrome: A Multiplex Cardiovascular Risk Factor. J. Clin. Endocrinol. Metab. 2007, 92, 399–404. [Google Scholar] [CrossRef]
- Lorenzo, C.; Okoloise, M.; Williams, K.; Stern, M.P.; Haffner, S.M. The metabolic syndrome as predictor of type 2 diabetes: The San Antonio heart study. Diabetes Care 2003, 26, 3153–3159. [Google Scholar] [CrossRef]
- Yamaoka, K.; Tango, T. Effects of lifestyle modification on metabolic syndrome: A systematic review and meta-analysis. BMC Med. 2012, 10, 138. [Google Scholar] [CrossRef]
- Gurusamy, J.; Gandhi, S.; Damodharan, D.; Ganesan, V.; Palaniappan, M.; Damodharan, D.; Venkatasubramanian, G.; Marimuthu, P. Exercise, diet and educational interventions for metabolic syndrome in persons with schizophrenia: A systematic review. Asian J. Psychiatry 2018, 36, 73–85. [Google Scholar] [CrossRef]
- Bassi, N.; Karagodin, I.; Wang, S.; Vassallo, P.; Priyanath, A.; Massaro, E.; Stone, N.J. Lifestyle Modification for Metabolic Syndrome: A Systematic Review. Am. J. Med. 2014, 127, 1242-e1. [Google Scholar] [CrossRef]
- Mahamid, M.; Khoury, T.; Amara, H.; Siadi, M.; Mohamed, J.; Mari, A.; Shalabi, R.; Sholy, H.; Nseir, W. Inadequate identification of fatty liver disease, obesity, and metabolic syndrome by family physicians. Diabetes Metab. Syndr. Obes. Targets Ther. 2018, 11, 515–519. [Google Scholar] [CrossRef]
- De Lédinghen, V.; Vergniol, J. Transient elastography (FibroScan). Gastroentérologie Clin. Biologique 2008, 32, 58–67. [Google Scholar] [CrossRef]
- Chang, P.E.; Goh, G.B.-B.; Ngu, J.H.; Tan, H.K.; Tan, C.K. Clinical applications, limitations and future role of transient elastography in the management of liver disease. World J. Gastrointest. Pharmacol. Ther. 2016, 7, 91–106. [Google Scholar] [CrossRef]
- Chivinge, A.; Harris, R.; Guha, N.; Aithal, G.; James, M.; Ryder, S.; Wilkes, E.; Holmes, M. Risk-stratified screening for chronic liver disease using vibration-controlled transient elastography (Fibroscan). Gastrointest. Nurs. 2018, 16, S15–S22. [Google Scholar] [CrossRef]
- Wong, G.L.-H. Transient elastography: Kill two birds with one stone? World J. Hepatol. 2013, 5, 264. [Google Scholar] [CrossRef]
- Recio, E.; Cifuentes, C.; Macías, J.; Mira, J.A.; Parra-Sánchez, M.; Rivero-Juárez, A.; Almeida, C.; Pineda, J.A.; Neukam, K. Interobserver concordance in controlled attenuation parameter measurement, a novel tool for the assessment of hepatic steatosis on the basis of transient elastography. Eur. J. Gastroenterol. Hepatol. 2013, 25, 905–911. [Google Scholar] [CrossRef]
- Fraquelli, M.; Rigamonti, C.; Casazza, G.; Conte, D.; Donato, M.F.; Ronchi, G.; Colombo, M. Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut 2007, 56, 968–973. [Google Scholar] [CrossRef] [Green Version]
- Boursier, J.; Konaté, A.; Gorea, G.; Reaud, S.; Quemener, E.; Oberti, F.; Hubert–Fouchard, I.; Dib, N.; Calès, P. Reproducibility of Liver Stiffness Measurement by Ultrasonographic Elastometry. Clin. Gastroenterol. Hepatol. 2008, 6, 1263–1269. [Google Scholar] [CrossRef]
- Castéra, L.; Vergniol, J.; Foucher, J.; Le Bail, B.; Chanteloup, E.; Haaser, M.; Darriet, M.; Couzigou, P.; De Lédinghen, V. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology 2005, 128, 343–350. [Google Scholar] [CrossRef]
- Li, Y.; Huang, Y.S.; Wang, Z.Z.; Yang, Z.R.; Sun, F.; Zhan, S.Y.; Liu, X.E.; Zhuang, H. Systematic review with meta-analysis: The diagnostic accuracy of transient elastography for the staging of liver fibrosis in patients with chronic hepatitis B. Aliment. Pharmacol. Ther. 2016, 43, 458–469. [Google Scholar] [CrossRef]
- Wong, V.W.; Vergniol, J.; Wong, G.L.; Foucher, J.; Chan, H.L.; Le Bail, B.; Choi, P.C.; Kowo, M.; Chan, A.W.; Merrouche, W.; et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology 2010, 51, 454–462. [Google Scholar] [CrossRef]
- Pavlov, C.S.; Casazza, G.; Nikolova, D.; Tsochatzis, E.; Burroughs, A.K.; Ivashkin, V.T.; Gluud, C. Transient elastography for diagnosis of stages of hepatic fibrosis and cirrhosis in people with alcoholic liver disease. Cochrane Database Syst. Rev. 2015, 1, 1–82. [Google Scholar] [CrossRef]
- Hartl, J.; Denzer, U.; Ehlken, H.; Zenouzi, R.; Peiseler, M.; Sebode, M.; Hübener, S.; Pannicke, N.; Weiler-Normann, C.; Quaas, A.; et al. Transient elastography in autoimmune hepatitis: Timing determines the impact of inflammation and fibrosis. J. Hepatol. 2016, 65, 769–775. [Google Scholar] [CrossRef]
- Roulot, D.; Costes, J.L.; Buyck, J.F.; Warzocha, U.; Gambier, N.; Czernichow, S.; Le Clesiau, H.; Beaugrand, M. Transient elastography as a screening tool for liver fibrosis and cirrhosis in a community-based population aged over 45 years. Gut 2011, 60, 977–984. [Google Scholar] [CrossRef]
- Foucher, J.; Reiller, B.; Jullien, V.; Leal, F.; Di Cesare, E.S.; Merrouche, W.; Delile, J.-M.; De Lédinghen, V. FibroScan used in street-based outreach for drug users is useful for hepatitis C virus screening and management: A prospective study. J. Viral Hepat. 2009, 16, 121–131. [Google Scholar] [CrossRef]
- Lédinghen, V.; Vergniol, J.; Foucher, J.; Merrouche, W.; Bail, B. Non-invasive diagnosis of liver steatosis using controlled attenuation parameter (CAP) and transient elastography. Liver Int. 2012, 32, 911–918. [Google Scholar] [CrossRef]
- Cardoso, A.C.; Beaugrand, M.; De Ledinghen, V.; Douvin, C.; Poupon, R.; Trinchet, J.-C.; Ziol, M.; Bedossa, P.; Marcellin, P. Diagnostic performance of controlled attenuation parameter for predicting steatosis grade in chronic hepatitis B. Ann. Hepatol. 2015, 14, 826–836. [Google Scholar] [CrossRef]
- Sansom, S.E.; Martin, J.; Adeyemi, O.; Burke, K.; Winston, C.; Markham, S.; Go, B.; Huhn, G. Steatosis Rates by Liver Biopsy and Transient Elastography with Controlled Attenuation Parameter in Clinical Experience of Hepatitis C Virus (HCV) and Human Immunodeficiency Virus/HCV Coinfection in a Large US Hepatitis Clinic. In Open Forum Infectious Diseases; Oxford University Press: New York, NY, USA, 2019; Volume 6, p. ofz099. [Google Scholar] [CrossRef]
- Hamaguchi, M.; Kojima, T.; Itoh, Y.; Harano, Y.; Fujii, K.; Nakajima, T.; Kato, T.; Takeda, N.; Okuda, J.; Ida, K.; et al. The Severity of Ultrasonographic Findings in Nonalcoholic Fatty Liver Disease Reflects the Metabolic Syndrome and Visceral Fat Accumulation. Am. J. Gastroenterol. 2007, 102, 2708–2715. [Google Scholar] [CrossRef]
- Baeza, I.; de la Serna, E.; Calvo-Escalona, R.; Merchan-Naranjo, J.; Rodriguez-Latorre, P.; Martinez-Cantarero, M.C.; Andres, P.; Alda, J.A.; Munoz-Samons, D.; Ilzarbe, D.; et al. One-Year Prospective Study of Liver Function Tests in Children and Adolescents on Second-Generation Antipsychotics: Is There a Link with Metabolic Syndrome? J. Child Adolesc. Psychopharmacol. 2018, 28, 463–473. [Google Scholar] [CrossRef]
- Clarke, W.T.; Miranda, J.; Neidich, E.; Hudock, R.; Peters, M.G.; Kelly, E.M. Metabolic syndrome and liver steatosis occur at lower body mass index in US Asian patients with chronic hepatitis B. J. Viral Hepat. 2019, 26, 1164–1169. [Google Scholar] [CrossRef]
- Laouirem, S.; Sannier, A.; Norkowski, E.; Cauchy, F.; Doblas, S.; Rautou, P.E.; Albuquerque, M.; Garteiser, P.; Sognigbé, L.; Raffenne, J.; et al. Endothelial fatty liver binding protein 4: A new targetable mediator in hepatocellular carcinoma related to metabolic syndrome. Oncogene 2019, 38, 3033–3046. [Google Scholar] [CrossRef]
- Chen, C.-H.; Yang, J.-H.; Chiang, C.W.; Hsiung, C.-N.; Wu, P.-E.; Chang, L.-C.; Chu, H.-W.; Chang, J.; Song, I.-W.; Yang, S.-L.; et al. Population structure of Han Chinese in the modern Taiwanese population based on 10,000 participants in the Taiwan Biobank project. Hum. Mol. Genet. 2016, 25, 5321–5331. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.-I.; Guh, J.-Y.; Wu, K.-D.; Chen, Y.-M.; Kuo, M.-C.; Hwang, S.-J.; Chen, T.-H.; Chen, H.-C. Modification of Diet in Renal Disease (MDRD) Study and CKD Epidemiology Collaboration (CKD-EPI) Equations for Taiwanese Adults. PLoS ONE 2014, 9, e99645. [Google Scholar] [CrossRef]
- Huang, P.L. A comprehensive definition for metabolic syndrome. Dis. Model. Mech. 2009, 2, 231–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alberti, K.G.M.M.; Zimmet, P.; Shaw, J. Metabolic syndrome-a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Arsang-Jang, S.; Kelishadi, R.; Motlagh, M.E.; Heshmat, R.; Mansourian, M. Temporal Trend of Non-Invasive Method Capacity for Early Detection of Metabolic Syndrome in Children and Adolescents: A Bayesian Multilevel Analysis of Pseudo-Panel Data. Ann. Nutr. Metab. 2019, 75, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Ceolin, J.; Engroff, P.; Mattiello, R.; Schwanke, C.H.A. Performance of Anthropometric Indicators in the Prediction of Metabolic Syndrome in the Elderly. Metab. Syndr. Relat. Disord. 2019, 17, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Gadelha, A.B.; Myers, J.; Moreira, S.; Dutra, M.T.; Safons, M.P.; Lima, R.M. Comparison of adiposity indices and cut-off values in the prediction of metabolic syndrome in postmenopausal women. Diabetes Metab. Syndr. Clin. Res. Rev. 2016, 10, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Vélez, R.; Correa-Bautista, J.E.; Sanders-Tordecilla, A.; Ojeda-Pardo, M.L.; Cobo-Mejía, E.A.; Castellanos-Vega, R.D.P.; García-Hermoso, A.; González-Jiménez, E.; Schmidt-RioValle, J.; González-Ruíz, K. Percentage of Body Fat and Fat Mass Index as a Screening Tool for Metabolic Syndrome Prediction in Colombian University Students. Nutrients 2017, 9, 1009. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Vélez, R.; Correa-Bautista, J.E.; Carrillo, H.A.; González-Jiménez, E.; Schmidt-RioValle, J.; Correa-Rodríguez, M.; Garcia-Hermoso, A.; González-Ruíz, K. Tri-Ponderal Mass Index vs. Fat Mass/Height3 as a Screening Tool for Metabolic Syndrome Prediction in Colombian Children and Young People. Nutrients 2018, 10, 412. [Google Scholar] [CrossRef]
- Tang, X.; Liu, Q. Prediction of the development of metabolic syndrome by the Markov model based on a longitudinal study in Dalian City. BMC Public Heal. 2018, 18, 707. [Google Scholar] [CrossRef]
- De Ledinghen, V.; Vergniol, J.; Capdepont, M.; Chermak, F.; Hiriart, J.B.; Cassinotto, C.; Merrouche, W.; Foucher, J.; Brigitte le, B. Controlled attenuation parameter (CAP) for the diagnosis of steatosis: A prospective study of 5323 examinations. J. Hepatol. 2014, 60, 1026–1031. [Google Scholar] [CrossRef]
- Boursier, J.; Zarski, J.-P.; De Ledinghen, V.; Rousselet, M.-C.; Sturm, N.; LeBail, B.; Fouchard-Hubert, I.; Gallois, Y.; Oberti, F.; Bertrais, S.; et al. Determination of reliability criteria for liver stiffness evaluation by transient elastography. Hepatology 2013, 57, 1182–1191. [Google Scholar] [CrossRef]
- Yeh, C.-J.; Chang, H.-Y.; Pan, W.-H. Time trend of obesity, the metabolic syndrome and related dietary pattern in Taiwan: From NAHSIT 1993–1996 to NAHSIT 2005–2008. Asia Pac. J. Clin. Nutr. 2011, 20, 292. [Google Scholar] [PubMed]

| Variable | Metabolic Syndrome | p-Value | |
|---|---|---|---|
| Median (IQR)/Count (%) | No (n = 1714) | Yes (n = 269) | |
| FibroScan measurements | |||
| E score (kPa) | 4.2 (3.5–4.9) | 5 (4.3–5.9) | <0.0001 * |
| CAP score (dB/m) | 236 (207–272) | 300 (264–333) | <0.0001 * |
| Demographics and anthropometrics | |||
| Age, years | 44 (36–52) | 47 (41–57) | <0.0001 * |
| Gender | |||
| Female | 958 (55.9) | 97 (36.1) | <0.0001 * |
| Male | 756 (44.1) | 172 (63.9) | |
| BMI (kg/m2) | 23.1 (21–25.5) | 27.4 (25.2–30) | <0.0001 * |
| Systolic blood pressure (mmHg) | 113 (104–124) | 135 (124–142) | <0.0001 * |
| Diastolic blood pressure (mmHg) | 72 (66–79) | 86 (78–91) | <0.0001 * |
| Comorbidity-related index | |||
| Hemoglobin A1c (%) | 5.3 (5.1–5.5) | 5.7 (5.4–6.1) | <0.0001 * |
| GOT (U/L) | 20 (17–24) | 23 (19–31) | <0.0001 * |
| GPT (U/L) | 18 (13–26) | 28 (19–44) | <0.0001 * |
| Uric acid (mg/dL) | 5.2 (4.3–6.4) | 6.3 (5.4–7.2) | <0.0001 * |
| GFR (ml/min/1.73 m2) | 97.03 (82.1–112.76) | 86.23 (72.53–99.24) | <0.0001 * |
| Creatinine (mg/dL) | 0.7 (0.6–0.9) | 0.9 (0.7–1) | <0.0001 * |
| Blood urine nitrogen (mg/dL) | 12 (10–15) | 13 (11–16) | 0.0005 * |
| HBsAg (IU/mL) | |||
| Negative | 1401 (81.7) | 231 (85.9) | 0.51 |
| Positive | 189 (11) | 27 (10) | |
| Anti-HBs (mIU/mL) | |||
| Negative | 543 (31.7) | 85 (31.6) | 0.731 |
| Positive | 1040 (60.7) | 171 (63.6) | |
| Anti-HCV (S/CO) | |||
| Negative | 1480 (86.3) | 228 (84.8) | 0.8731 |
| Positive | 93 (5.4) | 15 (5.6) | |
| Biochemical index | |||
| Red blood cell count (106/uL) | 4.68 (4.37–5.05) | 4.96 (4.63–5.23) | <0.0001 * |
| Hemoglobin (g/dL) | 13.9 (13–15) | 14.8 (13.8–15.6) | <0.0001 * |
| Hematocrit (%) | 41.3 (38.7–44.4) | 43.7 (41.2–45.9) | <0.0001 * |
| MCH (pg) | 30.1 (28.8–31.1) | 30.1 (28.9–31.1) | 0.5572 |
| MCV (fL) | 89.2 (85.9–91.8) | 88.6 (85.3–91.1) | 0.0662 |
| MCHC (g/dL) | 33.6 (33.1–34.2) | 33.9 (33.4–34.4) | <0.0001 * |
| Platelet count (103/uL) | 233 (197–271) | 234 (202–271) | 0.8476 |
| White blood cell count (103/uL) | 5.88 (4.87–7.15) | 6.76 (5.81–8.05) | <0.0001 * |
| Neu (%) | 57.55 (52.5–62.9) | 59.1 (54.4–64.6) | 0.0103 * |
| Lym (%) | 30.8 (26.1–35.7) | 30.2 (25.4–34.7) | 0.0538 |
| Mono (%) | 7.6 (6.5–8.7) | 7.2 (6.2–8.8) | 0.0411 * |
| Eso (%) | 2.2 (1.4–3.5) | 2.3 (1.5–3.4) | 0.9791 |
| Baso (%) | 0.7 (0.5–0.9) | 0.7 (0.5–0.9) | 0.0231 * |
| Total protein (g/dL) | 7.4 (7–7.7) | 7.4 (7.1–7.7) | 0.0577 |
| Albumin (g/dL) | 4.6 (4.4–4.8) | 4.6 (4.4–4.8) | 0.6006 |
| Globulin (g/dL) | 2.7 (2.5–3) | 2.8 (2.5–3.1) | 0.1019 |
| Albumin/globulin ratio | 1.7 (1.5–1.9) | 1.7 (1.5–1.9) | 0.2601 |
| Total bilirubin (mg/dL) | 0.6 (0.4–0.8) | 0.6 (0.4–0.8) | 0.4768 |
| Direct bilirubin (mg/dL) | 0.2 (0.2–0.3) | 0.2 (0.2–0.3) | 0.6328 |
| Alkaline phosphatase (U/L) | 60 (50–72) | 66 (55–80.5) | <0.0001 * |
| γ-Glutamyl transpeptidase (U/L) | 16 (12–25) | 27 (20–43.5) | <0.0001 * |
| Total cholesterol (mg/dL) | 187 (165–210) | 194 (165–220) | 0.0242 * |
| LDL (mg/dL) | 121 (101–143) | 133 (106–158) | <0.0001 * |
| HDL (mg/dL) | 55 (47–66) | 39 (35–45) | <0.0001 * |
| LDL/HDL ratio | 2.2 (1.6–2.9) | 3.4 (2.7–4.1) | <0.0001 * |
| Total cholesterol/HDL ratio | 3.3 (2.7–4.2) | 4.95 (4.1–5.8) | <0.0001 * |
| TG (mg/dL) | 85 (62–118) | 178 (143–233) | <0.0001 * |
| FBS (mg/dL) | 90 (85–95) | 102 (92–111) | <0.0001 * |
| Variables | Odds Ratio | 95% CI | p-Value | |
|---|---|---|---|---|
| FibroScan measurements | ||||
| E score (kPa) | 1.02 | 1.02 | 1.02 | <0.0001 * |
| CAP score (dB/m) | 1.02 | 1.01 | 1.04 | 0.003 * |
| Demographics | ||||
| Age (Years) | 1.03 | 1.02 | 1.04 | <0.0001 * |
| Gender, male vs. female | 1.59 | 1.17 | 2.15 | 0.003 * |
| Anthropometrics | ||||
| BMI (kg/m2) | 1.28 | 1.22 | 1.34 | <0.0001 * |
| Systolic blood pressure (mmHg) | 1.1 | 1.08 | 1.12 | <0.0001 * |
| Diastolic blood pressure (mmHg) | 1.06 | 1.05 | 1.07 | <0.0001 * |
| Comorbidity-related index | ||||
| Hemoglobin A1c (%) | 1.6 | 1.38 | 1.86 | <0.0001 * |
| GOT (U/L) | 1.01 | 1 | 1.03 | 0.007 * |
| GPT (U/L) | 1.01 | 1.01 | 1.02 | 0.0005 * |
| Uric acid (mg/dL) | 1.2 | 1.09 | 1.34 | 0.0005 * |
| GFR (ml/min/1.73 m2) | 0.99 | 0.98 | 1 | 0.005 * |
| Creatinine (mg/dL) | 2.47 | 1.42 | 4.3 | 0.001 * |
| Blood urine nitrogen (mg/dL) | 1.01 | 0.98 | 1.05 | 0.548 |
| HBsAg, positive vs. negative | 0.89 | 0.56 | 1.42 | 0.624 |
| Anti-HBs, positive vs. negative | 1.19 | 0.86 | 1.63 | 0.287 |
| Anti-HCV, positive vs. negative | 1.14 | 0.6 | 2.15 | 0.686 |
| Biochemical index | ||||
| Red blood cell count (106/uL) | 1.43 | 1.05 | 1.94 | 0.022 * |
| Hemoglobin (g/dL) | 1.34 | 1.16 | 1.55 | <0.0001 * |
| Hematocrit (%) | 1.09 | 1.03 | 1.15 | 0.001 * |
| MCH (pg) | 1.02 | 0.96 | 1.08 | 0.551 |
| MCV (fL) | 1 | 0.98 | 1.02 | 0.74 |
| MCHC (g/dL) | 1.32 | 1.11 | 1.57 | 0.002 * |
| Platelet count (103/uL) | 1 | 1 | 1 | 0.979 |
| White blood cell count (103/uL) | 1.08 | 1.01 | 1.15 | 0.027 * |
| Neu (%) | 1.04 | 1.02 | 1.06 | 0.0002 * |
| Lym (%) | 0.97 | 0.95 | 0.99 | 0.002 * |
| Mono (%) | 0.95 | 0.88 | 1.03 | 0.231 |
| Eso (%) | 0.93 | 0.86 | 1.01 | 0.077 |
| Baso (%) | 0.66 | 0.42 | 1.03 | 0.066 |
| Total protein (g/dL) | 1.18 | 0.85 | 1.65 | 0.324 |
| Albumin (g/dL) | 0.67 | 0.38 | 1.19 | 0.173 |
| Globulin (g/dL) | 1.42 | 0.99 | 2.02 | 0.055 |
| Albumin/globulin ratio | 0.67 | 0.39 | 1.14 | 0.139 |
| Total bilirubin (mg/dL) | 0.99 | 0.67 | 1.47 | 0.972 |
| Direct bilirubin (mg/dL) | 1.02 | 0.57 | 1.81 | 0.954 |
| Alkaline phosphatase (U/L) | 1 | 1 | 1.01 | 0.036 * |
| γ-Glutamyl transpeptidase (U/L) | 1.01 | 1 | 1.01 | 0.008 * |
| Total cholesterol (mg/dL) | 1 | 1 | 1 | 0.901 |
| LDL (mg/dL) | 1 | 1 | 1.01 | 0.323 |
| HDL (mg/dL) | 0.89 | 0.88 | 0.91 | <0.0001 * |
| LDL/HDL ratio | 2.15 | 1.82 | 2.54 | <0.0001 * |
| Total cholesterol/HDL ratio | 2.02 | 1.76 | 2.32 | <0.0001 * |
| Triglyceride (mg/dL) | 1.02 | 1.01 | 1.02 | <0.0001 * |
| Fasting glucose (mg/dL) | 1.03 | 1.02 | 1.04 | <0.0001 * |
| Models | Convenient Model | Final Model | ||||||
|---|---|---|---|---|---|---|---|---|
| Variables | OR | 95% CI | p-Value | OR | 95% CI | p-Value | ||
| CAP score (dB/m) | 1.01 | 1.01 | 1.02 | <0.0001 * | 1.01 | 1 | 1.01 | <0.0001 * |
| Gender, male vs. female | 1.57 | 1.14 | 2.15 | 0.0055 * | 0.86 | 0.53 | 1.39 | 0.5346 |
| Age (years) | 1.04 | 1.03 | 1.05 | <0.0001 * | 1.03 | 1.01 | 1.05 | 0.0005 * |
| BMI (kg/m2) | 1.28 | 1.22 | 1.34 | <0.0001 * | 1.28 | 1.21 | 1.36 | <0.0001 * |
| Hemoglobin A1c (%) | 1.57 | 1.35 | 1.82 | <0.0001 * | ||||
| GPT (U/L) | 1.01 | 1.01 | 1.02 | 0.0016 * | ||||
| GFR (ml/min/1.73 m2) | 0.99 | 0.98 | 1 | 0.0233 * | ||||
| Hemoglobin (g/dL) | 1.25 | 1.07 | 1.46 | 0.0056 * | ||||
| Neutrophils (%) | 1.03 | 1 | 1.05 | 0.0217 * | ||||
| AUC † | 0.85 | 0.83 | 0.88 | <0.0001 * | 0.88 | 0.85 | 0.9 | - |
| Models | Prediction Performance Parameters | Gender- and BMI-Specific Cut-Off Points of CAP † | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thd | Sen | Spe | PPV | NPV | PLr | NLr | (Male) | (Female) | |||||||
| Whole | (18.5) | (24) | (27) | Whole | (18.5) | (24) | (27) | ||||||||
| Convenient | 0.09 | 0.89 | 0.66 | 0.28 | 0.97 | 2.64 | 0.17 | 254.14 | 372.03 | 254.57 | 191.65 | 292.28 | 410.17 | 292.71 | 229.78 |
| Final | 0.15 | 0.78 | 0.82 | 0.39 | 0.96 | 4.47 | 0.26 | 390.84 | 564.95 | 391.48 | 298.54 | 371.79 | 545.9 | 372.42 | 279.49 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-J.; Lin, C.-H.; Wang, S.-T.; Lin, S.-Y.; Chang, S.-S. Noninvasive and Convenient Screening of Metabolic Syndrome Using the Controlled Attenuation Parameter Technology: An Evaluation Based on Self-Paid Health Examination Participants. J. Clin. Med. 2019, 8, 1775. https://doi.org/10.3390/jcm8111775
Lin Y-J, Lin C-H, Wang S-T, Lin S-Y, Chang S-S. Noninvasive and Convenient Screening of Metabolic Syndrome Using the Controlled Attenuation Parameter Technology: An Evaluation Based on Self-Paid Health Examination Participants. Journal of Clinical Medicine. 2019; 8(11):1775. https://doi.org/10.3390/jcm8111775
Chicago/Turabian StyleLin, Yu-Jiun, Chang-Hsien Lin, Sen-Te Wang, Shiyng-Yu Lin, and Shy-Shin Chang. 2019. "Noninvasive and Convenient Screening of Metabolic Syndrome Using the Controlled Attenuation Parameter Technology: An Evaluation Based on Self-Paid Health Examination Participants" Journal of Clinical Medicine 8, no. 11: 1775. https://doi.org/10.3390/jcm8111775
APA StyleLin, Y.-J., Lin, C.-H., Wang, S.-T., Lin, S.-Y., & Chang, S.-S. (2019). Noninvasive and Convenient Screening of Metabolic Syndrome Using the Controlled Attenuation Parameter Technology: An Evaluation Based on Self-Paid Health Examination Participants. Journal of Clinical Medicine, 8(11), 1775. https://doi.org/10.3390/jcm8111775




