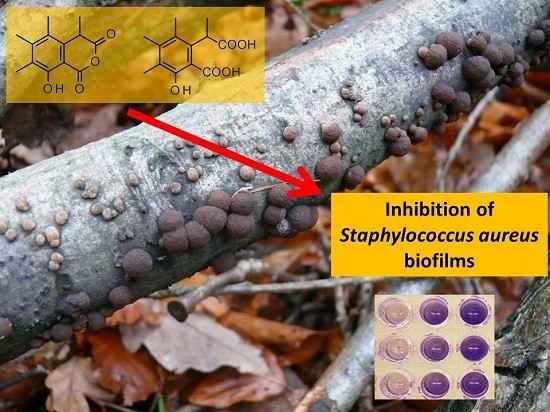
Bioactive Compounds Produced by Hypoxylon fragiforme against Staphylococcus aureus Biofilms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Microorganisms
2.3. Isolation and Fungal Identification
2.4. Fermentation
2.5. Inhibition of Biofilm Formation
2.6. Dispersion of Pre-Formed Biofilms
2.7. Cytotoxicity Assay
2.8. Purification and Structure Elucidation of Compounds
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Aswathanarayan, J.B.; Vittal, R.R. Microbial biofilms and their control by various antimicrobial strategies. In Microbial Pathogens and Strategies for Combating Them: Science, Technology and Education; Formatex Research Center: Badajoz, Spain, 2013; pp. 124–153. [Google Scholar]
- Potera, C. Studying slime. Environ. Health Perspect. 1998, 106, 604–606. [Google Scholar] [CrossRef]
- Abraham, W.-R. Going beyond the control of quorum-sensing to combat biofilm infections. Antibiotics 2016, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Bjarnsholt, T. The role of bacterial biofilms in chronic infections. APMIS 2013, 121, 1–58. [Google Scholar] [CrossRef] [PubMed]
- Estrela, A.; Abraham, W.-R. Fungal metabolites for the control of biofilm infections. Agriculture 2016, 6, 37. [Google Scholar] [CrossRef]
- Trautner, B.W.; Darouiche, R.O. Role of biofilm in catheter-associated urinary tract infection. Am. J. Infect. Control 2004, 32, 177–183. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin-Borlace, L.; Stapleton, F.; Matheson, M.; Dart, J.K. Bacterial biofilm on contact lenses and lens storage cases in wearers with microbial keratitis. J. Appl. Microbiol. 1998, 84, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Schaefer, A.L.; Parsek, M.R.; Moningerk, T.O.; Welsh, M.J.; Greenberg, E.P. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Lett. Nat. 2000, 407, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-H.; Tian, X. Quorum sensing and bacterial social interactions in biofilms. Sensors 2012, 12, 2519–2538. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Wang, L.; Zhang, L.-H. Quorum-quenching microbial infections: Mechanisms and implications. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2007, 362, 1201–1211. [Google Scholar] [CrossRef] [PubMed]
- Yuyama, K.T.; Neves, T.S.P.C.; Memória, M.T.; Tartuci, I.T.; Abraham, W.-R. Aurantiogliocladin inhibits biofilm formation at subtoxic concentrations. AIMS Microbiol. 2017, 3, 50–60. [Google Scholar] [CrossRef]
- De Carvalho, M.P.; Türck, P.; Abraham, W.-R. Secondary metabolites control the associated bacterial communities of saprophytic basidiomycotina fungi. Microbes Environ. 2015, 30, 196–198. [Google Scholar] [CrossRef] [PubMed]
- Wendt, L.; Sir, E.B.; Kuhnert, E.; Heitkämper, S.; Lambert, C.; Hladki, A.I.; Romero, A.I.; Luangsa-Ard, J.J.; Srikitikulchai, P.; Peršoh, D.; et al. Resurrection and emendation of the Hypoxylaceae, recognised from a multigene phylogeny of the Xylariales. Mycol. Prog. 2017, 1–40. [Google Scholar] [CrossRef]
- Daranagama, D.A.; Hyde, K.D.; Sir, E.B.; Thambugala, K.M.; Tian, Q.; Samarakoon, M.C.; McKenzie, E.H.C.; Jayasiri, S.C.; Tibpromma, S.; Bhat, J.D.; et al. Towards a natural classification and backbone tree for Graphostromataceae, Hypoxylaceae, Lopadostomataceae and Xylariaceae. Fungal Divers. 2017, 1–165. [Google Scholar] [CrossRef]
- Stadler, M.; Quang, D.N.; Tomita, A.; Hashimoto, T.; Asakawa, Y. Changes in secondary metabolism during stromatal ontogeny of Hypoxylon fragiforme. Mycol. Res. 2006, 110, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Asakawa, Y. Biologically active substances of Japanese inedible mushrooms. Heterocycles 1998, 47, 1067–1100. [Google Scholar]
- White, T.J.; Bruns, T.D.; Lee, S.B.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Academic Press Inc: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes—Application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, K.; Cigelnik, E. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol. Phylogenet. Evol. 1997, 7, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Pontecorvo, G.; Roper, J.A.; Chemmons, L.M.; Macdonald, K.D.; Bufton, A.W.J. The Genetics of Aspergillus nidulans. Adv. Genet. 1953, 5, 141–238. [Google Scholar] [CrossRef] [PubMed]
- Edwards, R.L.; Fawcett, V.; Maitland, D.J.; Nettleton, R.; Shields, L.; Whalley, A.J.S. Hypoxyxylerone. A novel green pigment from the fungus Hypoxylon fragiforme. J. Chem. Soc. Chem. Commun. 1991, 1009–1010. [Google Scholar] [CrossRef]
- O’Toole, G.A. Microtiter dish biofilm formation assay. J. Vis. Exp. 2011, 47. [Google Scholar] [CrossRef] [PubMed]
- Pažoutová, S.; Follert, S.; Bitzer, J.; Keck, M.; Surup, F.; Šrůtka, P.; Holuša, J.; Stadler, M. A new endophytic insect-associated Daldinia species, recognised from a comparison of secondary metabolite profiles and molecular phylogeny. Fungal Divers. 2013, 60, 107–123. [Google Scholar] [CrossRef]
- Chepkirui, C.; Matasyoh, J.C.; Decock, C.; Stadler, M. Two cytotoxic triterpenes from cultures of a Kenyan Laetiporus sp. (Basidiomycota). Phytochem. Lett. 2017, 20, 106–110. [Google Scholar] [CrossRef]
- Fournier, J.; Köpcke, B.; Stadler, M. New species of Hypoxylon from western Europe and Ethiopia. Mycotaxon 2010, 113, 209–235. [Google Scholar] [CrossRef]
- Turner, W.B. Fungal Metabolites; Academic Press: New York, NY, USA, 1971; p. 118. [Google Scholar]
- Pedras, M.S.C.; Ahiahonu, P.W.K. Phytotoxin production and phytoalexin elicitation by the phytopathogenic fungus Sclerotinia sclerotiorum. J. Chem. Ecol. 2004, 30, 2163–2179. [Google Scholar] [CrossRef] [PubMed]
- Satomura, Y.; Sato, A. Isolation and physiological activity of sclerin, a metabolite of Sclerotinia fungus. Agric. Biol. Chem. 1965, 29, 337–344. [Google Scholar] [CrossRef]
- Matsui, M.; Sugimura, Y.; Yamashita, K.; Mori, K.; Ogawa, T. Synthesis of isochroman-1,3-diones. A new synthesis of (±) sclerin. Agric. Biol. Chem. Biol. Chem. 1968, 32, 492–495. [Google Scholar] [CrossRef]
- Starratt, A.N.; Lazarovits, G. High-performance liquid chromatography with photodiode array detection for analysis of the fungal metabolite sclerin. J. Chromatogr. A 1996, 741, 131–134. [Google Scholar] [CrossRef]
- Ju, Y.-M.; Rogers, J. A Revision of the Genus Hypoxylon; The American Phytopathological Society: Saint Paul, MN, USA, 1996. [Google Scholar]
- Stadler, M.; Hellwig, V. Chemotaxonomy of the Xylariaceae and remarkable bioactive compounds from Xylariales and their associated asexual stages. Recent Res. Dev. Phytochem. 2005, 9, 41–93. [Google Scholar]
- Anderson, J.R.; Edwards, R.L.; Whalley, A.J.S. Metabolites of the higher fungi. Part 21. 3-Methyl-3,4-dihydroisocoumarins and related compounds from the Ascomycete family Xylariaceae. J. Chem. Soc. Perkin Trans. I 1983, 2165–2192. [Google Scholar] [CrossRef]
- Piettre, A.; Chevenier, E.; Massardier, C.; Gimbert, Y.; Greene, A.E. Synthetic approach to hypoxyxylerone, novel inhibitor of topoisomerase I. Org. Lett. 2002, 4, 3139–3142. [Google Scholar] [CrossRef] [PubMed]
- Dombrowski, A.W.; Bills, G.F.; Sabnis, G.; Koupal, L.R.; Meyer, R.; Ondeyka, J.G.; Giacobbec, R.A.; Monaghanc, R.L.; Lingham, R.B. L-696,474, a novel cytochalasin as an inhibitor of HIV-1 protease. I. The production organism and its fermentation. J. Antibiot. 1991, 45, 679–685. [Google Scholar]
- Chien, M.M.; Schiff, P.L.; Slatkin, D.J.; Knapp, J.E. Metabolites of aspergilli III. The isolation of citrinin, dihydrocitrinone and sclerin from Aspergilus carneus. Lloydia 1977, 40, 301–302. [Google Scholar] [PubMed]
- Satoh, A.; Ogawa, H.; Satomura, Y. Effect of sclerin on production of the aminoglycoside antibiotics accompanied by salvage function in Streptomyces. Agric. Biol. Chem. 1975, 398, 1593–1598. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Satomura, Y. Effects of sclerin on energy-linked functions and phospholipase activity in mitochondria isolated from rat liver and some plants. Agric. Biol. Chem. 1974, 38, 1289–1296. [Google Scholar] [CrossRef]
- Deng, Y.; Lim, A.; Lee, J.; Chen, S.; An, S.; Dong, Y.-H.; Zhang, L.-H. Diffusible signal factor (DSF) quorum sensing signal and structurally related molecules enhance the antimicrobial efficacy of antibiotics against some bacterial pathogens. BMC Microbiol. 2014, 14, 51. [Google Scholar] [CrossRef] [PubMed]
- Yuyama, K.; Abraham, W.-R. cis-2-Alkenoic acids as promising drugs for the control of biofilm infections. Med. Chem. 2016, 13, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Estrela, A.B.; Abraham, W.-R. Combining biofilm-controlling compounds and antibiotics as a promising new way to control biofilm infections. Pharmaceuticals 2010, 3, 1374–1393. [Google Scholar] [CrossRef] [PubMed]
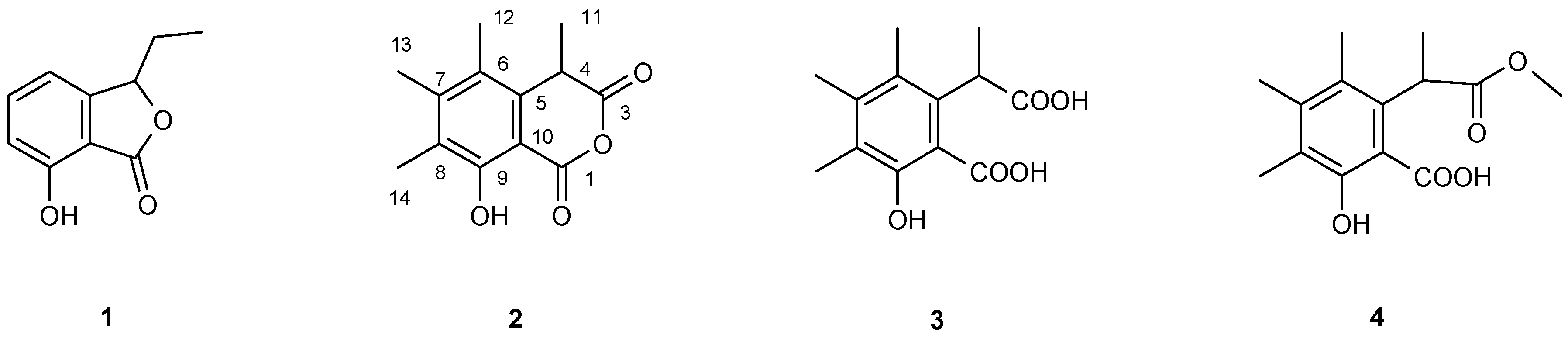
| (2) | (3) | (4) | ||||
|---|---|---|---|---|---|---|
| 1H | 13C | 1H | 13C | 1H | 13C | |
| C-1 | - | 166.22 | - | 172.94 | 179.91 | |
| C-3 | - | 168.68 | - | 176.36 | 171.40 | |
| C-4 | 4.160 (1H, q, J = 7.4 Hz) | 38.65 | 4.468 (1H, q, J = 7.4 Hz) | 42.01 | 4.315 (1H, q, J = 7.6 Hz) | 42.22 |
| C-5 | - | 134.53 | - | 137.43 | 136.17 | |
| C-6 | - | 123.89 | - | 126.39 | 123.88 | |
| C-7 | - | 147.70 | - | 142.84 | 143.40 | |
| C-8 | - | 124.61 | - | 123.56 | 126.75 | |
| C-9 | 10.754 (1H, s) 1 | 158.82 | 7.487 (1H, s) | 157.54 | 10.767 (1H, s) | 157.76 |
| C-10 | - | 101.41 | - | 109.98 | 109.52 | |
| C-11 | 1.570 (3H, d, J = 7.4 Hz) | 22.18 | 1.537 (3H, d, J = 7.4 Hz) | 17.12 | 1.526 (3H, d, J = 7.6 Hz) | 22.10 |
| C-12 | 2.306 (s) | 17.38 | 2.233 (s) | 16.61 | 2.240 (s) | 16.70 |
| C-13 | 2.189 (s) | 14.40 | 2.125 (s) | 15.94 | 2.283 (s) | 16.83 |
| C-14 | 2.252 (s) | 11.83 | 2.189 (s) | 11.91 | 2.240 (s) | 12.38 |
| OMe | - | - | - | - | 3.871 (s) | 51.36 |
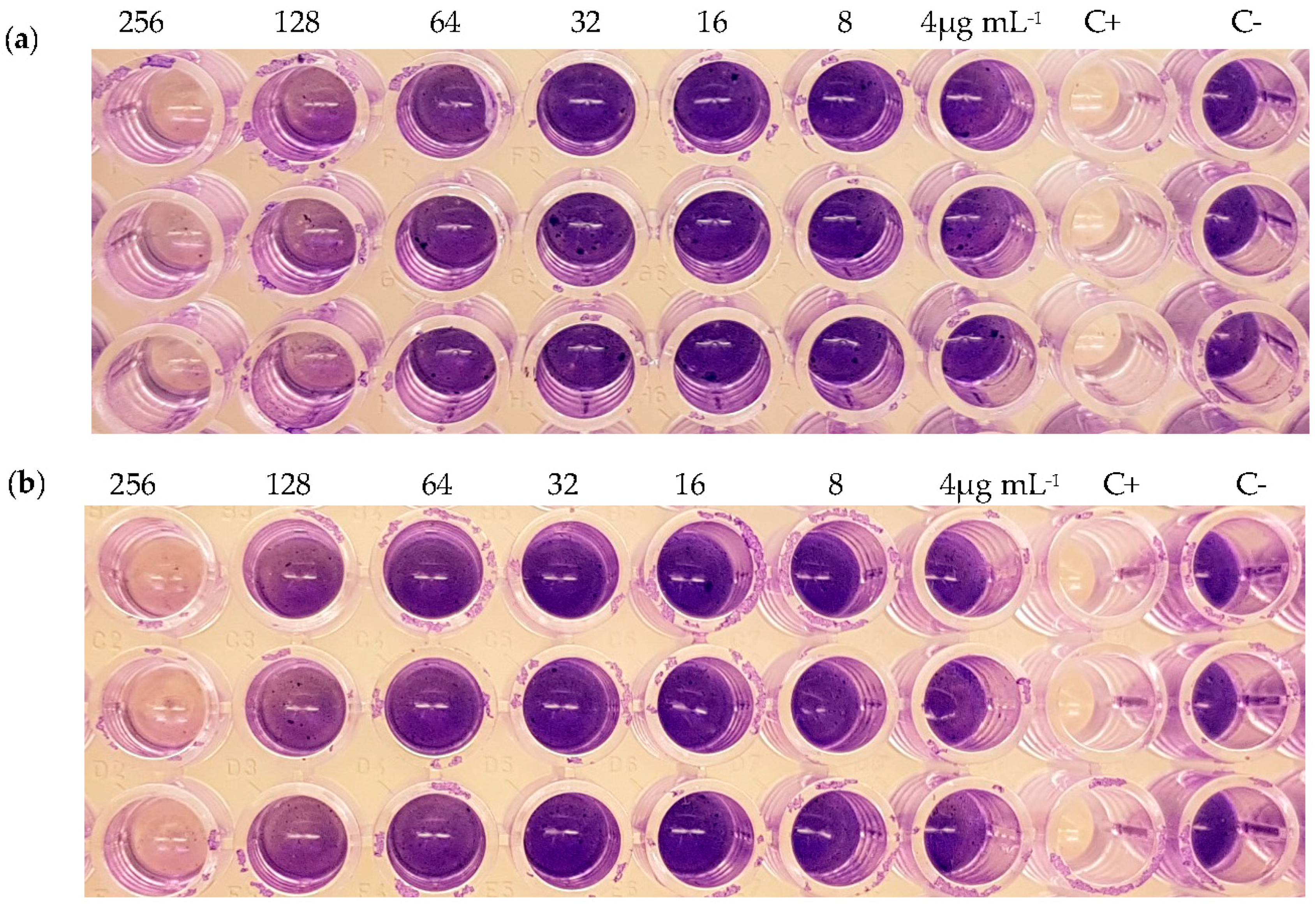
| Strain | Compound | MIC (μg mL−1) | Inhibition of Biofilm Formation (%) | Inhibition of Pre-Formed Biofilm (%) |
|---|---|---|---|---|
| S. aureus | (1) | >256 | - 2 | - |
| (2) | >256 | 86 (256 μg mL−1) 51 (128 μg mL−1) | - | |
| (3) | >256 | 80 (256 μg mL−1) 34 (128 μg mL−1) | - | |
| (4) | >256 | - | - | |
| E. coli | T 1 | >256 | - | nt 3 |
| B. cereus | T | >256 | - | nt |
| S. mutans | T | >256 | - | nt |
| S. epidermidis | T | >256 | - | nt |
| P. aeruginosa | T | >256 | - | nt |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuyama, K.T.; Chepkirui, C.; Wendt, L.; Fortkamp, D.; Stadler, M.; Abraham, W.-R. Bioactive Compounds Produced by Hypoxylon fragiforme against Staphylococcus aureus Biofilms. Microorganisms 2017, 5, 80. https://doi.org/10.3390/microorganisms5040080
Yuyama KT, Chepkirui C, Wendt L, Fortkamp D, Stadler M, Abraham W-R. Bioactive Compounds Produced by Hypoxylon fragiforme against Staphylococcus aureus Biofilms. Microorganisms. 2017; 5(4):80. https://doi.org/10.3390/microorganisms5040080
Chicago/Turabian StyleYuyama, Kamila Tomoko, Clara Chepkirui, Lucile Wendt, Diana Fortkamp, Marc Stadler, and Wolf-Rainer Abraham. 2017. "Bioactive Compounds Produced by Hypoxylon fragiforme against Staphylococcus aureus Biofilms" Microorganisms 5, no. 4: 80. https://doi.org/10.3390/microorganisms5040080
APA StyleYuyama, K. T., Chepkirui, C., Wendt, L., Fortkamp, D., Stadler, M., & Abraham, W.-R. (2017). Bioactive Compounds Produced by Hypoxylon fragiforme against Staphylococcus aureus Biofilms. Microorganisms, 5(4), 80. https://doi.org/10.3390/microorganisms5040080