A Novel Thermostable and Alkaline Protease Produced from Bacillus stearothermophilus Isolated from Olive Oil Mill Sols Suitable to Industrial Biotechnology
Abstract
:1. Introduction
2. Results
2.1. Effect of Different Parameters on Cell Growth and Protease Production from B. stearothermophilus
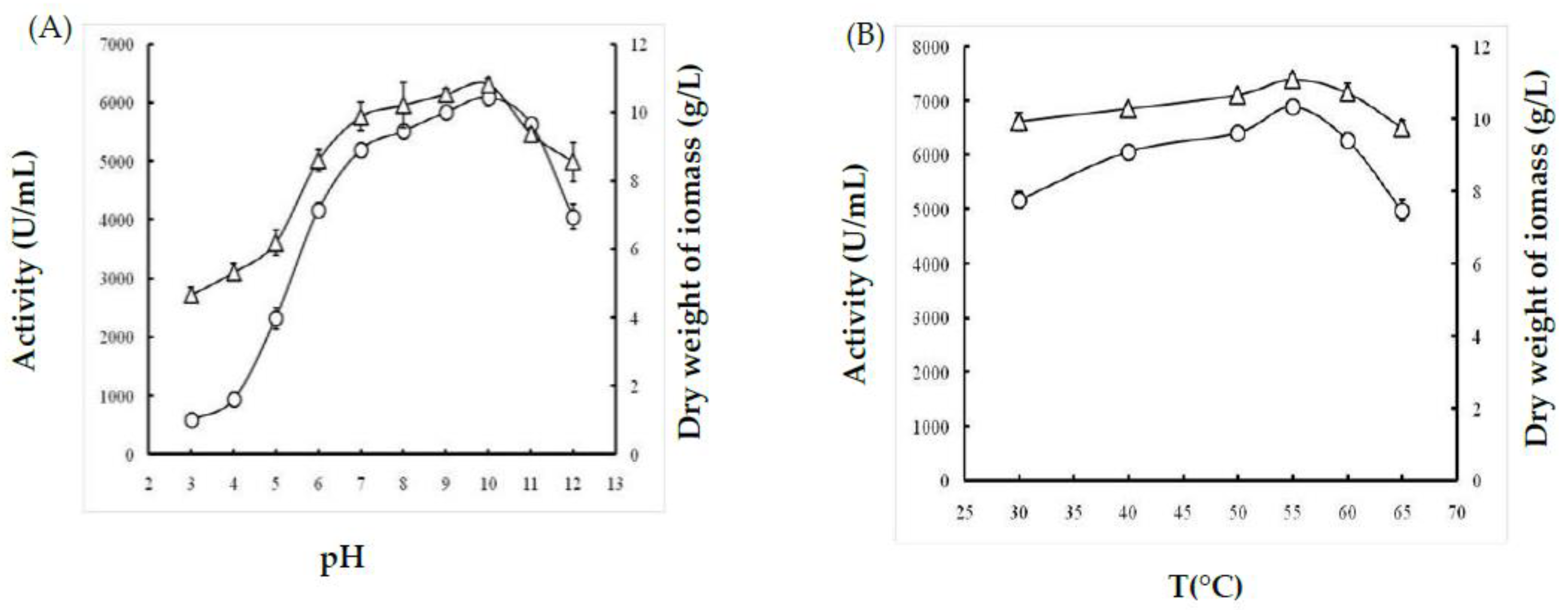
2.1.1. Effect of Temperature
2.1.2. Effect of pH
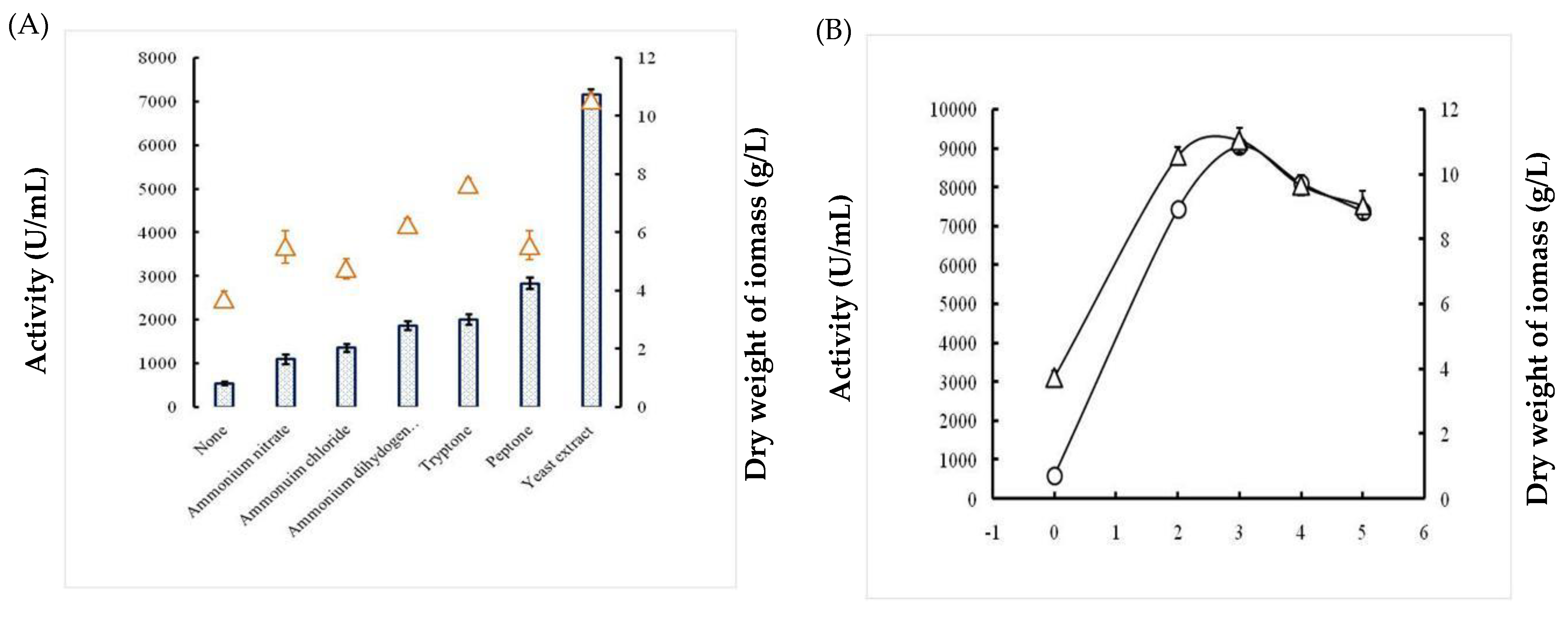
2.1.3. Effects of the Carbon Source
2.1.4. Effects of Nitrogen Sources
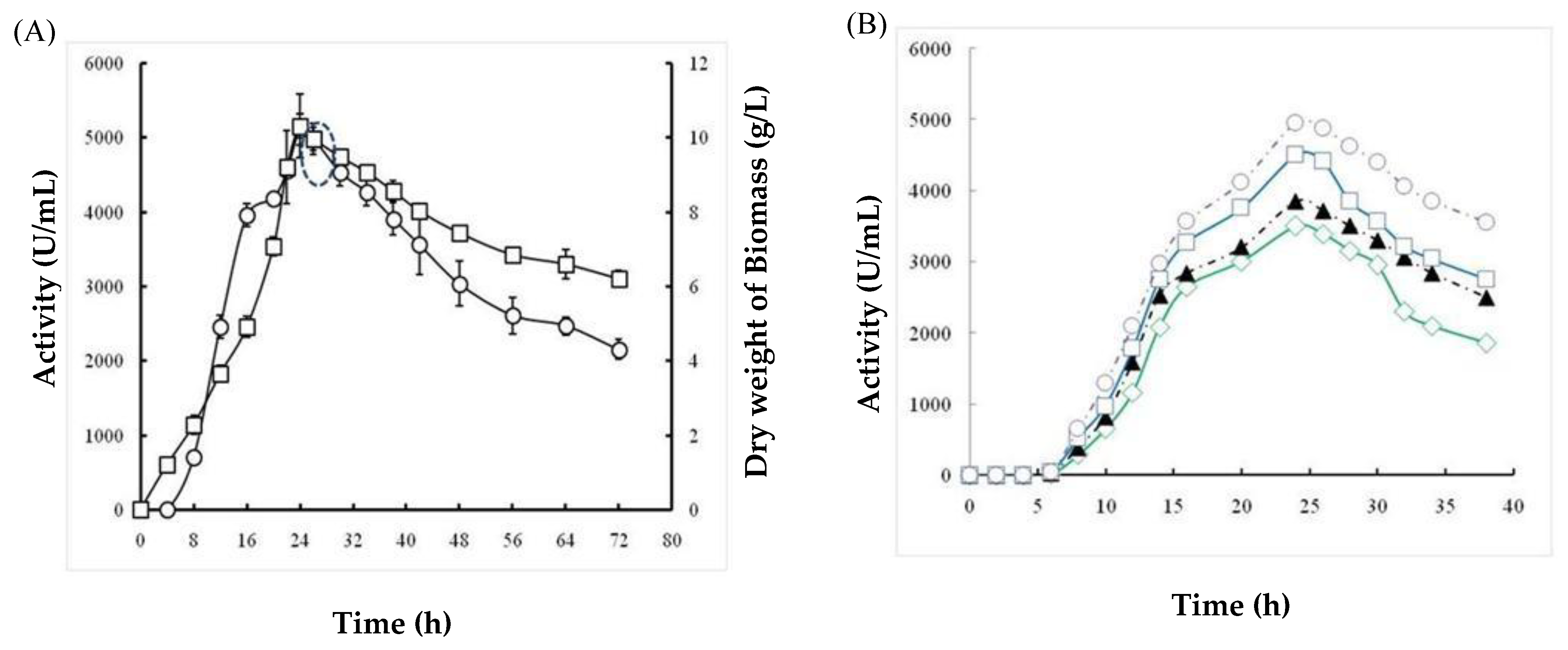
2.1.5. Effects of Incubation Time and Inoculum Size
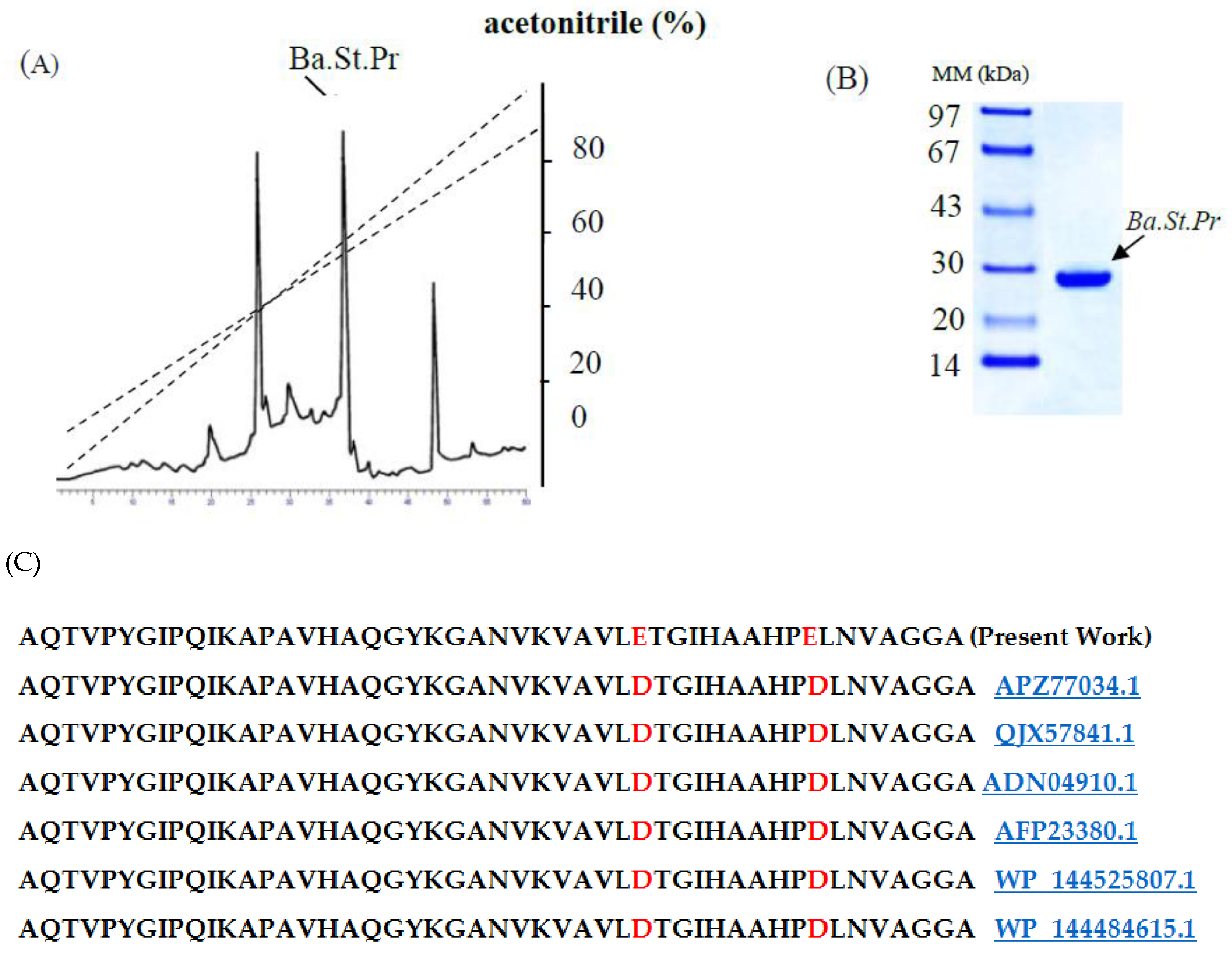
2.2. Purification Procedure of Ba.St.Pr and Determination of NH2-Terminal Amino Acid Sequence
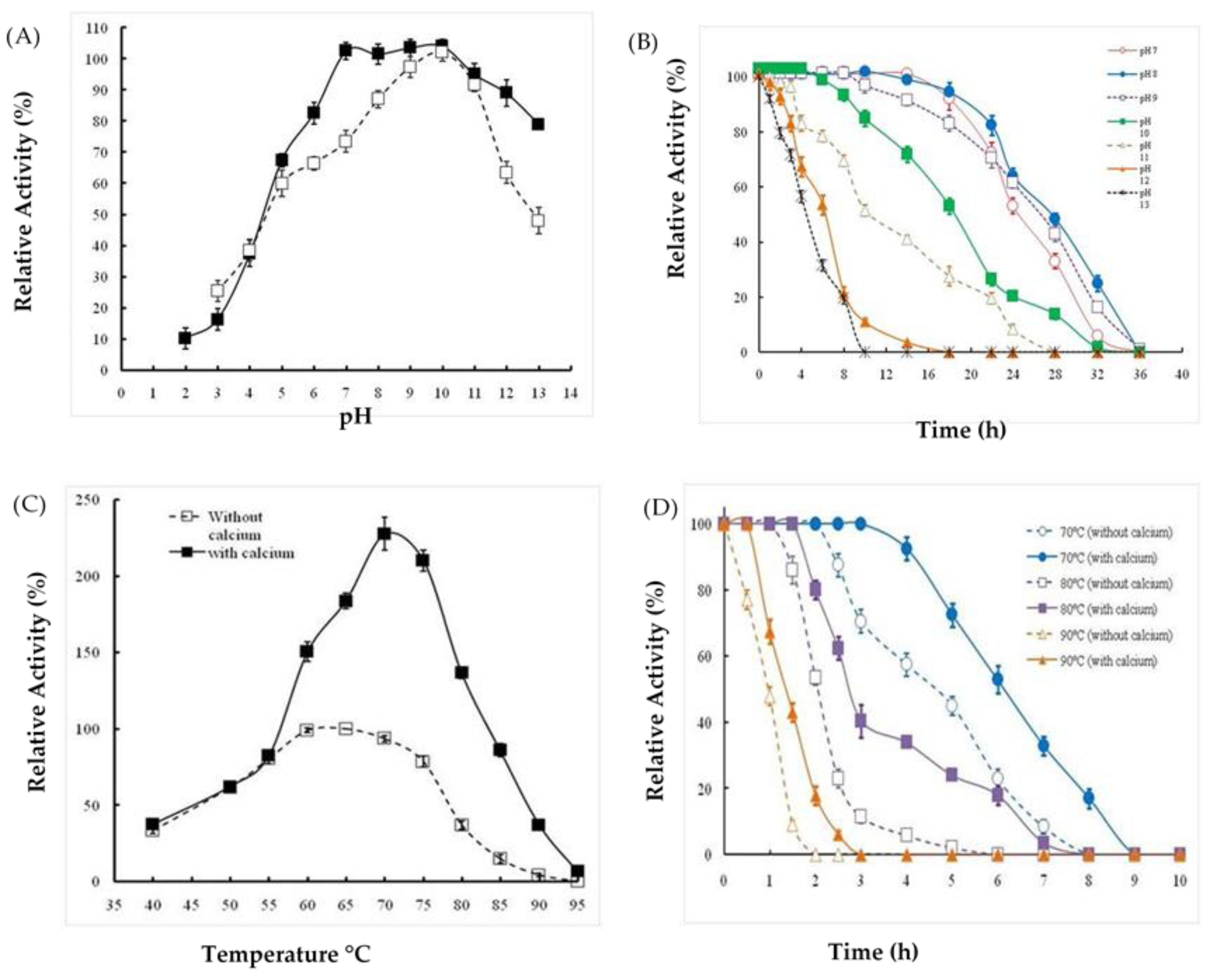
2.3. Effects of Extreme pH and Temperature on Protease Activity and Stability
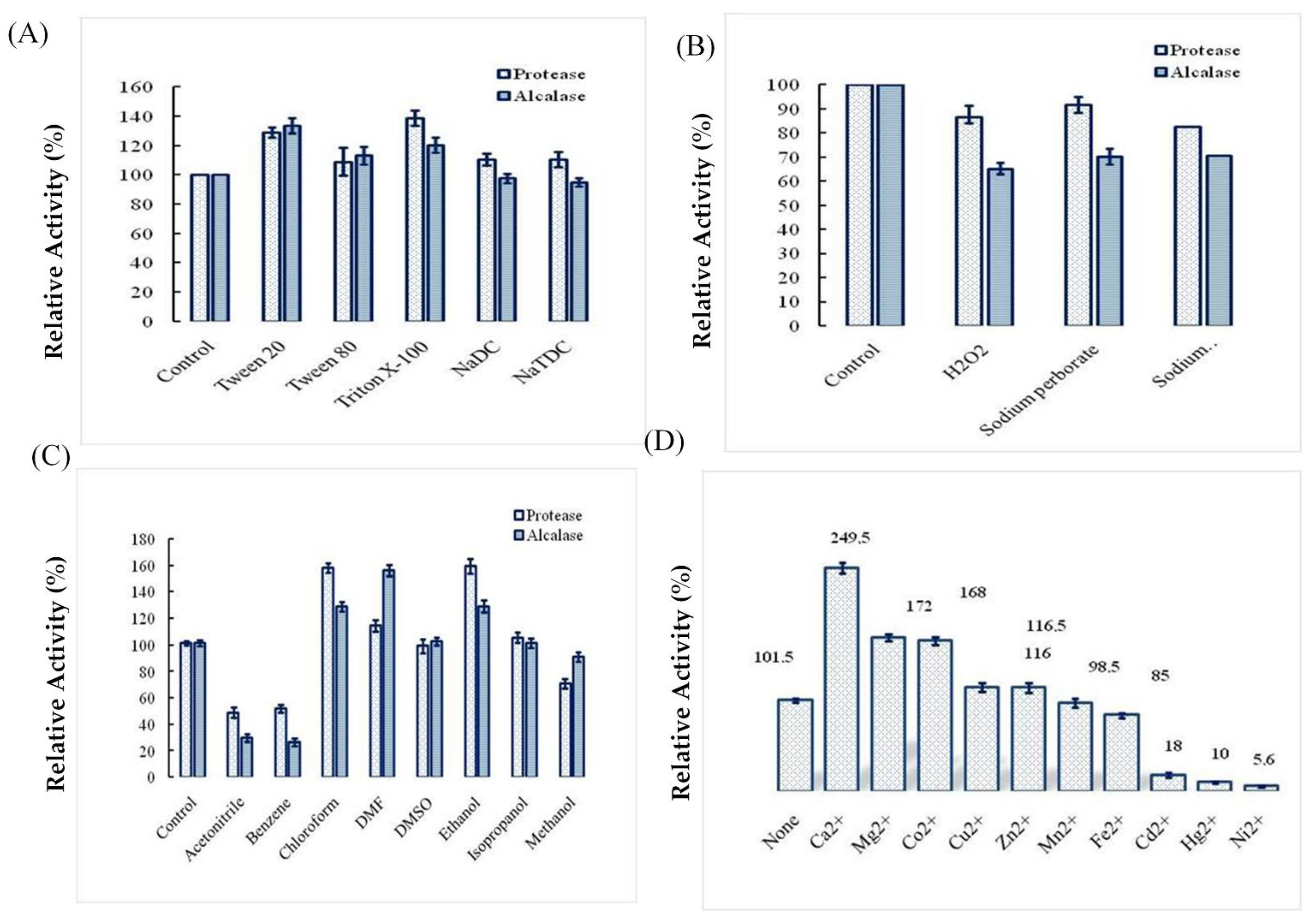
2.4. Effects of Surfactants, Oxidizing Agents, Organic Solvents, and Ions on Ba.St.Pr Stability
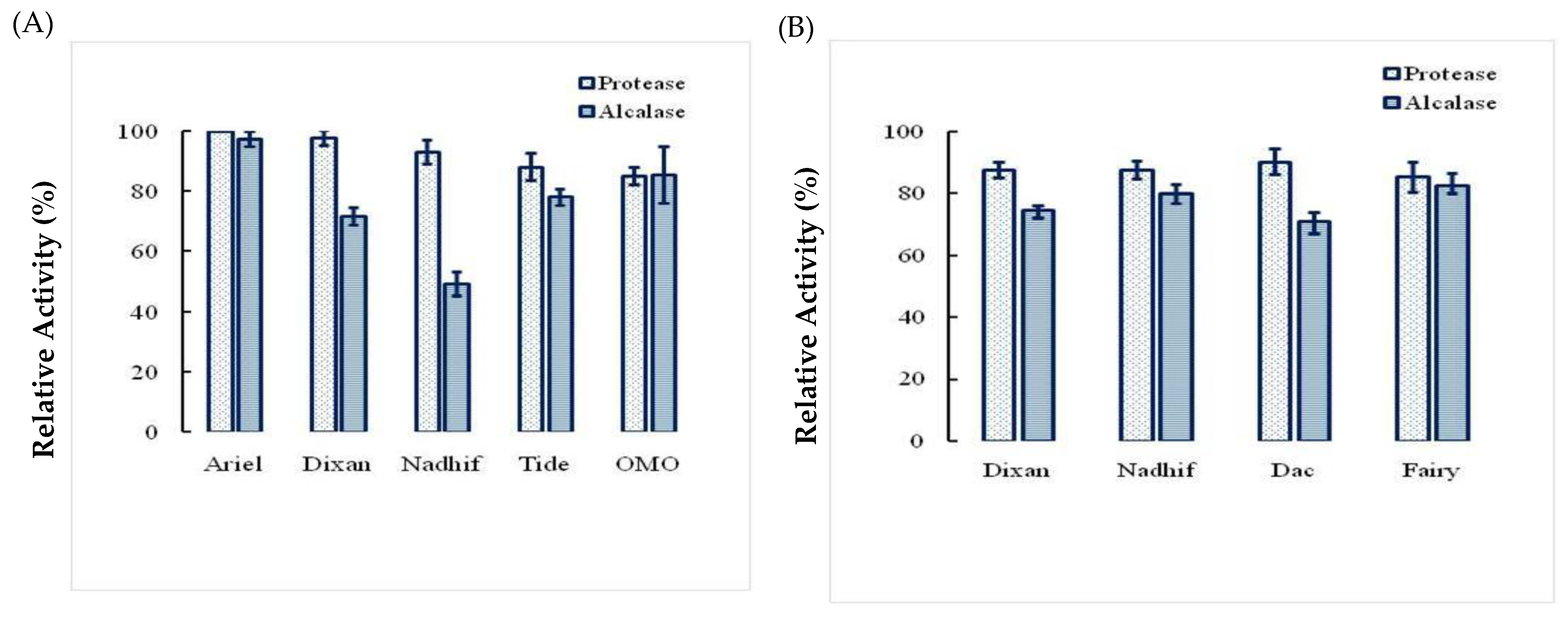
2.5. Stability and Compatibility of Ba.St.Pr with Solid and Liquid Commercial Laundry Detergents
3. Discussion
4. Materials and Methods
4.1. Protease Assay
4.2. Effects of Culture Conditions on Bacterial Growth and Protease Production of the Isolate B. stearothermophilus
4.2.1. Effect of Inoculum Size
4.2.2. Effects of pH and Temperature:
4.2.3. Effects of Carbon Sources:
4.2.4. Effects of Nitrogen Sources
4.2.5. Effects of Incubation Time
4.3. Protease Purification Procedure
4.4. Protein Analysis
4.5. Amino Acid Sequencing
4.6. Effects of pH and Temperature on Ba.St.Pr Activity and Stability
4.7. Performance Evaluation of Purified Ba.St.Pr
4.7.1. Effect of Organic Solvents on Enzyme Stability
4.7.2. Effects of Surfactants and Oxidizing Agents on Enzyme Stability
4.7.3. Effects of Liquid and Solid Detergent on Enzyme Stability
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kuddus, M.S.; Singh, P.; Thomas, G.; Al-Hazimi, A. Recent developments in production biotechnological applications of, C-phycocyanin. BioMed Res. Int. 2013, 2013, 742859. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, Z.D.; Donkor, O.; Street, W.A.; Vasiljevic, T. Proteolytic activities in fillets of selected underutilized Australian fish species. Food Chem. 2013, 140, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.M.; Kumar, R.; Panwar, S.; Kumar, A. Microbial alkaline proteases: Optimization of production parameters and their properties. J. Genet. Eng. Biotechnol. 2017, 15, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Tufvesson, P.; Lima-Ramos, J.; Nordblad, M.; Woodley, J.M. Guidelines and cost analysis for catalyst production in biocatalytic processes. Org. Process. Res. Dev. 2011, 15, 266–274. [Google Scholar] [CrossRef]
- Belmessikh, A.B.; Boukhalfa, H.; Mechakra-Maza, A.; Gheribi-Aoulmi, Z.; Amrane, A. statistical optimization of culture medium for neutral protease production by Aspergillus oryzae. Comparative study between solid and submerged fermentations on tomato pomace. J. Taiwan Inst. Chem. Eng. 2013, 44, 377–385. [Google Scholar] [CrossRef]
- Bach, E.S.A.; Sant’Anna, V.; Daroit, D.J.; Corrêa, A.P.F.; Segalin, J.; Brandelli, A. Production, one-step purification, and characterization of a keratinolytic protease from Serratia marcescens P3. Process. Biochem. 2012, 47, 2455–2462. [Google Scholar] [CrossRef] [Green Version]
- Helal, M.M.I.; Amer, H.; Abdelwahed, N.A.M.; Ghobashy, M.O.I. Physiological and microbiological studies on production of alkaline protease from locally isolated Bacillus subtilis. Aust. J. Basic Appl. Sci. 2012, 6, 193–203. [Google Scholar]
- Harwood, C.R.; Cranenburgh, R. Bacillus protein secretion: An unfolding story. Trends Microbiol. 2008, 16, 73–79. [Google Scholar] [CrossRef]
- Duman, R.E.; Löwe, J. Crystal structures of € Bacillus subtilis lon protease. J. Mol. Biol. 2010, 401, 653–670. [Google Scholar] [CrossRef]
- Feldman, L.A. Preparation of Microbial Alkaline Protease by Fermentation with Bacillus Subtilis, Variety Licheniformis. U.S. Patent 3623957, 30 November 1971. [Google Scholar]
- Rehman, R.A.; Ahmed, M.; Siddique, A.; Hasan, F.; Hameed, A.; Jamal, A. Catalytic role of thermostable metalloproteases from Bacillus subtilis KT004404 as dehairing and destaining agent. Appl. Biochem. Biotechnol. 2017, 181, 434–450. [Google Scholar] [CrossRef]
- Anandharaj, M.S.; Sivasankari, B.; Siddharthan, N.A.; Rani, R.P.; Sivakumar, S. Production, purification, and biochemical characterization of thermostable metallo-protease from novel Bacillus alkalitelluris TWI3 isolated from tannery waste. Appl. Biochem. Biotechnol. 2016, 178, 1666–1686. [Google Scholar] [CrossRef]
- Annamalai, N.R.; Rajeswari, M.V.; Thavasi, R.; Vijayalakshmi, S.; Balasubramanian, T. Optimization, purification and characterization of novel thermostable, haloalkaline, solvent stable protease from Bacillus halodurans CAS6 using marine shellfish wastes: A potential additive for detergent and antioxidant synthesis. Bioprocess. Biosyst. Eng. 2013, 36, 873–883. [Google Scholar] [CrossRef]
- Bougatef, A.B.; Balti, R.; Haddar, A.; Jellouli, K.; Souissi, N.; Nasri, M. Protein hydrolysates from bluefin tuna (Thunnus thynnus) heads as influenced by the extent of enzymatic hydrolysis. Biotechnol. Bioprocess Eng. 2012, 17, 841–852. [Google Scholar] [CrossRef]
- Ozcan, T.K.; Kurdal, E. The effects of using a starter culture, lipase, and protease enzymes on ripening of Mihalic cheese. Int. J. Dairy Technol. 2012, 65, 585–593. [Google Scholar] [CrossRef]
- Caille, J.C.; Govindan, C.K.; Junga, H.; Lalonde, J.; Yao, Y. Hetero diels-alderbiocatalysis approach for the synthesis of (s)-3-[2-{(methylsulfonyl) oxy} ethoxy]-4-(triphenylmethoxy)-1-butanol methanesulfonate, a key intermediate for the synthesis of the pkc inhibitor. Org. Process. Res. Dev. 2002, 6, 471–476. [Google Scholar] [CrossRef]
- Rahman, R.; Salleh, A.; Basri, M. Production of Protease from Bacillus stearothermophilus F1. U.S. Patent 20050186661, 18 February 2005. [Google Scholar]
- Hawumba, J.F.T.; Theron, J.; Brözel, V.S. Thermophilic Protease-Producing Geobacillus from Buranga Hot Springs in Western Uganda. Curr. Microbiol. 2002, 45, 144–150. [Google Scholar] [CrossRef]
- Shanthakumari, A.R.; Bominathan, M. Studies on screening of Bacillus sp. for protease production. Int. J. Theor. Appl. Sci. 2017, 9, 294–299. [Google Scholar]
- Khusro, A. One Factor at A Time based optimization of protease from poultry associated Bacillus licheniformis. J. Appl. Pharm. Sci. 2016, 6, 88–95. [Google Scholar] [CrossRef] [Green Version]
- Thebti, W.; Riahi, Y.; Belhadj, O. Purification and characterization of a new thermostable, Haloalkaline, solvent stable, and detergent compatible serine protease from Geobacillus toebii Strain LBT 77. BioMed Res. Int. 2016, 2016, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akhavan Sepahy, A.; Jabalameli, L. Effect of culture conditions on the production of an extracellular protease by Bacillus sp. Isolated from soil sample of Lavizan jungle park. Enzym. Res. 2011, 2011, 1–7. [Google Scholar]
- Derekova, A.M.; Mandeva, R.; Kambourova, M. Phylogenetic diversity of thermophilic carbohydrate degrading bacilli from Bulgarian hot springs. World J. Microbiol. Biotechnol. 2008, 24, 1697–1702. [Google Scholar] [CrossRef]
- Malathi, S.C.; Appl, R. Production of alkaline protease by a new Aspergillus flavus isolate under solid-substrate fermentation conditions for use as a depilation agent. Environ. Microbiol. 1991, 57, 712–716. [Google Scholar] [CrossRef] [Green Version]
- Tsuchiya, K.; Sakashita, H.; Nakamura, Y.; Kimura, T. Production of thermostable alkaline protease by alkalophilic Thermoactinomyces sp. HS682. Agric. Biol. Chem. 1991, 55, 3125–3127. [Google Scholar] [CrossRef]
- Phadatare, S.U.D.; Deshpande, V.V.; Srinivasan, M.C. High activity alkaline protease from Conidiobolus coronatus (NCL 86.8.20): Enzyme production and compatibility with commercial detergents. Enzym. Microb. Technol. 1993, 15, 72–76. [Google Scholar] [CrossRef]
- Frankena, J.; Van Verseveld, H.W.; Stouthamer, A.H. A continuous culture study of the bioenergetic aspects of growth and production of exocellular protease in Bacillus licheniformis. Appl. Microbiol. Biotechnol. 1985, 22, 169–176. [Google Scholar] [CrossRef]
- Hidayat, M.Y.; Saud, H.M.; Samsudin, A.A. Isolation and characterisation of Sulphur oxidizing bacteria isolated from hot spring in Malaysia for biological deodorisation of hydrogen sulphide in chicken manure. Media Peternak. 2017, 40, 178–187. [Google Scholar] [CrossRef]
- Yang, S.H.; Cho, J.K.; Lee, S.Y.; Abanto, O.D.; Kim, S.K.; Ghosh, C.; Lim, J.S.; Hwang, S.G. Isolation and characterization of Novel denitrifying bacterium Geobacillus sp. SG-01 strain from wood chips composted with swine manure. Asian-Australas. J. Anim. Sci. 2013, 26, 1651–1658. [Google Scholar]
- Rekik, H.; Jaouadi, N.Z.; Gargouri, F.; Bejar, W.; Frikha, F.; Jmal, N.; Bejar, S.; Jaouadi, B. Production, purification and biochemical characterization of a novel detergent-stable serine alkaline protease from Bacillus safensis strain RH12. IJBM 2019, 121, 1227–1239. [Google Scholar] [CrossRef] [PubMed]
- Alcaraz, L.D.; Olmedo, G.; Bonilla, G.; Cerritos, R.; Hernández, G.; Cruz, A.; Ramírez, E.; Putonti, C.; Jiménez, B.; Martínez, E.; et al. The genome of Bacillus coahuilensis reveals adaptations essential for survival in the relic of an ancient marine environment. Proc. Nat. Acad. Sci. USA 2008, 105, 5803–5808. [Google Scholar] [CrossRef] [Green Version]
- Yamane, T.K.; Kani, T.; Hatanaka, T.; Suzuki, A.; Ashida, T.; Kobatashi, T.; Ito, S.; Yamashita, O. Structure of a new alkaline serine protease (M-protease) from Bacillus sp. KSM-K16. Acta Cryst. Sect. D Acta Cryst. 1995, 51, 199–206. [Google Scholar] [CrossRef]
- McDonald, C.E.C.; Chen, L.L. The Lowry modification of the Folin reagent for determination of proteinase activity. Anal. Biochem. 1965, 10, 175–177. [Google Scholar] [CrossRef]
- Sinha, R.K.; Khare, S.K. Characterization of detergent compatible protease of a halophilic Bacillus sp. EMB9: Differential role of metal ions in stability and activity. Bioresour. Technol. 2013, 145, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Hewick, R.M. I-Iunkapiller, MW; Hood, LE; Dreyer, WJ. Bioi. GCIII 1981, 256, 7990–7997. [Google Scholar]

| Purification Step | Total Activity (Units) | Protein (mg) | Specific Activity (U/mg) | Activity Recovery (%) | Purification Factor |
|---|---|---|---|---|---|
| Culture supernatant | 2,775,000 | 3780 | 734 | 100 | 1 |
| (NH4)2SO4 Precipitation (30–65%) | 2,358,750 | 1120 | 2106 | 85 | 2.87 |
| Heat treatment (80 °C for 30 min) | 2,090,500 | 119 | 19,248 | 75.3 | 26.2 |
| RP-HPLC (C-8) | 1,534,575 | 26 | 59,022 | 55.3 | 80.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karray, A.; Alonazi, M.; Horchani, H.; Ben Bacha, A. A Novel Thermostable and Alkaline Protease Produced from Bacillus stearothermophilus Isolated from Olive Oil Mill Sols Suitable to Industrial Biotechnology. Molecules 2021, 26, 1139. https://doi.org/10.3390/molecules26041139
Karray A, Alonazi M, Horchani H, Ben Bacha A. A Novel Thermostable and Alkaline Protease Produced from Bacillus stearothermophilus Isolated from Olive Oil Mill Sols Suitable to Industrial Biotechnology. Molecules. 2021; 26(4):1139. https://doi.org/10.3390/molecules26041139
Chicago/Turabian StyleKarray, Aida, Mona Alonazi, Habib Horchani, and Abir Ben Bacha. 2021. "A Novel Thermostable and Alkaline Protease Produced from Bacillus stearothermophilus Isolated from Olive Oil Mill Sols Suitable to Industrial Biotechnology" Molecules 26, no. 4: 1139. https://doi.org/10.3390/molecules26041139
APA StyleKarray, A., Alonazi, M., Horchani, H., & Ben Bacha, A. (2021). A Novel Thermostable and Alkaline Protease Produced from Bacillus stearothermophilus Isolated from Olive Oil Mill Sols Suitable to Industrial Biotechnology. Molecules, 26(4), 1139. https://doi.org/10.3390/molecules26041139








