Step by Step about Germ Cells Development in Canine
Abstract
:Simple Summary
Abstract
1. Introduction
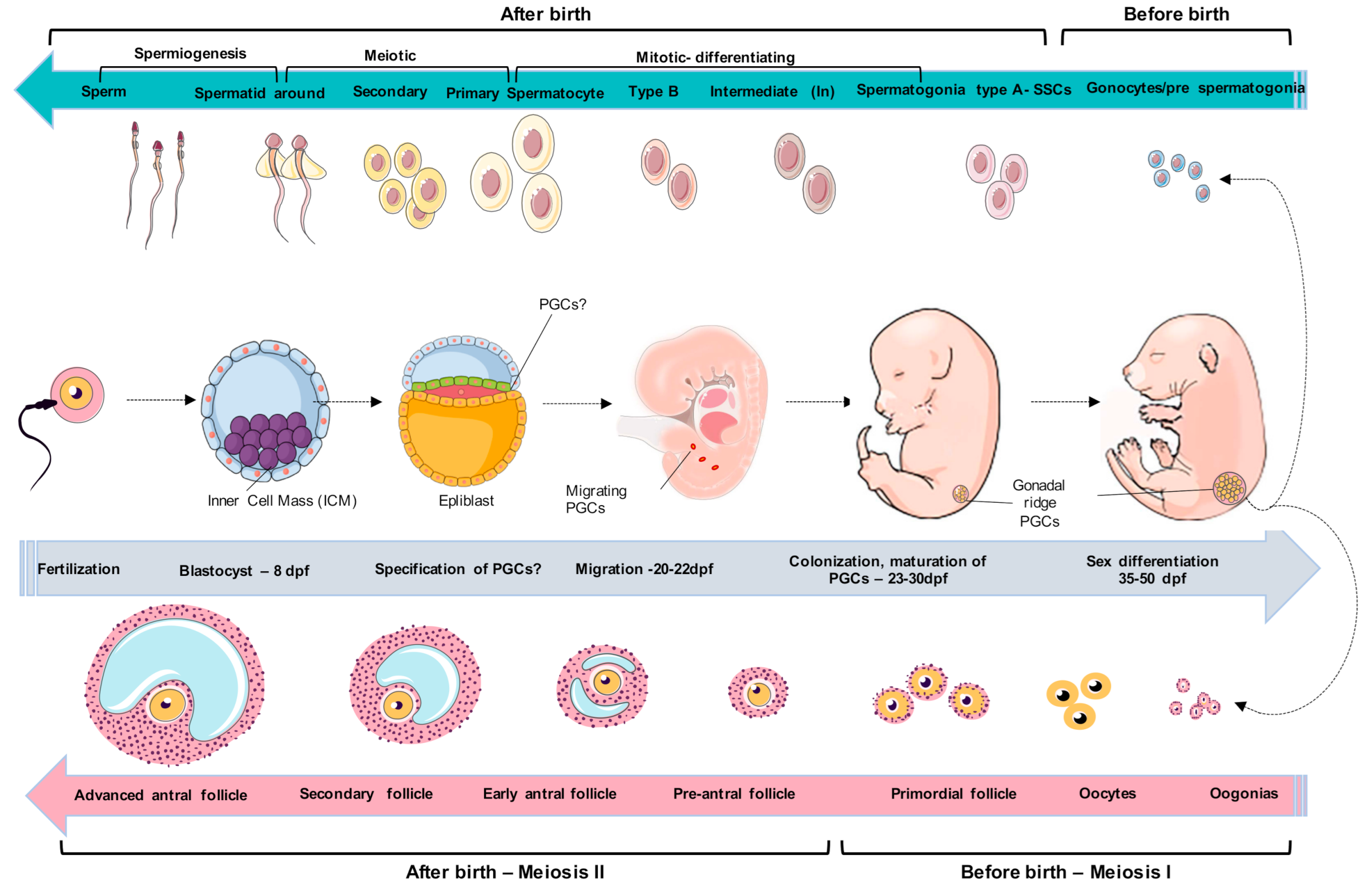
2. Embryological and Primordial Germ Cells Development
3. Spermatogonial Canine Stem Cells
4. Germ Cell Signals
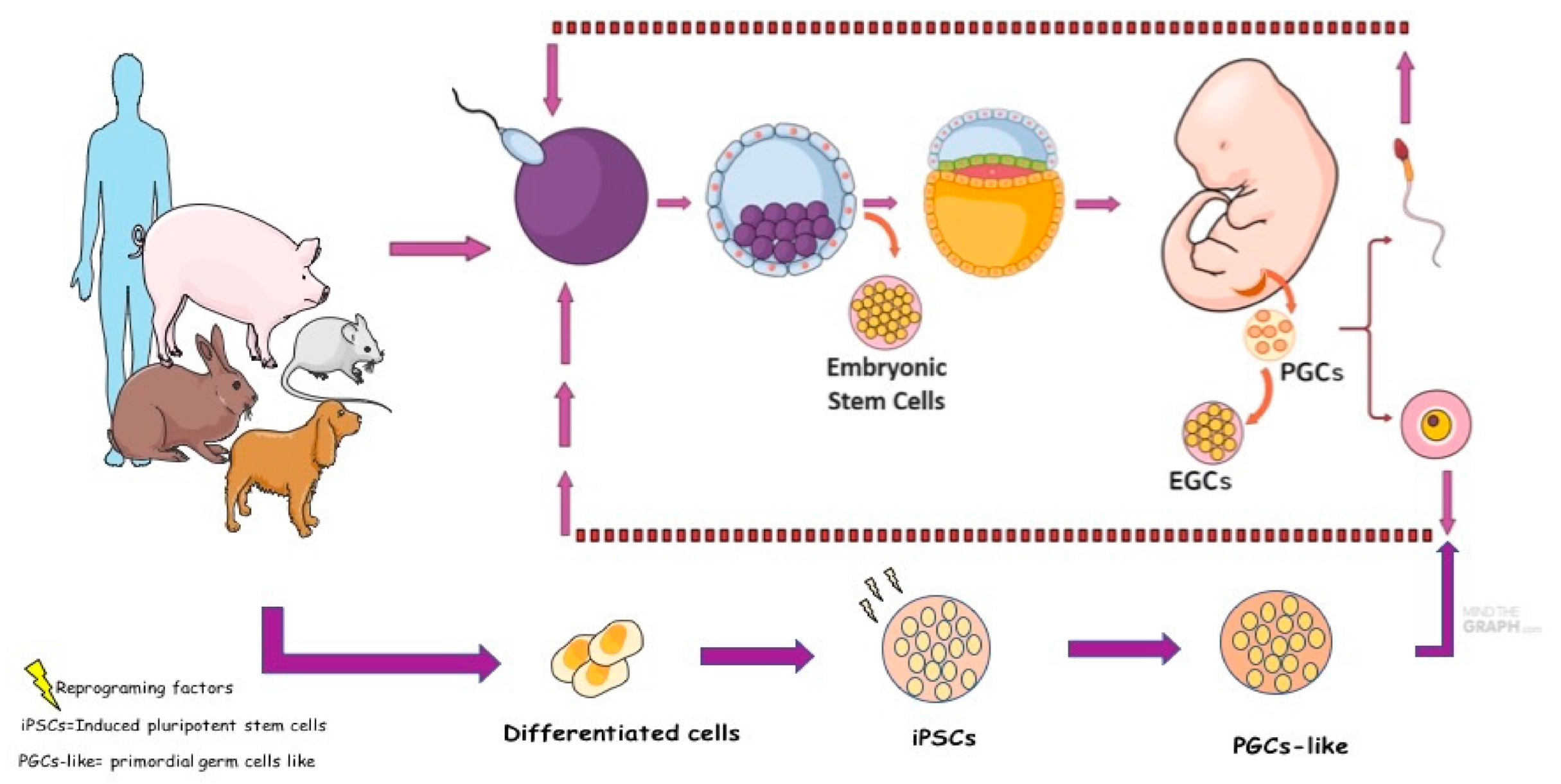
5. Canine Germ Cells in Vitro
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Cinalli, R.M.; Rangan, P.; Lehmann, R. Germ Cells Are Forever. Cell 2008, 132, 559–562. [Google Scholar] [CrossRef] [Green Version]
- Chuva, S.M.; Lopes, D.S.; Roelen, B.A.J. On the formation of germ cells: The good, the bad and the ugly. Differentiation 2010, 79, 131–140. [Google Scholar] [CrossRef]
- Shaffer, L.G. Special issue on canine genetics: Animal models for human disease and gene therapies, new discoveries for canine inherited diseases, and standards and guidelines for clinical genetic testing for domestic dogs. Qual. Life Res. 2019, 138, 437–440. [Google Scholar] [CrossRef] [Green Version]
- Canovas, S.; Cuerva, R.C.; Aguilar, E.; Cibelli, J.B. Progress towards human primordial germ cell specificationin vitro. Mol. Hum. Reprod. 2016, 23, 4–15. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.-I.; Lee, S.-Y.; Hwang, D.-Y. Extracellular Matrix-Dependent Generation of Integration- and Xeno-Free iPS Cells Using a Modified mRNA Transfection Method. Stem Cells Int. 2016, 2016, 6853081. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Turner, D.; Nelson, J.; Dobrinski, I.; McEntee, M.; Travis, A.J. Production of donor-derived sperm after spermatogonial stem cell transplantation in the dog. Reproduction 2008, 136, 823–831. [Google Scholar] [CrossRef]
- De Souza, A.F.; Pieri, N.C.G.; Roballo, K.; Bressan, F.F.; Casals, J.B.; Ambrósio, C.E.; Perecin, F.; Martins, D.D.S. Dynamics of male canine germ cell development. PLoS ONE 2018, 13, e0193026. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Fang, J.; Cai, S.; Lv, C.; Zhang, S.; Hua, J. Primordial germ cell-like cells derived from canine adipose mesenchymal stem cells. Cell Prolif. 2016, 49, 503–511. [Google Scholar] [CrossRef]
- Kirkness, E.F.; Bafna, V.; Halpern, A.L.; Levy, S.; Remington, K.; Rusch, D.B.; Delcher, A.L.; Pop, M.; Wang, W.; Fraser, C.M.; et al. The Dog Genome: Survey Sequencing and Comparative Analysis. Science 2003, 301, 1898–1903. [Google Scholar] [CrossRef]
- Poul, H.; Fred, S.; Morten, V. Essential of Domestic Animal Embryology; Elservier: London, UK, 2010; pp. 32–55. [Google Scholar]
- Miglino, M.A.; Ambrósio, C.E.; Martins, D.D.S.; Wenceslau, C.V.; Pfarrer, C.; Leiser, R. The carnivore pregnancy: The development of the embryo and fetal membranes. Theriogenology 2006, 66, 1699–1702. [Google Scholar] [CrossRef]
- Martins, A.; Favaron, P.O.; Schäfer, B.; Miglino, M.; Oliveira, L.D.J.; Oliveira, F. Development of the cardiorespiratory system in dogs from days 16 to 46 of pregnancy. Reprod. Domest. Anim. 2016, 51, 804–812. [Google Scholar] [CrossRef]
- Pieri, N.; Souza, A.F.; Casals, J.B.; Roballo, K.; Ambrósio, C.E.; Martins, D.S. Comparative Development of Embryonic Age by Organogenesis in Domestic Dogs and Cats. Reprod. Domest. Anim. 2015, 50, 625–631. [Google Scholar] [CrossRef]
- Gier, H.T.; Marion, G.B. Development of Mammalian Testes and Genital Ducts1. Biol. Reprod. 1969, 1, 1–23. [Google Scholar] [CrossRef]
- McLaren, A. Primordial germ cells in the mouse. Dev. Biol. 2003, 262, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Heeren, A.M.; He, N.; De Souza, A.F.; Goercharn-Ramlal, A.; Van Iperen, L.; Roost, M.S.; Fernandes, M.M.G.; Van Der Westerlaken, L.A.J.; Lopes, S.M.C.D.S. On the development of extragonadal and gonadal human germ cells. Biol. Open 2016, 5, 185–194. [Google Scholar] [CrossRef] [Green Version]
- Pretzer, S. Canine embryonic and fetal development: A review. Theriogenology 2008, 70, 300–303. [Google Scholar] [CrossRef]
- De Souza, A.F.; De Ramos, E.C.; Cury, F.S.; Pieri, N.C.G.; Martins, D.D.S. The timeline development of female canine germ cells. Reprod. Domest. Anim. 2019, 54, 964–971. [Google Scholar] [CrossRef]
- Biason-Lauber, A. Control of sex development. Best Pr. Res. Clin. Endocrinol. Metab. 2010, 24, 163–186. [Google Scholar] [CrossRef] [Green Version]
- Jiménez, R. Ovarian Organogenesis in Mammals: Mice Cannot Tell Us Everything. Sex. Dev. 2009, 3, 291–301. [Google Scholar] [CrossRef]
- Takagi, Y.; Talbot, N.C.; Rexroad, C.E.; Pursel, V.G., Jr. Identification of pig primordial germ cells by immunocytochemistry and lectin binding. Mol. Reprod. Dev. 1997, 46, 567–580. [Google Scholar] [CrossRef]
- Andersen, A.C.; Simpson, M.E. The Ovary and Reproductive Cycle of the Dog (Beagle); Geron-X Inc.: Los Altos, CA, USA, 1973. [Google Scholar]
- Barber, M.; Lee, S.; Steffens, W.; Ard, M.; Fayrer-Hosken, R. Immunolocalization of zona pellucida antigens in the ovarian follicle of dogs, cats, horses and elephants. Theriogenology 2001, 55, 1705–1717. [Google Scholar] [CrossRef]
- Durrant, B.; Pratt, N.C.; Russ, K.D.; Bolamba, D. Isolation and characterization of canine advanced preantral and early antral follicles. Theriogenology 1998, 49, 917–932. [Google Scholar] [CrossRef]
- Concannon, P.W.; McCann, J.P.; Temple, M. Biology and endocrinology of ovulation, pregnancy and parturition in the dog. J. Reprod. Fertil. Suppl. 1989, 39, 3–25. [Google Scholar]
- Songsasen, N.; Wildt, D. Oocyte biology and challenges in developing in vitro maturation systems in the domestic dog. Anim. Reprod. Sci. 2007, 98, 2–22. [Google Scholar] [CrossRef] [Green Version]
- Yamashiro, C.; Sasaki, K.; Yabuta, Y.; Kojima, Y.; Nakamura, T.; Okamoto, I.; Yokobayashi, S.; Murase, Y.; Ishikura, Y.; Shirane, K.; et al. Generation of human oogonia from induced pluripotent stem cells in vitro. Science 2018, 362, 356–360. [Google Scholar] [CrossRef] [Green Version]
- De Rooij, D.G. Proliferation and differentiation of spermatogonial stem cells. Reproduction 2001, 121, 347–354. [Google Scholar] [CrossRef]
- Yoshida, S.; Sukeno, M.; Nakagawa, T.; Ohbo, K.; Nagamatsu, G.; Suda, T.; Nabeshima, Y.-I. The first round of mouse spermatogenesis is a distinctive program that lacks the self-renewing spermatogonia stage. Development 2006, 133, 1495–1505. [Google Scholar] [CrossRef] [Green Version]
- Hafez, E.S.E. Reprodução Animal, 7th ed.; Manole: São Paulo, Brazil, 2004; p. 27. [Google Scholar]
- Schäfer-Somi, S.; Kaya, D.; Gültiken, N.; Aslan, S. Suppression of Fertility in Pre-pubertal Dogs and Cats. Reprod. Domest. Anim. 2014, 49, 21–27. [Google Scholar] [CrossRef]
- Lee, W.-Y.; Lee, R.; Park, H.-J.; Do, J.T.; Seo, H.G.; Kim, J.-H.; Jhun, H.; Lee, J.-H.; Hur, T.; Song, H. Characterization of male germ cell markers in canine testis. Anim. Reprod. Sci. 2017, 182, 1–8. [Google Scholar] [CrossRef]
- Johnston, S.D.; Root Kustritz, M.V.; Olson, P.N.S. The canine estrous cycle. In Canine and Feline Theriogenology, 1st ed.; Johnston, S.D., Root Kustritz, M.V., Olson, P.N.S., Eds.; WB Saunders Company: Philadelphia, PA, USA, 2001; pp. 16–31. [Google Scholar]
- Mialot, J.; Guerin, C.; Begon, D. Growth, Testicular Development and Sperm Output in the Dog from Birth to Post Pubertal Period. Andrologia 2009, 17, 450–460. [Google Scholar] [CrossRef]
- Ramaswamy, S.; Weinbauer, G.F. Endocrine control of spermatogenesis: Role of FSH and LH/ testosterone. Spermatogenesis 2014, 4, e996025. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.M.; Avelar, G.F.; De França, L.R. The seminiferous epithelium cycle and its duration in different breeds of dog (Canis familiaris). J. Anat. 2009, 215, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Hess, R.A.; De Franca, L.R. Spermatogenesis and Cycle of the Seminiferous Epithelium. Adv. Exp. Med. Biol. 2009, 636, 1–15. [Google Scholar] [CrossRef]
- Schlatt, S.; Ehmcke, J. Regulation of spermatogenesis: An evolutionary biologist’s perspective. Semin. Cell Dev. Biol. 2014, 29, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Kubota, H.; Brinster, R.L. Spermatogonial stem cells†. Biol. Reprod. 2018, 99, 52–74. [Google Scholar] [CrossRef] [Green Version]
- Phillips, B.T.; Gassei, K.; Orwig, K.E. Spermatogonial stem cell regulation and spermatogenesis. Philos. Trans. R. Soc. B 2010, 365, 1663–1678. [Google Scholar] [CrossRef] [Green Version]
- Fayomi, A.P.; Orwig, K.E. Spermatogonial stem cells and spermatogenesis in mice, monkeys and men. Stem Cell Res. 2018, 29, 207–214. [Google Scholar] [CrossRef]
- Chen, S.-R.; Liu, Y.-X. Regulation of spermatogonial stem cell self-renewal and spermatocyte meiosis by Sertoli cell signaling. Reproduction 2015, 149, R159–R167. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Zhang, Y.; Qu, R.; He, Y.; Tian, X.; Zeng, W. Spermatogonial stem cells from domestic animals: Progress and prospects. Reproduction 2014, 147, R65–R74. [Google Scholar] [CrossRef] [Green Version]
- Valli, H.; Phillips, B.T.; Gassei, K.; Nagano, M.C.; Orwig, K.E. Spermatogonial Stem Cells and Spermatogenesis; Elsevier: San Diego, CA, USA, 2015; pp. 595–635. [Google Scholar]
- Hayashi, K.; Lopes, S.M.C.D.S.; Surani, M.A.; Chung, H.-Y.; Weinberger, M.B.; Levine, J.B.; Kavner, A.; Yang, J.-M.; Tolbert, S.H.; Kaner, R.B. Germ Cell Specification in Mice. Science 2007, 316, 394–396. [Google Scholar] [CrossRef]
- Pieri, N.C.G.; De Souza, A.F.; Mançanares, A.C.F.; Roballo, K.; Casals, J.; Martins, D.D.S.; Ambrósio, C.E. Immunolocalization of proteins in the spermatogenesis process of canine. Reprod. Domest. Anim. 2016, 52, 170–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakata, H. Morphology of mouse seminiferous tubules. Anat. Sci. Int. 2018, 94, 1–10. [Google Scholar] [CrossRef]
- Sá, R.; Graça, I.; Silva, J.; Malheiro, I.; Carvalho, F.; Barros, A.; Sousa, M.; Graça, I. Quantitative Analysis of Cellular Proliferation and Differentiation of the Human Seminiferous Epithelium In Vitro. Reprod. Sci. 2012, 19, 1063–1074. [Google Scholar] [CrossRef]
- Amann, R.P. The cycle of the seminiferous epithelium in humans: A need to revisit? J Androl. 2008, 29, 469–487. [Google Scholar] [CrossRef] [PubMed]
- Clermont, Y. Kinetics of spermatogenesis in mammals: Seminiferous epithelium cycle and spermatogonial renewal. Physiol. Rev. 1972, 52, 198–236. [Google Scholar] [CrossRef] [PubMed]
- França, L.R.; Ogawa, T.; Avarbock, M.R.; Brinster, R.L.; Russell, L.D. Germ Cell Genotype Controls Cell Cycle during Spermatogenesis in the Rat1. Biol. Reprod. 1998, 59, 1371–1377. [Google Scholar] [CrossRef]
- França, L.R.; Avelar, G.F.; Almeida, F.F.l. Spermatogenesis and sperm transit through the epididymis in mammals with emphasis on pigs. Theriogenology 2005, 15, 300–318. [Google Scholar] [CrossRef]
- Bertocchini, F.; Lopes, S.M.C.D.S. Germline development in amniotes: A paradigm shift in primordial germ cell specification. BioEssays 2016, 38, 791–800. [Google Scholar] [CrossRef] [Green Version]
- Aguero, T.; Kassmer, S.; Alberio, R.; Johnson, A.D.; King, M.L. Mechanisms of Vertebrate Germ Cell Determination. Neurotransm. Interact. Cogn. Funct. 2016, 953, 383–440. [Google Scholar] [CrossRef]
- Ohinata, Y.; Payer, B.; O’Carroll, D.; Ancelin, K.; Ono, Y.; Sano, M.; Barton, S.C.; Obukhanych, T.; Nussenzweig, M.C.; Tarakhovsky, A.; et al. Blimp1 is a critical determinant of the germ cell lineage in mice. Nat. Cell Biol. 2005, 436, 207–213. [Google Scholar] [CrossRef]
- Yamaji, M.; Tanaka, T.; Shigeta, M.; Chuma, S.; Saga, Y.; Saitou, M. Functional reconstruction of NANOS3 expression in the germ cell lineage by a novel transgenic reporter reveals distinct subcellular localizations of NANOS3. Reproduction 2010, 139, 381–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, W.W.; Dietmann, S.; Irie, N.; Leitch, H.G.; Floros, V.I.; Bradshaw, C.R.; Hackett, J.A.; Chinnery, P.F.; Surani, M.A. A Unique Gene Regulatory Network Resets the Human Germline Epigenome for Development. Cell 2015, 161, 1453–1467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Julaton, V.T.A.; Pera, R.A.R. NANOS3 function in human germ cell development. Hum. Mol. Genet. 2011, 20, 2238–2250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magnúsdóttir, E.; Gillich, A.; Grabole, N.; Surani, M.A.; Surani, M.A. Combinatorial control of cell fate and reprogramming in the mammalian germline. Curr. Opin. Genet. Dev. 2012, 22, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Ohinata, Y.; Ohta, H.; Shigeta, M.; Yamanaka, K.; Wakayama, T.; Saitou, M. A Signaling Principle for the Specification of the Germ Cell Lineage in Mice. Cell 2009, 137, 571–584. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, K.; Kobayashi, T.; Umino, T.; Goitsuka, R.; Matsui, Y.; Kitamura, D. SMAD1 signaling is critical for initial commitment of germ cell lineage from mouse epiblast. Mech. Dev. 2002, 118, 99–109. [Google Scholar] [CrossRef]
- Arnold, S.J.; Maretto, S.; Islam, A.; Bikoff, E.K.; Robertson, E.J. Dose-dependent Smad1, Smad5 and Smad8 signaling in the early mouse embryo. Dev. Biol. 2006, 296, 104–118. [Google Scholar] [CrossRef]
- Chang, H.; Matzuk, M.M. Smad5 is required for mouse primordial germ cell development. Mech. Dev. 2001, 104, 61–67. [Google Scholar] [CrossRef]
- Martins, D.D.S.; Ambrósio, C.E.; Saraiva, N.Z.; Wenceslau, C.V.; Morini, A.C.; Kerkis, I.; Garcia, J.M.; A Miglino, M. Early development and putative primordial germ cells characterization in dogs. Reprod. Domest. Anim. 2011, 46. [Google Scholar] [CrossRef]
- Pesce, M.; Wang, X.; Wolgemuth, D.J.; Schöler, H.R. Differential expression of the Oct-4 transcription factor during mouse germ cell differentiation. Mech. Dev. 1998, 71, 89–98. [Google Scholar] [CrossRef]
- Nichols, J.; Zevnik, B.; Anastassiadis, K.; Niwa, H.; Klewe-Nebenius, D.; Chambers, I.; Schöler, H.; Smith, A. Formation of Pluripotent Stem Cells in the Mammalian Embryo Depends on the POU Transcription Factor Oct4. Cell 1998, 95, 379–391. [Google Scholar] [CrossRef] [Green Version]
- Kehler, J.; Tolkunova, E.; Koschorz, B.; Pesce, M.; Gentile, L.; Boiani, M.; Lomelí, H.; Nagy, A.; McLaughlin, K.J.; Schöler, H.R.; et al. Oct4 is required for primordial germ cell survival. EMBO Rep. 2004, 5, 1078–1083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.-J.; Shim, H.; Anderson, G. Short Communication: Lack of Stage-Specific Embryonic Antigen-1 Expression by Bovine Embryos and Primordial Germ Cells. J. Dairy Sci. 1999, 82, 516–519. [Google Scholar] [CrossRef]
- Wrobel, K.-H.; Kritzenberger, M. Histochemical in situ identification of bovine embryonic blood cells reveals differences to the adult haematopoietic system and suggests a close relationship between haematopoietic stem cells and primordial germ cells. Histochem. Cell Biol. 2004, 121, 273–289. [Google Scholar] [CrossRef] [PubMed]
- Ledda, S.; Bogliolo, L.; Bebbere, D.; Ariu, F.; Pirino, S. Characterization, isolation and culture of primordial germ cells in domestic animals: Recent progress and insights from the ovine species. Theriogenology 2010, 74, 534–543. [Google Scholar] [CrossRef]
- Bucay, N.; Yebra, M.; Cirulli, V.; Afrikanova, I.; Kaido, T.; Hayek, A.; Montgomery, A.M. A Novel Approach for the Derivation of Putative Primordial Germ Cells and Sertoli Cells from Human Embryonic Stem Cells. Stem Cells 2009, 27, 68–77. [Google Scholar] [CrossRef]
- Payer, B.; Saitou, M.; Barton, S.C.; Thresher, R.; Dixon, J.P.; Zahn, D.; Colledge, W.H.; Carlton, M.B.; Nakano, T.; Surani, M. stella Is a Maternal Effect Gene Required for Normal Early Development in Mice. Curr. Biol. 2003, 13, 2110–2117. [Google Scholar] [CrossRef] [Green Version]
- Vincent, J.J.; Li, Z.; Lee, S.A.; Liu, X.; Etter, M.O.; Diaz-Perez, S.V.; Taylor, S.K.; Gkountela, S.; Lindgren, A.G.; Clark, A. Single Cell Analysis Facilitates Staging of Blimp1-Dependent Primordial Germ Cells Derived from Mouse Embryonic Stem Cells. PLoS ONE 2011, 6, e28960. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Stojkovic, P.; Przyborski, S.; Cooke, M.; Armstrong, L.; Lako, M.; Stojkovic, M. Derivation of Human Embryonic Stem Cells from Developing and Arrested Embryos. Stem Cells 2006, 24, 2669–2676. [Google Scholar] [CrossRef]
- Lin, W.; Modiano, J.F.; Ito, D. Stage-specific embryonic antigen: Determining expression in canine glioblastoma, melanoma, and mammary cancer cells. J. Veter. Sci. 2017, 18, 101–104. [Google Scholar] [CrossRef] [Green Version]
- Whitworth, D.J.; Ovchinnikov, D.A.; Wolvetang, E.J. Generation and Characterization of LIF-dependent Canine Induced Pluripotent Stem Cells from Adult Dermal Fibroblasts. Stem Cells Dev. 2012, 21, 2288–2297. [Google Scholar] [CrossRef] [PubMed]
- Rossi, P. Transcriptional control of KIT gene expression during germ cell development. Int. J. Dev. Biol. 2013, 57, 179–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Høyer, P.E.; Byskov, A.G.; Møllgård, K. Stem cell factor and c-Kit in human primordial germ cells and fetal ovaries. Mol. Cell. Endocrinol. 2005, 234, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dolci, S.; Williams, D.E.; Ernst, M.K.; Resnick, J.L.; Brannan, C.I.; Lock, L.F.; Lyman, S.D.; Boswell, H.S.; Donovan, P.J. Requirement for mast cell growth factor for primordial germ cell survival in culture. Nature 1991, 352, 809–811. [Google Scholar] [CrossRef] [PubMed]
- Hen, G.; Friedman-Einat, M.; Sela-Donenfeld, D. Primordial germ cells in the dorsal mesentery of the chicken embryo demonstrate left–right asymmetry and polarized distribution of the EMA1 epitope. J. Anat. 2014, 224, 556–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Encinas, G.; Zogbi, C.; Stumpp, T. Detection of Four Germ Cell Markers in Rats during Testis Morphogenesis: Differences and Similarities with Mice. Cells Tissues Organs 2012, 195, 443–455. [Google Scholar] [CrossRef]
- Medrano, J.V.; Ramathal, C.; Nguyen, H.N.; Simón, C.; Pera, R.A.R. Divergent RNA-binding Proteins, DAZL and VASA, Induce Meiotic Progression in Human Germ Cells Derived in Vitro. Stem Cells 2012, 30, 441–451. [Google Scholar] [CrossRef] [Green Version]
- Nicholls, P.K.; Schorle, H.; Naqvi, S.; Hu, Y.-C.; Fan, Y.; Carmell, M.A.; Dobrinski, I.; Watson, A.L.; Carlson, D.F.; Fahrenkrug, S.C.; et al. Mammalian germ cells are determined after PGC colonization of the nascent gonad. Proc. Natl. Acad. Sci. USA 2019, 116, 25677–25687. [Google Scholar] [CrossRef]
- Kee, K.; Angeles, V.T.; Flores, M.; Nguyen, H.N.; Pera, R.A.R. Human DAZL, DAZ and BOULE genes modulate primordial germ-cell and haploid gamete formation. Nat. Cell Biol. 2009, 462, 222–225. [Google Scholar] [CrossRef]
- Chen, H.-H.; Welling, M.; Bloch, D.B.; Muñoz, J.; Mientjes, E.; Chen, X.; Tramp, C.; Wu, J.; Yabuuchi, A.; Chou, Y.-F.; et al. DAZL Limits Pluripotency, Differentiation, and Apoptosis in Developing Primordial Germ Cells. Stem Cell Rep. 2014, 3, 892–904. [Google Scholar] [CrossRef] [Green Version]
- Hummitzsch, K.; Irving-Rodgers, H.F.; Hatzirodos, N.; Bonner, W.; Sabatier, L.; Reinhardt, D.P.; Sado, Y.; Ninomiya, Y.; Wilhelm, D.; Rodgers, R.J. A New Model of Development of the Mammalian Ovary and Follicles. PLoS ONE 2013, 8, e55578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, R.A.; Fulton, N.; Cowan, G.; Coutts, S.; Saunders, P.T.K. Conserved and divergent patterns of expression of DAZL, VASA and OCT4 in the germ cells of the human fetal ovary and testis. BMC Dev. Biol. 2007, 7, 136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Barros, F.R.; Worst, R.; Saurin, G.; Mendes, C.; Assumpção, M.E.O.D.; Visintin, J. α-6 Integrin Expression in Bovine Spermatogonial Cells Purified by Discontinuous Percoll Density Gradient. Reprod. Domest. Anim. 2012, 47, 887–890. [Google Scholar] [CrossRef] [PubMed]
- Costoya, J.A.; Hobbs, R.M.; Barna, M.; Cattoretti, G.; Manova, K.; Sukhwani, M.; Orwig, K.E.; Wolgemuth, D.J.; Pandolfi, P.P. Essential role of Plzf in maintenance of spermatogonial stem cells. Nat. Genet. 2004, 36, 653–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azizi, H.; Niazi, T.A.; Skutella, T.; Govahi, M. In Vitro and In Vivo Determinations of The Anti-GDNF Family Receptor Alpha 1 Antibody in Mice by Immunochemistry and RT-PCR. Int. J. Fertil. Steril. 2020, 14, 228–233. [Google Scholar] [CrossRef]
- Lee, K.H.; Lee, R.; Lee, W.Y.; Kim, D.H.; Chung, H.J.; Kim, J.-H.; Kim, N.H.; Choi, S.H.; Kim, J.H.; Song, H. Identification and In Vitro Derivation of Spermatogonia in Beagle Testis. PLoS ONE 2014, 9, e109963. [Google Scholar] [CrossRef]
- Harkey, M.A.; Asano, A.; Zoulas, M.E.; Torok-Storb, B.; Nagashima, J.; Travis, A. Isolation, genetic manipulation, and transplantation of canine spermatogonial stem cells: Progress toward transgenesis through the male germ-line. Reproduction 2013, 146, 75–90. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.Y.; Wong, E.W.; Yan, H.H.N.; Mruk, D.D. Regulation of spermatogenesis in the microenvironment of the seminiferous epithelium: New insights and advances. Mol. Cell. Endocrinol. 2010, 315, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Oatley, J.M.; Brinster, R.L. The Germline Stem Cell Niche Unit in Mammalian Testes. Physiol. Rev. 2012, 92, 577–595. [Google Scholar] [CrossRef] [Green Version]
- Park, M.H.; Kim, M.S.; Yun, J.I.; Choi, J.H.; Lee, E.; Lee, S.T. Integrin Heterodimers Expressed on the Surface of Porcine Spermatogonial Stem Cells. DNA Cell Biol. 2018, 37, 253–263. [Google Scholar] [CrossRef]
- Naughton, C.K.; Jain, S.; Strickland, A.M.; Gupta, A.; Milbrandt, J. Glial Cell-Line Derived Neurotrophic Factor-Mediated RET Signaling Regulates Spermatogonial Stem Cell Fate1. Biol. Reprod. 2006, 74, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Grasso, M.; Fuso, A.; Dovere, L.; De Rooij, D.G.; Stefanini, M.; Boitani, C.; Vicini, E. Distribution of GFRA1-expressing spermatogonia in adult mouse testis. Reprodution 2012, 143, 325–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, R.C.; Costa, G.M.J.; Lacerda, S.M.S.N.; Batlouni, S.R.; Soares, J.M.; Avelar, G.F.; Böttger, K.B.; Silva, S.F.; Nogueira, M.S.; Andrade, L.M.; et al. Germ Cell Transplantation in Felids: A Potential Approach to Preserving Endangered Species. J. Androl. 2011, 33, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Tiptanavattana, N.; Thongkittidilok, C.; Techakumphu, M.; Tharasanit, T. Characterization and In Vitro Culture of Putative Spermatogonial Stem Cells Derived from Feline Testicular Tissue. J. Reprod. Dev. 2013, 59, 189–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayes, B.; Fagerlie, S.R.; Ramakrishnan, A.; Baran, S.; Harkey, M.; Graf, L.; Bar, M.; Bendoraite, A.; Tewari, M.; Torok-Storb, B. Derivation, Characterization, and In Vitro Differentiation of Canine Embryonic Stem Cells. Stem Cells 2008, 26, 465–473. [Google Scholar] [CrossRef]
- Bedford-Guaus, S.; Kim, S.; Mulero, L.; Vaquero, J.; Morera, C.; Adan-Milanès, R.; Veiga, A.; Raya, A. Molecular markers of putative spermatogonial stem cells in the domestic cat. Reprod. Domest. Anim. 2016, 52, 177–186. [Google Scholar] [CrossRef] [Green Version]
- Baltus, A.E.; Menke, D.B.; Hu, Y.-C.; Goodheart, M.L.; Carpenter, A.E.; De Rooij, D.G.; Page, D.C. In germ cells of mouse embryonic ovaries, the decision to enter meiosis precedes premeiotic DNA replication. Nat. Genet. 2006, 38, 1430–1434. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, Y.; Nie, R.; Friel, P.; Mitchell, D.; Evanoff, R.M.; Pouchnik, D.; Banasik, B.; McCarrey, J.R.; Small, C.; et al. Expression of Stimulated by Retinoic Acid Gene 8 (Stra8) and Maturation of Murine Gonocytes and Spermatogonia Induced by Retinoic Acid In Vitro. Biol. Reprod. 2008, 78, 537–545. [Google Scholar] [CrossRef]
- Feng, C.W.; Bowles, J.; Koopman, P. Control of mammalian germ cell entry into meiosis. Mol. Cell. Endocrinol. 2014, 382, 488–497. [Google Scholar] [CrossRef]
- Endo, T.; Romer, K.A.; Anderson, E.L.; Baltus, A.E.; De Rooij, D.G.; Page, D.C. Periodic retinoic acid–STRA8 signaling intersects with periodic germ-cell competencies to regulate spermatogenesis. Proc. Natl. Acad. Sci. USA 2015, 112, E2347–E2356. [Google Scholar] [CrossRef] [Green Version]
- Collier, B.; Gorgoni, B.; Loveridge, C.; Cooke, H.J.; Gray, N.K. The DAZL family proteins are PABP-binding proteins that regulate translation in germ cells. EMBO J. 2005, 24, 2656–2666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Z.; Kokkinaki, M.; Jiang, J.; Dobrinski, I.; Dym, M. Isolation, Characterization, and Culture of Human Spermatogonia1. Biol. Reprod. 2010, 82, 363–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seandel, M.; James, D.; Shmelkov, S.V.; Falciatori, I.; Kim, J.; Chavala, S.; Scherr, D.S.; Zhang, F.; Torres, R.; Gale, N.W.; et al. Generation of functional multipotent adult stem cells from GPR125+ germline progenitors. Nat. Cell Biol. 2007, 449, 346–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grieco, V.; Banco, B.; Giudice, C.; Mosca, F.; Finazzi, M. Immunohistochemical Expression of the KIT Protein (CD117) in Normal and Neoplastic Canine Testes. J. Comp. Pathol. 2010, 142, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Schrans-Stassen, B.H.G.J.; Van De Kant, H.J.G.; De Rooij, D.G.; Van Pelt, A.M.M. Differential Expression of c-kit in Mouse Undifferentiated and Differentiating Type A Spermatogonia. Endocrinology 1999, 140, 5894–5900. [Google Scholar] [CrossRef]
- Filipponi, D.; Hobbs, R.M.; Ottolenghi, S.; Rossi, P.; Jannini, E.A.; Pandolfi, P.P.; Dolci, S. Repression of kit Expression by Plzf in Germ Cells. Mol. Cell. Biol. 2007, 27, 6770–6781. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Tang, J.; Haines, C.J.; Feng, H.; Lai, L.; Teng, X.; Han, Y. c-kit expression profile and regulatory factors during spermatogonial stem cell differentiation. BMC Dev. Biol. 2013, 13, 38. [Google Scholar] [CrossRef] [Green Version]
- Kwon, J.; Wang, Y.-L.; Setsuie, R.; Sekiguchi, S.; Sakurai, M.; Sato, Y.; Lee, W.-W.; Ishii, Y.; Kyuwa, S.; Noda, M.; et al. Developmental Regulation of Ubiquitin C-Terminal Hydrolase Isozyme Expression During Spermatogenesis in Mice. Biol. Reprod. 2004, 71, 515–521. [Google Scholar] [CrossRef]
- Sutovsky, P. Ubiquitin-dependent proteolysis in mammalian spermatogenesis, fertilization, and sperm quality control: Killing three birds with one stone. Microsc. Res. Tech. 2003, 61, 88–102. [Google Scholar] [CrossRef]
- Ivanova, N.; Dobrin, R.; Lu, R.; Kotenko, I.; Levorse, J.; Decoste, C.; Schafer, X.; Lun, Y.; Lemischka, I.R. Dissecting self-renewal in stem cells with RNA interference. Nat. Cell Biol. 2006, 442, 533–538. [Google Scholar] [CrossRef]
- Bhartiya, D.; Kasiviswanathan, S.; Unni, S.K.; Pethe, P.; Dhabalia, J.V.; Patwardhan, S.; Tongaonkar, H.B. Newer Insights Into Premeiotic Development of Germ Cells in Adult Human Testis Using Oct-4 as a Stem Cell Marker. J. Histochem. Cytochem. 2010, 58, 1093–1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izadyar, F.; Wong, J.; Maki, C.; Pacchiarotti, J.; Ramos, T.; Howerton, K.; Yuen, C.; Greilach, S.; Zhao, H.H.; Chow, M.; et al. Identification and characterization of repopulating spermatogonial stem cells from the adult human testis. Hum. Reprod. 2011, 26, 1296–1306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Kim, H.K.; Rho, J.-Y.; Han, Y.-M.; Kim, J. The Human OCT-4 Isoforms Differ in Their Ability to Confer Self-renewal. J. Biol. Chem. 2006, 281, 33554–33565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuijk, E.; De Gier, J.; Lopes, S.M.C.D.S.; Chambers, I.; Van Pelt, A.M.M.; Colenbrander, B.; Roelen, B.A.J. A Distinct Expression Pattern in Mammalian Testes Indicates a Conserved Role for NANOG in Spermatogenesis. PLoS ONE 2010, 5, e10987. [Google Scholar] [CrossRef] [PubMed]
- Nagamatsu, G.; Kosaka, T.; Saito, S.; Takubo, K.; Akiyama, H.; Sudo, T.; Horimoto, K.; Oya, M.; Suda, T. Tracing the Conversion Process from Primordial Germ Cells to Pluripotent Stem Cells in Mice1. Biol. Reprod. 2012, 86, 182. [Google Scholar] [CrossRef] [PubMed]
- Turnpenny, L.; Spalluto, C.M.; Perrett, R.M.; O’Shea, M.; Hanley, K.P.; Cameron, I.T.; Wilson, D.I.; Hanley, N.A. Evaluating Human Embryonic Germ Cells: Concord and Conflict as Pluripotent Stem Cells. Stem Cells 2006, 24, 212–220. [Google Scholar] [CrossRef]
- Resnick, J.L.; Ortiz, M.; Keller, J.R.; Donovan, P.J. Role of Fibroblast Growth Factors and Their Receptors in Mouse Primordial Germ Cell Growth1. Biol. Reprod. 1998, 59, 1224–1229. [Google Scholar] [CrossRef] [Green Version]
- Durcova-Hills, G.; Surani, A. Reprogramming Primordial Germ Cells (PGC) to Embryonic Germ (EG) Cells. Curr. Protoc. Stem Cell Biol. 2008, 5, 1A.3.1–1A.3.20. [Google Scholar] [CrossRef]
- Cong, Y.; Ma, J.; Sun, R.; Wang, J.; Xue, B.; Wang, J.; Xie, B.; Wang, J.; Hu, K.; Liu, Z. Derivation of Putative Porcine Embryonic Germ Cells and Analysis of Their Multi-Lineage Differentiation Potential. J. Genet. Genom. 2013, 40, 453–464. [Google Scholar] [CrossRef]
- Kakegawa, R.; Teramura, T.; Takehara, T.; Anzai, M.; Mitani, T.; Matsumoto, K.; Saeki, K.; Sagawa, N.; Fukuda, K.; Hosoi, Y. Isolation and culture of rabbit primordial germ cells. J. Reprod. Dev. 2008, 54, 352–357. [Google Scholar] [CrossRef] [Green Version]
- Huang, B.; Xie, T.S.; Shi, D.S.; Li, T.; Wang, X.L.; Mo, Y.; Wang, Z.Q.; Li, M.M. Isolation and characterization of EG-like cells from Chinese swamp buffalo (Bubalus bubalis). Cell Biol. Int. 2007, 31, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Leitch, H.G.; Blair, K.; Mansfield, W.; Ayetey, H.; Humphreys, P.; Nichols, J.; Surani, M.A.; Smith, A. Embryonic germ cells from mice and rats exhibit properties consistent with a generic pluripotent ground state. Development 2010, 137, 2279–2287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, W.; Yang, W.; Lei, A.; Gao, Z.; Yang, C.; Hua, J.; Huang, W.; Ma, X.; Wang, H.L.H.; Dou, Z. A caprine chimera produced by injection of embryonic germ cells into a blastocyst. Theriogenology 2008, 69, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Ohta, H.; Kurimoto, K.; Aramaki, S.; Saitou, M. Reconstitution of the Mouse Germ Cell Specification Pathway in Culture by Pluripotent Stem Cells. Cell 2011, 146, 519–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irie, N.; Weinberger, L.; Tang, W.W.; Kobayashi, T.; Viukov, S.; Manor, Y.S.; Dietmann, S.; Hanna, J.H.; Surani, M.A. SOX17 Is a Critical Specifier of Human Primordial Germ Cell Fate. Cell 2015, 160, 253–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasaki, K.; Yokobayashi, S.; Nakamura, T.; Okamoto, I.; Yabuta, Y.; Kurimoto, K.; Ohta, H.; Moritoki, Y.; Iwatani, C.; Tsuchiya, H.; et al. Robust In Vitro Induction of Human Germ Cell Fate from Pluripotent Stem Cells. Cell Stem Cell 2015, 17, 178–194. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Wei, Y.; Sun, H.-X.; Mahdi, A.K.; Arteaga, C.A.P.; Sakurai, M.; Schmitz, D.A.; Zheng, C.; Ballard, E.D.; Li, J.; et al. Derivation of Intermediate Pluripotent Stem Cells Amenable to Primordial Germ Cell Specification. Cell Stem Cell 2020. [Google Scholar] [CrossRef]
- Betts, D.H.; Tobias, I.C. Canine Pluripotent Stem Cells: Are They Ready for Clinical Applications? Front. Veter. Sci. 2015, 2, 41. [Google Scholar] [CrossRef] [Green Version]
- Fang, J.; Wei, Y.; Lv, C.; Peng, S.; Zhao, S.; Hua, J. CD61 promotes the differentiation of canine ADMSCs into PGC-like cells through modulation of TGF-β signaling. Sci. Rep. 2017, 7, srep43851. [Google Scholar] [CrossRef] [Green Version]
- Creemers, L.B.; Ouden, K.D.; Van Pelt, A.M.M.; De Rooij, D.G. Maintenance of adult mouse type A spermatogonia in vitro: Influence of serum and growth factors and comparison with prepubertal spermatogonial cell culture. Reproduction 2002, 124, 791–799. [Google Scholar] [CrossRef]
- Huleihel, M.; Abuelhija, M.; Lunenfeld, E. In vitroculture of testicular germ cells: Regulatory factors and limitations. Growth Factors 2007, 25, 236–252. [Google Scholar] [CrossRef] [PubMed]
- Ibtisham, F.; Awang-Junaidi, A.H.; Honaramooz, A. The study and manipulation of spermatogonial stem cells using animal models. Cell Tissue Res. 2020, 380, 393–414. [Google Scholar] [CrossRef] [PubMed]
- Aponte, P.M. Spermatogonial stem cells: Current biotechnological advances in reproduction and regenerative medicine. World J. Stem Cells 2015, 7, 669–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Classification | Genes | 3–5 Weeks | 5–8 Weeks | 9–10 Weeks (Female) | 9–10 Weeks (Males) |
|---|---|---|---|---|---|
| PLURIPOTENT | POU5F1 | + | + | + | + |
| NANOG | + | + | + | + | |
| PRMD14 | + | − | − | − | |
| GERM CELLS (INITIAL) | BLIMP1 | + | − | − | − |
| CD38 | − | + | − | − | |
| NANOS3 | − | + | − | − | |
| GERM CELLS (LATES) | DDX4 | − | + | + | + |
| DAZL | − | − | − | + | |
| SCP3 | − | − | + | − | |
| ENDODERMIC | SOX17 | + | − | − | − |
| MESODERMIC | T | + | − | − | − |
| CKIT | − | + | + | − |
| Classification | Genes | E 3.5 | E 5–6.0 | E 7.25–7.5 | E 10.5 | E 14.5 Female | E 14.5 Male |
|---|---|---|---|---|---|---|---|
| PLURIPOTENT | POU5F1 | + | + | + | + | − | + |
| NANOG | + | − | + | + | + | + | |
| SOX2 | + | + | + | + | + | + | |
| GERM CELLS (INITIAL) | BLIMP1 | − | − | + | + | − | − |
| FRAGILIS | + | + | + | + | + | ? | |
| NANOS3 | -- | − | + | + | ? | ? | |
| GERM CELLS (LATES) | DDX4 | − | + | + | + | +/− | |
| DAZL | − | − | − | − | + | + | |
| SCP3 | − | − | − | − | + | + | |
| MESODERMIC | T | − | +/− | + | − | − | − |
| Classification | Genes | Bovine | Ovine | Porcine | Rabbit | Canine |
|---|---|---|---|---|---|---|
| PLURIPOTENT | POU5F1 | + | + | + | ? | + |
| NANOG | ? | + | + | ? | + | |
| SOX2 | ? | + | ? | ? | ? | |
| STELLA | ? | ? | ? | ? | + | |
| GERM CELLS | PG 2 | ? | ? | ? | + | ? |
| CKIT | + | + | ? | ? | ? | |
| SSEA1 | + | + | + | ? | ? | |
| SSEA4 | ? | + | ? | ? | ? | |
| EMA1 | ? | ? | + | ? | ? | |
| DDX4 | ? | ? | + | ? | + | |
| DAZL | ? | ? | ? | ? | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza, A.F.; Pieri, N.C.G.; Martins, D.d.S. Step by Step about Germ Cells Development in Canine. Animals 2021, 11, 598. https://doi.org/10.3390/ani11030598
de Souza AF, Pieri NCG, Martins DdS. Step by Step about Germ Cells Development in Canine. Animals. 2021; 11(3):598. https://doi.org/10.3390/ani11030598
Chicago/Turabian Stylede Souza, Aline Fernanda, Naira Caroline Godoy Pieri, and Daniele dos Santos Martins. 2021. "Step by Step about Germ Cells Development in Canine" Animals 11, no. 3: 598. https://doi.org/10.3390/ani11030598
APA Stylede Souza, A. F., Pieri, N. C. G., & Martins, D. d. S. (2021). Step by Step about Germ Cells Development in Canine. Animals, 11(3), 598. https://doi.org/10.3390/ani11030598







