Synthesis and Potent Antimicrobial Activity of Some Novel N-(Alkyl)-2-Phenyl-1H-Benzimidazole-5-Carboxamidines
Abstract
:Introduction
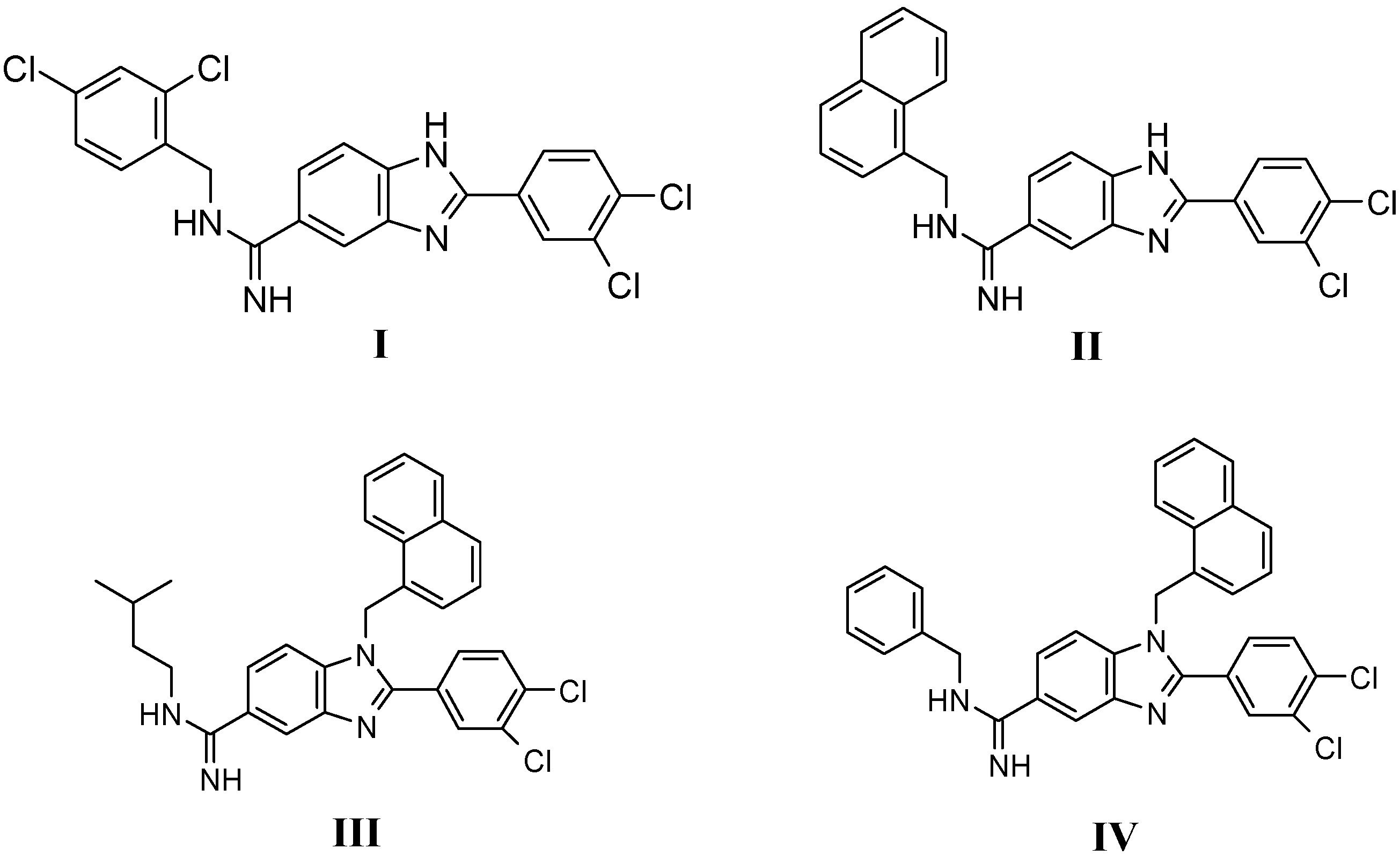
Results and Discussion
Chemistry
| 40 | Cl | >50 | >50 | >50 | >50 | >50 | >50 | ||||
| 41 | CH(CH3)2 | Cl | Cl | >50 | >50 | >50 | >50 | >50 | >50 | ||
| 42 | CH(CH3)2 | F | >50 | >50 | >50 | >50 | >50 | >50 | |||
| 43 | (Et)2NCH2CH2 | F | >50 | >50 | >50 | >50 | >50 | >50 | |||
| 44 | (Me)2NCH2CH2 | Cl | >50 | >50 | >50 | >50 | >50 | >50 | |||
| 45 | (Me)2NCH2CH2 | Cl | Cl | 12.5 | 12.5 | 12.5 | >50 | 50 | 12.5 | ||
| 46 | CH(CH3)2 | CH3 | CN | >50 | >50 | >50 | >50 | >50 | >50 | ||
| 47 | CH(CH3)2 | CH3 | OCH3 | OCH3 | >50 | >50 | >50 | >50 | >50 | >50 | |
| 48 | Cyclopropyl | COOCH3 | >50 | >50 | >50 | >50 | >50 | >50 | |||
| 49 | PhCH2 | COOH | >50 | >50 | >50 | >50 | >50 | >50 | |||
| 50 | PhCH2 | COOCH3 | 12.5 | 25 | 3.12 | >50 | 50 | 25 | |||
| 51 | Cyclohexyl | COOH | >50 | >50 | >50 | >50 | >50 | >50 | |||
| 52 | n-butyl | >50 | >50 | >50 | >50 | >50 | >50 | ||||
| 53 | (Me)2NCH2CH2 | n-butyl | F | >50 | >50 | >50 | >50 | >50 | >50 | ||
| 54 | CH(CH3)2 | CH(CH3)2 | F | >50 | >50 | >50 | >50 | >50 | >50 | ||
| 55 | (Me)2NCH2CH2 | Ph | Cl | Cl | 12.5 | 3.12 | 12.5 | >50 | 25 | 12.5 | |
| 56 | (Me)2NCH2CH2 | PhCH2 | Cl | Cl | 12.5 | 12.5 | 12.5 | >50 | 25 | 12.5 | |
| 57 | (Et)2NCH2CH2 | PhCH2 | Cl | Cl | 12.5 | 12.5 | 6.25 | 50 | 12.5 | 12.5 | |
| 58 | (Me)2NCH2CH2 | PhCH2 | Cl | Cl | 50 | 12.5 | 12.5 | >50 | 50 | 25 | |
| 59 | (Et)2NCH2CH2 | 2,4-di-Cl- benzyl | Cl | Cl | 3.12 | 3.12 | 3.12 | 12.5 | 6.25 | 3.12 | |
| 60 | (Et)2NCH2CH2 | PhCH2CH2 | OCH3 | OCH3 | >50 | >50 | >50 | >50 | >50 | >50 | |
| 61 | Isobutyl | PhCH2CH2 | Cl | Cl | 6.25 | 6.25 | 6.25 | >50 | 12.5 | 12.5 | |
| Ref | OH | t-butyl | 0.78 | 0.78 | 0.78 | ||||||
| Sult | 0.39 | 25 | 25 | 1.56 | |||||||
| Amp | 0.78 | 50 | 0.78 | ||||||||
| Cip | 0.39 | ||||||||||
| Flu | 1.56 |
Antimicrobial Activity
Conclusions
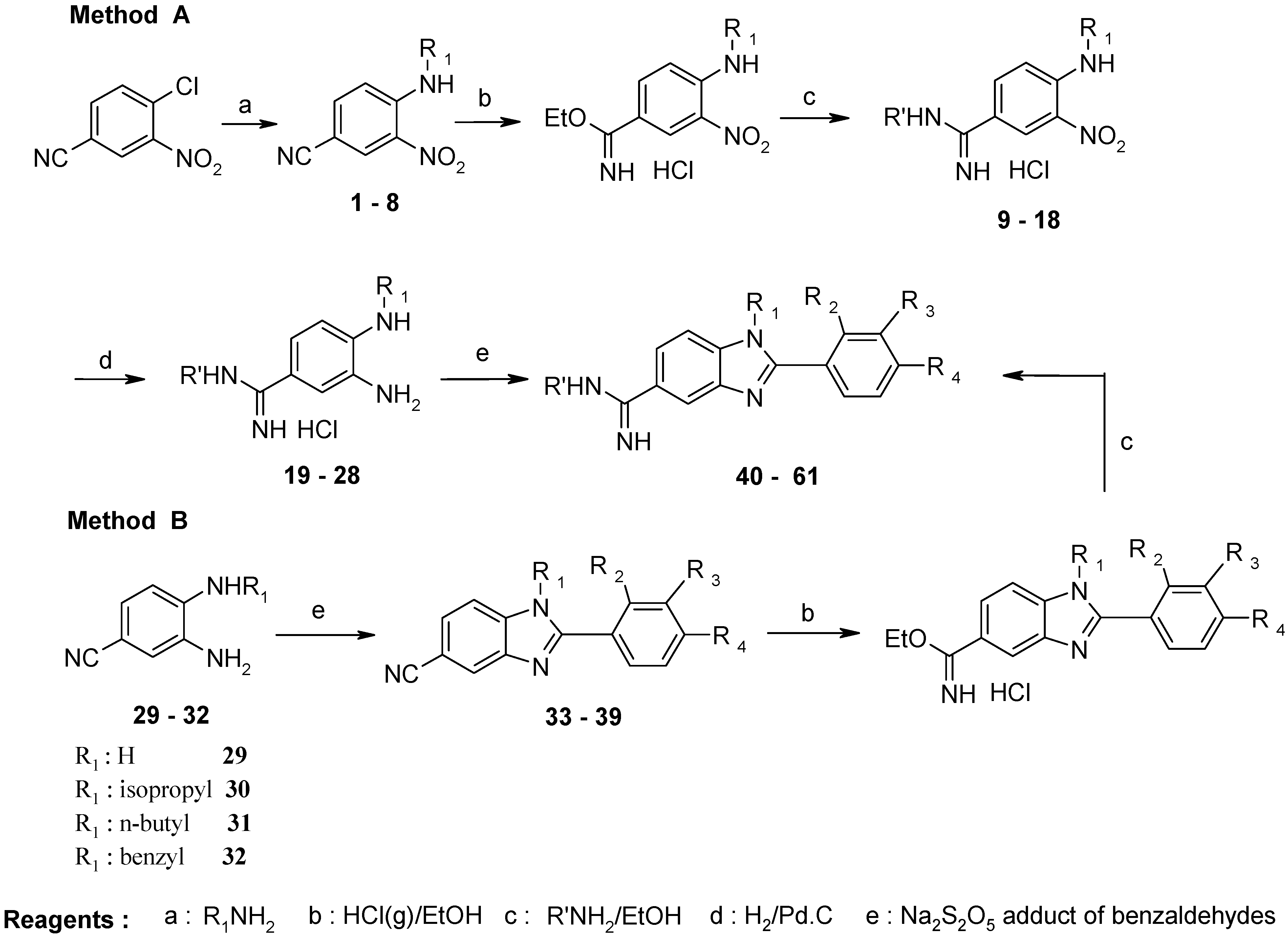
Experimental
General
| No | Mp (OC) | Yield (%) | Formula Calculated Found | 1H-NMR δ ppm (DMSO-d6) (if not stated otherwise) | MS (ESI+) m/z | Synthesis Method and Isolation Column Chromatography if not stated otherwise | |
| 40 | >300 | 35 | C14H11ClN4 . 2HCl . H2O C: 46.50 H: 4.18 N: 15.5 C: 46.24 H: 3.99 N: 15.3 | 7.59 - 7.97 (arom. 7H), 8.31 (s), 9.30 (s), 9.53 (s) | 271 (100) 273 (33) | (B) Crys. Ethanolic HCl | |
| 41 | >290 | 40 | C17H16Cl2N4. 2HCl . 1.5H2O C: 45.66 H: 4.73 N: 12.5 C: 45.67 H: 4.45 N: 12.4 | 1.21 (d, 6H), 4.03 (m, 1H), 7.6 (m, 2H), 7.8(d, J=8.5, 1H), 7.83 (d, J=2, 1H), 7.92 (d, J=8.4, 1H), 8.05 (d, J=1.5, 1H), 9.06 (s), 9.42 (s), 9.54 (d) | 347 (100) 349 (65) 351 (11) | (B) CH2Cl2 : Isopropanol : NH3 (100 : 60 : 4) | |
| 42 | >300 | 28 | C17H17FN4. 2HCl . 1.5H2O C: 51.52 H: 5.59 N: 14.1 C: 51.90 H: 5.31 N: 14.1 | 1.29 (d, 6H), 4.08 (m, 1H), 7.51 (t, 2H), 7.67 (d, J=8.3, 1H), 7.87 (d, J=8.4, 1H), 8.08 (s, 1H), 8.44 (m, 2H), 9.08 (s), 9.48 (s), 9.62 (d) | 297 (100) | (B) CH2Cl2 : Isopropanol : NH3 (100 : 60 : 3) | |
| 43 | >300 | 41 | C20H24FN5. 3HCl . 2H2O C: 48.15 H: 6.26 N: 14.1 C: 48.01 H: 6.02 N: 13.99 | 1.29 (t, 6H), 3.26 (4H), 3.46 (2H), 4.00 (2H), 7.56 (t, 2H), 7.84 (d, J=8.4, 1H), 7.92 (d, J=8.4, 1H), 8.28 (s, 1H), 8.52 (m, 2H), 9.67 (s), 9.93 (s), 10.21 (1H), 10.96 (s) | 354 (100) | (B) CH2Cl2 : Isopropanol : NH3 (100 : 60 : 3) | |
| 44 | 100-110 bubb | 37 | *
C18H20ClN5 . 4HCl . 1.5H2O. 0.5C2H6O C: 42.27 H: 5.97 N: 12.97 C: 42.54 H: 6.04 N: 13.01 | (CD3OD): 3.05 (6H), 3.66 (t, 2H), 4.03 (t, 2H), 7.69 (m, 1H), 7.82 (m, 2H), 7.99 (d, 1H), 8.102 (s, 2H), 8.54 (s, 1H) | 342 (100) 344 (33) | (B) CH2Cl2 : Isopropanol : NH3 (100 : 60 : 3) | |
| 45 | 95-100 bubb | 18 | C18H19Cl2N5. 1.5HCl 0.25H2O . 0.25C3H8O C: 49.99 H: 5.14 N: 15.54 C: 49.97 H: 5.14 N: 15.23 | 2.23 (s, 6H), 2.58 (t, 2H), 3.53 (t, 2H), 7.56 (dd, J=8.4, 1.6, 1H), 7.80 (d, J=8.8, 1H), 7.86 (d, J=8.4, 1H), 8.06 (s, 1H), 8.25 (dd, J= 8.4 1.8, 1H), 8.50 (d, J=1.8, 1H) | 376 (100) 378 (69) 380 (11) | (A) CH2Cl2 : Isopropanol : Ethylamine (100 : 50 : 3) | |
| 46 | 200-210 bubb | 30 | C19H19N5 . 1.5H2O . 0.1C3H8O C: 66.15 H: 6.55 N: 19.98 C: 66.19 H: 5.97 N: 19.82 | 1.23 (d, 6H), 3.95 (m, 1H), 3.97 (s, 3H), 7.76 (m, 2H), 8.09 (m, 5H) | 318 (100) | (A) CH2Cl2 : Isopropanol : Propylamine(100 : 50 : 4) | |
| 47 | 100-110 bubb | 26 | C20H24N4O2 . 1.8H2O C: 62.42 H: 7.22 N: 14.55 C: 62.62 H: 6.94 N: 14.21 | (DMSO-d6 + 1 drop D2O): 1.17 (d, 6H), 3.85-3.90 (10H), 7.15 (d, J=8.4, 1H), 7.4 (s, 2H), 7.62 (m, 2H), 7.97 (s, 1H) | 353 (100) | (A) CH2Cl2 : Isopropanol : Propylamine (100 : 50 : 3) | |
| 48 | 285-290 bubb | 60 | C19H18N4O2 . 3H2O . 0.3C3H8O C: 58.80 H: 6.54 N: 13.78 C: 58.72 H: 6.20 N: 13.36 | 0.77 (2H), 0.91 (d, 2H), 2.79 (1H), 3.89 (s, 3H), 7.53 (d, J=8, 1H), 7.67 (d, J=8.4, 1H), 8.04 (s, 1H), 8.09 (d, J=7.8, 2H), 8.38 (d, J=8, 2H) | 335 (100) | (A) Cryst. Isopropanol | |
| 49 | 260-270 bubb | 34 | C22H18N4O2 . 2H2O C: 65.01 H: 5.45 N: 13.77 C: 64.95 H: 5.23 N: 13.78 | 4.73 (s, 2H), 7.35-8.30 (aromat. 12H) | 371 (100) | (A) Cryst. EtOH | |
| 50 | 265-275 bubb | 52 | C23H20N4O2 . 0.25H2O C: 71.03 H: 5.31 N: 14.40 C: 71.07 H: 5.14 N: 14.32 | 3.88 (s, 3H), 4.53 (s, 2H), 7.27-7.56 (m, 7H), 8.03-8.06 (m, 3H), 8.37 (d, J=8.4, 2H), | 385 (100) | (A) CH2Cl2 : Isopropanol : Propylamine (100 : 100 : 6) | |
| 51 | >300 | 64 | C21H22N4O2 . 2HCl . 1.5H2O C: 54.55 H: 5.88 N: 12.12 C: 54.26 H: 5.89 N: 12.62 | 1.21-1.42-1.63-1.77-1.98 (m, 10H), 3.78 (1H), 7.65 (d, J=8.4, 1H), 7.87 (d, J=8.4, 1H), 8.1 (s, 1H), 8.15 (d, J=8, 2H), 8.47 (d, J=7.6, 2H), 9.19 (s), 9.50 (s), 9.63 (d) | 363 (100) | (A) Cryst. Ethanolic HCl | |
| 52 | 150-155 bubb | 22 | C18H20N4. 2HCl . 2.2H2O 0.5C3H8O C: 53.84 H: 7.04 N: 12.88 C: 53.98 H: *** N: 12.81 | (Base) : 0.64 (t, 3H), 1.01 (m, 2H), 1.56 (m, 2H), 4.33 (t, 2H), 7.56 (m, 3H), 7.76 (m, 3H), 7.95 (d, J=8.6, 1H), 8.23 (d, J=1.4, 1H), 9.18 (s), 9.41 (s) | 293 (100) | (A) CH2Cl2 : Isopropanol : NH3 (100 : 50 : 4) | |
| 53 | 205-210 bubb | 32 | C22H28FN5 . 3HCl . 2H2O C: 50.14 H: 6.69 N: 13.29 C: 50.34 H: 6.52 N: 12.92 | 0.78 (t, 3H), 1.20 (m, 2H), 1.70 (m, 2H), 2.90 (s, 6H), 3.51 (t, 2H), 4.03 (t, 2H), 4.45 (t, 2H), 7.55 (m, 2H), 8.01 (m, 3H), 8.10 (d, J=8.6, 1H), 8.42 (d, J=1.3, 1H), 9.77 (s), 9.97 (s), 10.28 (s) | 382 (100) | (B) CH2Cl2 : Isopropanol : Ethylamine (100 : 50 : 3) | |
| 54 | 115-120 bubb | 20 | C20H23FN4 . 3HCl . 1.25H2O C: 51.07 H: 6.11 N: 11.91 C: 51.05 H: 6.31 N: 11.55 | 1.34 (d, 6H), 1.66 (d, 6H), 4.2 (m, 1H), 4.8 (m, 1H), 7.54 (m, 2H), 7.74 (d, J=8.7, 1H), 7.86 (m, 2H), 8.22 (2H), 9.21 (s), 9.58 (s), 9.68 (d) | 339 (100) | (B) CH2Cl2 : Isopropanol : Ethylamine (100 : 50 : 3) | |
| 55 | 295-300 bubb | 30 | C24H23Cl2N5 . 3HCl . 1.25H2O C: 49.34 H: 4.91 N: 11.98 C: 49.45 H: *** N: 11.87 | 2.85 (s, 6H), 3.44 (t, 2H), 3.92 (t, 2H), 7.37-7.80 (m, 10H), 8.41 (d, J=1.2, 1H), 9.52 (s), 9.83 (s), 10.07 (s), 10.81 (s) | 452 (100) 454 (68) 456 (12) | (A) CH2Cl2 : Isopropanol : NH3 (100 : 50 : 15) | |
| 56 | 130-135 bubb | 49 | C25H25Cl2N5 . 3HCl . 1.25H2O C: 50.18 H: 5.14 N: 11.7 C: 50.06 H: 5.22 N: 11.49 | 2.90 (6H), 3.50 (t, 2H), 4.03 (q, 2H), 5.74 (s, 2H), 7.04 (m, 2H),7.34 (m, 3H), 7.82 (m, 4H), 8.02 (d, J=1.9, 1H), 8.42 (d, J=1.4, 1H), 9.71 (s), 9.86 (s), 10.15 (d), 11.14 (s) | 466 (100) 468 (68) 470 (11) | (B) CH2Cl2 : Isopropanol : Ethylamine (100 : 50 : 5) | |
| 57 | 120-130 bubb | 46 | C27H29Cl2N5 . 3HCl . 2H2O C: 50.68 H: 5.67 N: 10.94 C: 50.66 H: 5.62 N: 10.92 | 1.27 (t, 6H), 3.24 (4H), 3.45 (2H), 3.99 (2H), 5.72 (s, 2H), 6.98 (m, 2H), 7.27 (m, 3H), 7.81 (m, 4H), 8.01 (d, J=2, 1H), 8.35 (s, 1H), 9.61 (s), 9.81 (s), 10.11 (s), 10.97 (s) | 494 (100) 496 (68) 498 (12) | (B) CH2Cl2 : Isopropanol : Ethylamine (100 : 50 : 3) | |
| 58 | ** | 33 | ** C25H25Cl2N5 . HCl | (CD3OD): 3.29 (6H), 3.63 (t, 2H), 3.99 (t, 2H), 5.57 (s, 2H), 7.03 (m, 2H), 7.26 (m, 3H), 7.61 (dd, J=8.4 , 2, 1H), 7.68 (d, J=8.1, 1H), 7.82 (d, J=2, 1H), 7.95 (m, 2H), 8.42 (s, 1H) | 466 (100) 468 (71) 470 (13) | (B) CH2Cl2 : Isopropanol : NH3 (100 : 50 : 0.5) | |
| 59 | 120-125 bubb | 35 | C27H27Cl4N5 . 3HCl . H2O C: 46.64 H: 4.71 N: 10.07 C: 46.80 H: 4.82 N: 9.86 | 1.25 (t, 6H), 3.23 (m, 4H), 3.41 (t, 2H), 3.84 (2H), 5.71 (s, 2H), 6.73 (d, J=8.8, 1H), 7.30 (dd, J=8.4 2, 1H), 7.62-7.81 (m, 5H), 7.93 (d, J=2, 1H), 8.35 (s, 1H), 9.64 (s), 9.83 (s), 10.13 (s), 10.96 (s) | 562 (80) 564 (100) 566 (51) 568 (14) | (A) CH2Cl2 : Isopropanol : NH3 (100 : 50 : 0.5) | |
| 60 | 127-130 bubb. | 30 | C30H37N5O2 . 3HCl . H2O C: 54.34 H: 6.99 N: 10.56 C: 54.40 H: 6.80 N: 10.45 | 1.29 (t, 6H), 3.08 (t, 2H), 3.25 (4H), 3.48 (d, 2H), 3.84 (s,3H), 3.89 (s,3H), 4.04 (q, 2H), 4.83 (t, 2H), 6.9 (m, 2H), 7.12-7.31 (m, 6H), 8.03 (d, J=8.8, 1H), 8.27 (d, J=8.8, 1H), 8.36 (s, 1H), 9.87 (s), 10.08 (s), 10.44 (s), 11.1 (s) | 500 (100) | (A) CH2Cl2 : Isopropanol : iso-propylamine(90 : 30 : 2) | |
| 61 | 288-290 | 19 | C26H26Cl2N4 . 1.5HCl C: 60.04 H: 5.33 N: 10.77 C: 60.05 H: 5.50 N: 10.66 | 0.96 (d,6H), 2.06 (m, 1H), 2.93 (t, 2H), 3.16 (t, 2H), 4.63 (t, 2H), 6.67 (d, J=7.2, 2H), 7.03 (t, J=7.2, 2H), 7.11 (m, 1H), 7.45 (2H), 7.70 (m, 2H), 8.01 (d, J=8.8, 1H), 8.12 (s, 1H), 9.02 (s), 9.44 (s), 9.75 (s) | 465 (100) 467 (67) 469 (11) | (A) CH2Cl2 : Isopropanol : ethylamine(100 : 50 : 0.5) | |
4-(2,4-Dichlorobenzyl)amino-3-nitrobenzonitrile (7)
4-(2-Phenylethyl)amino-3-nitrobenzonitrile (8)
General Procedure for Synthesis of 9–18, 40–44, 53, 54, 56–58
| Comp | R’ | R1 | Formula | NMR δ ppm (DMSO-d6) | Mass (ESI+) | mp (oC) | Yield (%) |
| 9 | butyl | C11H16N4O2 | 0.895 (t, 3H), 1.34 (m, 2H), 1.56 (m, 2H), 3.41 (q, 2H), 7.22 (d, J=9.2, 1H), 7.95 (dd, J=9.2, 2.4, 1H), 8.6 (t, 1H), 8.67 (d, J=2.4, 1H), 9.2 (br.s) | 237 (100) | 200-4 | 62 |
| Comp | R’ | R1 | Formula | NMR δ ppm (DMSO-d6) | MS (ESI+) | mp (oC) | Yield (%) |
| 19 | butyl | C11H18N4 | 0.88 (t, 3H), 1.36 (m, 2H), 1.56 (m, 2H), 3.1 (t, 2H), 5.01 (br.s, 2H), 5.57 (br.s, 2H), 6.46 (d, J=8, 1H), 6.9 (s, 1H), 7.07 (d, J=8.4, 1H), 8.58 (s, 2H), 8.74 (s, 2H). | 207(100) | 285 | 94 |
General Procedure for Synthesis of 19 – 28
| Comp | R1 | R2 | R3 | R4 | Formula | NMR δ ppm (CDCl3) | Mass (ESI+) | mp (oC) | Yield (%) | İsolation |
| 33 | Cl | C14H8ClN3 | Lit [2] |
General Procedure for Synthesis of 33–39, 45–52, 55, 59–61
Acknowledgments
References
- Göker, H.; Özden, S.; Yildiz, S.; Boykin, D.W. Synthesis and potent antibacterial activity against MRSA of some novel 1,2-Disubstituted-1H-Benzimidazole-N-alkylated-5-carboxamidines. Eur. J. Med. Chem. 2005, 40, 1062–1069. [Google Scholar]
- Göker, H.; Kus, C.; Boykin, D.W.; Yildiz, S.; Altanlar, N. Synthesis of some new 2-substituted-phenyl-1H-benzimidazole-5-carbonitriles and their potent activity against Candida species. Bioorg. Med. Chem. 2002, 10, 2589–2596. [Google Scholar]
- Weidner-Wells, M.A.; Ohemeng, K.A.; Nguyen, V.N.; Fraga-Spano, S.; Macielag, M.J.; Werblood, H.M.; Foleno, B.D.; Webb, G.C.; Barrett, J.F.; Hlasta, D.J. Amidino benzimidazole inhibitors of bacterial two-component systems. Bioorg. Med. Chem. Lett. 2001, 11, 1545–1548. [Google Scholar]
- a)National Committee for Clinical Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, 5th ed; Approved Standard (M7A5); National Committee for Clinical Laboratory Standards: Wayne, PA, 2000. [Google Scholar]; b)Shadomy, S.; Pfaller, M.A. Laboratory Studies with Antifungal Agents: Susceptibility Tests and Quantitation in Body Fluids. In “Manual of Clinical Microbiology 5th Ed.”; Balows, A., Hausler, W.J., Hermann, K.L., Isenberg, H.D., Shadomy, H.J., Eds.; Washington DC, 1991; Chapter 117; p. 1173. [Google Scholar].
- Ritter, H. DE 723751, 1942.
- Roesner, M.; Raethner, W. DE 2839989, 1980.
- Sample Availability : Available from the authors.
© 2005 by MDPI (http:www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Göker, H.; Alp, M.; Yıldız, S. Synthesis and Potent Antimicrobial Activity of Some Novel N-(Alkyl)-2-Phenyl-1H-Benzimidazole-5-Carboxamidines. Molecules 2005, 10, 1377-1386. https://doi.org/10.3390/10111377
Göker H, Alp M, Yıldız S. Synthesis and Potent Antimicrobial Activity of Some Novel N-(Alkyl)-2-Phenyl-1H-Benzimidazole-5-Carboxamidines. Molecules. 2005; 10(11):1377-1386. https://doi.org/10.3390/10111377
Chicago/Turabian StyleGöker, Hakan, Mehmet Alp, and Sulhiye Yıldız. 2005. "Synthesis and Potent Antimicrobial Activity of Some Novel N-(Alkyl)-2-Phenyl-1H-Benzimidazole-5-Carboxamidines" Molecules 10, no. 11: 1377-1386. https://doi.org/10.3390/10111377
APA StyleGöker, H., Alp, M., & Yıldız, S. (2005). Synthesis and Potent Antimicrobial Activity of Some Novel N-(Alkyl)-2-Phenyl-1H-Benzimidazole-5-Carboxamidines. Molecules, 10(11), 1377-1386. https://doi.org/10.3390/10111377








