Influence of Additive Firing on the Surface Characteristics, Streptococcus mutans Viability and Optical Properties of Zirconia
Abstract
1. Introduction
2. Materials and Methods
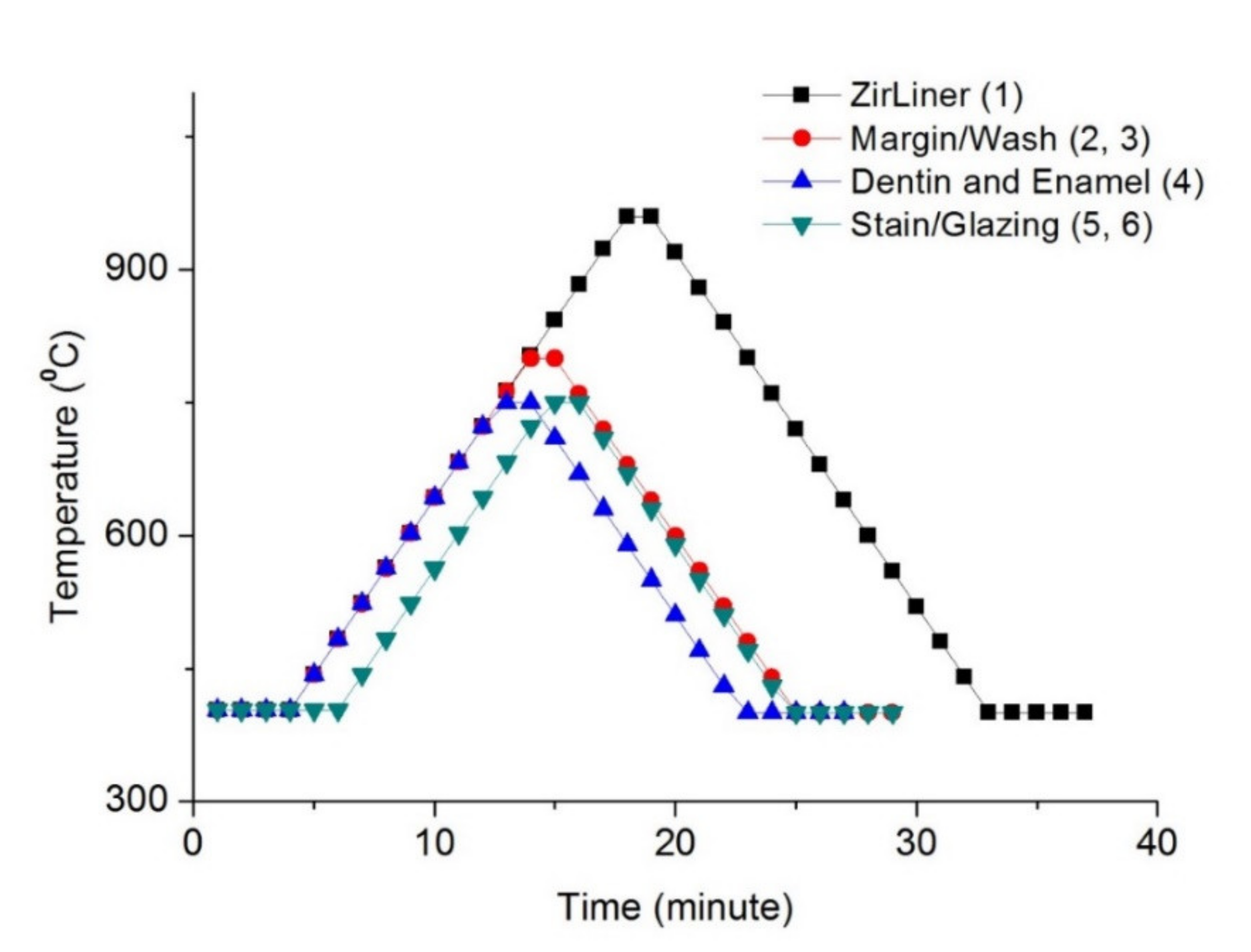
2.1. Preparation of Specimen
2.2. Surface Characteristics
2.3. Streptococcus Mutans Viability
2.4. Calculation of Translucency
2.5. Statistical Analysis
3. Results
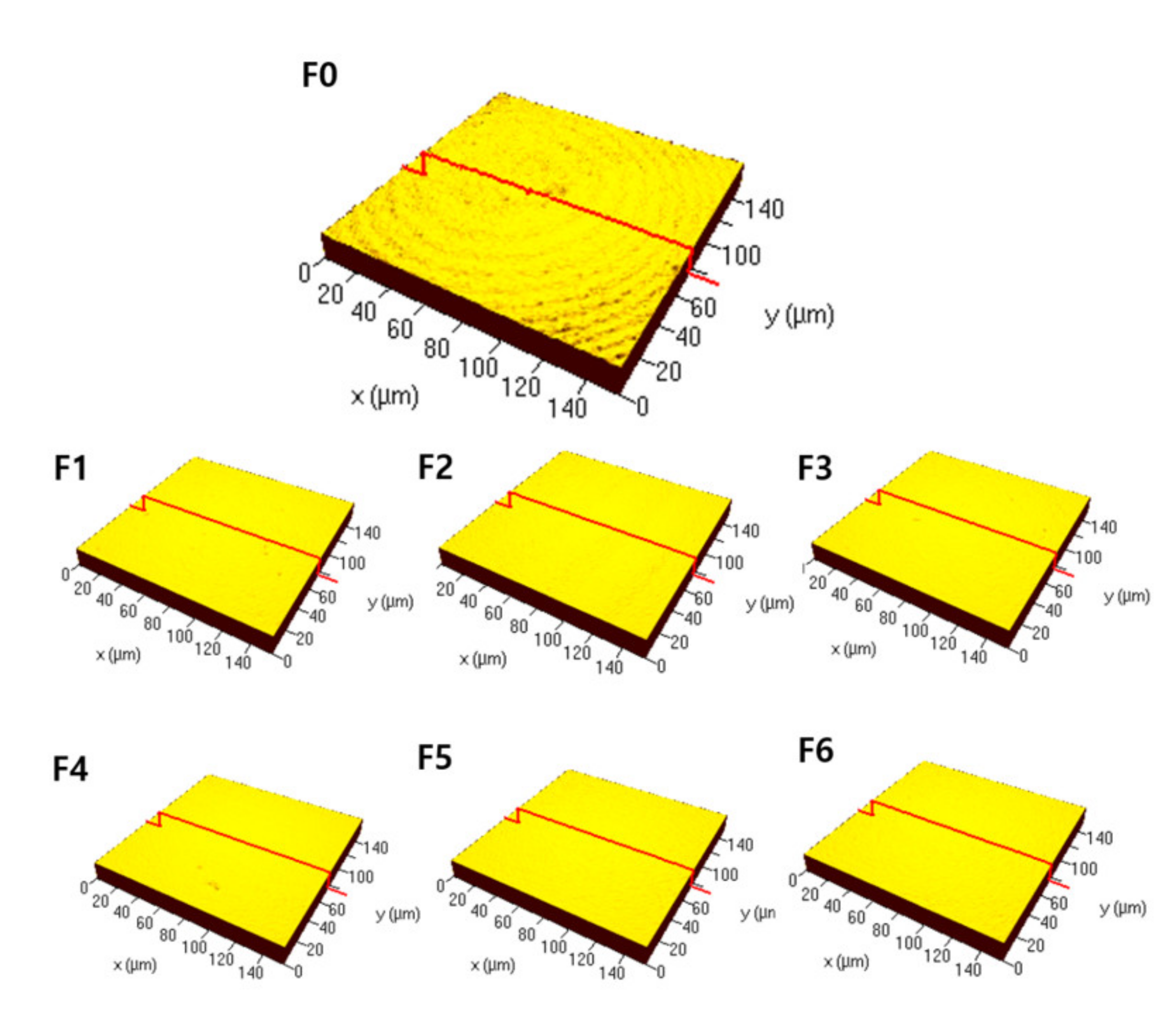
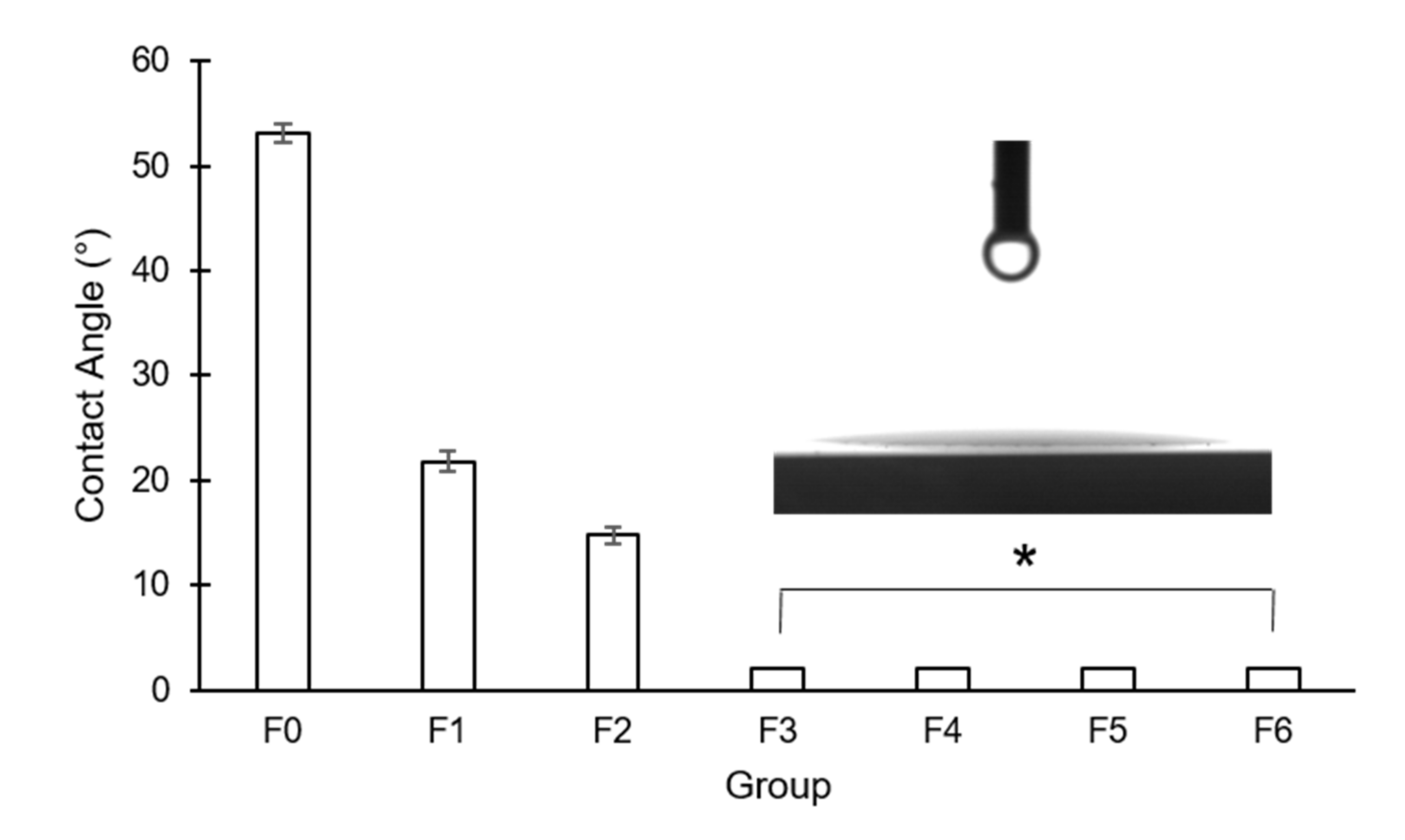
3.1. Surface Characteristics
3.2. Streptococcus Mutans Viability
3.3. Optical Property
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pittayachawan, P.; McDonald, A.; Young, A.; Knowles, J.C. Flexural strength, fatigue life, and stress-induced phase trans-formation study of Y-TZP dental ceramic. J. Biomed. Mater. Res. B 2009, 88, 366–377. [Google Scholar] [CrossRef]
- Guazzato, M.; Albakry, M.; Ringer, S.P.; Swain, M.V. Strength, fracture toughness and microstructure of a selection of all-ceramic materials. Part II. Zirconia-based dental ceramics. Dent. Mater. 2004, 20, 449–456. [Google Scholar] [CrossRef]
- Ardlin, B.I. Transformation-toughened zirconia for dental inlays, crowns and bridges: Chemical stability and effect of low-temperature aging on flexural strength and surface structure. Dent. Mater. 2002, 18, 590–595. [Google Scholar] [CrossRef]
- Kosmač, T.; Oblak, C.; Jevnikar, P.; Funduk, N.; Marion, L. The effect of surface grinding and sandblasting on flexural strength and reliability of Y-TZP zirconia ceramic. Dent. Mater. 1999, 15, 426–433. [Google Scholar] [CrossRef]
- Øilo, M.; Gjerdet, N.R.; Tvinnereim, H.M. The firing procedure influences properties of a zirconia core ceramic. Dent. Mater. 2008, 24, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, J. What future for zirconia as a biomaterial? Biomaterials 2006, 27, 535–543. [Google Scholar] [CrossRef]
- Kohorst, P.; Brinkmann, H.; Dittmer, M.P.; Borchers, L.; Stiesch, M. Influence of the veneering process on the marginal fit of zirconia fixed dental prostheses. J. Oral Rehabil. 2010, 37, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Pang, L.; Yao, J. The effects of firing numbers on the opening total pore volume, translucency parameter and color of dental all-ceramic systems. Hua Xi Kou Qiang Yi Xue Za Zhi 2012, 30, 417–419. [Google Scholar]
- Nejatidanesh, F.; Azadbakht, K.; Savabi, O.; Sharifi, M.; Shirani, M. Effect of repeated firing on the translucency of CAD-CAM monolithic glass-ceramics. J. Prosthet. Dent. 2020, 123, 530.e1–530.e6. [Google Scholar] [CrossRef]
- Ryan, B.J.; Poduska, K.M. Roughness effects on contact angle measurements. Am. J. Phys. 2008, 76, 1074–1077. [Google Scholar] [CrossRef]
- Yang, C.; Tartaglino, U.; Persson, B.N.J. Influence of Surface Roughness on Superhydrophobicity. Phys. Rev. Lett. 2006, 97, 116103. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhou, J.; Liu, X.; Zheng, M.; Yang, J.; Tan, J. Ultraviolet light-treated zirconia with different roughness affects function of human gingival fibroblasts in vitro: The potential surface modification developed from implant to abutment. J. Biomed. Mater. Res. B 2015, 103, 116–124. [Google Scholar] [CrossRef]
- Pantea, M.; Antoniac, I.; Trante, O.; Ciocoiu, R.; Fischer, C.A.; Traistaru, T. Correlations between connector geometry and strength of zirconia-based fixed partial dentures. Mater. Chem. Phys. 2019, 222, 96–109. [Google Scholar] [CrossRef]
- Abdullah, A.O.; Muhammed, F.K.; Yu, H.; Pollington, S.; Xudong, S.; Liu, Y. The impact of laser scanning on zirconia coating and shear bond strength using veneer ceramic material. Dent. Mater. J. 2019, 38, 452–463. [Google Scholar] [CrossRef]
- Khan, A.A.; Mohamed, B.A.; Mirza, E.H.; Syed, J.; Divakar, D.D.; Vallittu, P.K. Surface wettability and nano roughness at different grit blasting operational pressures and their effects on resin cement to zirconia adhesion. Dent. Mater. J. 2019, 38, 388–395. [Google Scholar] [CrossRef]
- Rohr, N.; Zeller, B.; Matthisson, L.; Fischer, J. Surface structuring of zirconia to increase fibroblast viability. Dent. Mater. 2020, 36, 779–786. [Google Scholar] [CrossRef]
- Han, A.; Tsoi, J.K.; Matinlinna, J.P.; Zhang, Y.; Chen, Z. Effects of different sterilization methods on surface characteristics and biofilm formation on zirconia in vitro. Dent. Mater. 2018, 34, 272–281. [Google Scholar] [CrossRef]
- Chen, L.; Yang, S.; Yu, P.; Wu, J.; Guan, H.; Wu, Z. Comparison of bacterial adhesion and biofilm formation on zirconia fabricated by two different approaches: An in vitro and in vivo study. Adv. Appl. Ceram. 2020, 119, 323–331. [Google Scholar] [CrossRef]
- Kunrath, M.F.; Monteiro, M.S.; Gupta, S.; Hubler, R.; De Oliveira, S.D. Influence of titanium and zirconia modified surfaces for rapid healing on adhesion and biofilm formation of Staphylococcus epidermidis. Arch. Oral Biol. 2020, 117, 104824. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.-H.; Choi, H.; Yoo, Y.-J.; Kim, J.-H.; Park, Y.-B.; Moon, H.-S. Effect of polishing method on surface roughness and bacterial adhesion of zirconia-porcelain veneer. Ceram. Int. 2017, 43, 5382–5387. [Google Scholar] [CrossRef]
- Go, H.; Park, H.; Lee, J.; Seo, H.; Lee, S. Effect of various polishing burs on surface roughness and bacterial adhesion in pediatric zirconia crowns. Dent. Mater. J. 2019, 38, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Hsu, L.C.; Fang, J.; Borca-Tasciuc, D.A.; Worobo, R.W.; Moraru, C.I.J.A. Effect of micro-and nanoscale to-pography on the adhesion of bacterial cells to solid surfaces. Appl. Environ. Microbiol. 2013, 79, 2703–2712. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Carrera, C.; Chen, R.; Li, J.; Lenton, P.; Rudney, J.D.; Jones, R.S.; Aparicio, C.; Fok, A. Degradation in the dentin–composite interface subjected to multi-species biofilm challenges. Acta Biomater. 2014, 10, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Song, C.; Jung, J.; Ahn, S.; Ferracane, J.; Ferracane, J. The Effects of Surface Roughness of Composite Resin on Biofilm Formation of Streptococcus mutans in the Presence of Saliva. Oper. Dent. 2012, 37, 532–539. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, K.; Zhang, N.; Melo, M.; Weir, M.; Zhou, X.; Bai, Y.; Reynolds, M.; Xu, H.H.K. Developing a new gen-eration of antimicrobial and bioactive dental resins. J. Dent. Res. 2017, 96, 855–863. [Google Scholar] [CrossRef]
- Forssten, S.D.; Björklund, M.; Ouwehand, A.C. Streptococcus mutans, Caries and Simulation Models. Nutrients 2010, 2, 290–298. [Google Scholar] [CrossRef]
- Lee, B.-C.; Jung, G.-Y.; Kim, D.-J.; Han, J.-S. Initial bacterial adhesion on resin, titanium and zirconiain vitro. J. Adv. Prosthodont. 2011, 3, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.C.M.; Mota, R.R.C.; Sordi, M.B.; Passoni, B.B.; Benfatti, C.A.M.; Magini, R.S. Biofilm Formation on Different Materials Used in Oral Rehabilitation. Braz. Dent. J. 2016, 27, 141–147. [Google Scholar] [CrossRef]
- Lee, D.-H.; Mai, H.-N.; Thant, P.P.; Hong, S.-H.; Kim, J.; Jeong, S.-M.; Lee, K.-W. Effects of different surface finishing protocols for zirconia on surface roughness and bacterial biofilm formation. J. Adv. Prosthodont. 2019, 11, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Wang, C.; Zhou, J.; Jiang, L.; Xue, J.; Li, W.J.B.R.I. Influence of surface properties on adhesion forces and attachment of Streptococcus mutans to zirconia in vitro. Biomed. Res. Int. 2016, 2016, 1–10. [Google Scholar]
- Kaizer, M.D.R.; Diesel, P.G.; Mallmann, A.; Jacques, L.B. Ageing of silorane-based and methacrylate-based composite resins: Effects on translucency. J. Dent. 2012, 40, e64–e71. [Google Scholar] [CrossRef]
- Queiroz, J.R.C.; Benetti, P.; Massi, M.; Junior, L.N.; Della Bona, A. Effect of multiple firing and silica deposition on the zirconia–porcelain interfacial bond strength. Dent. Mater. 2012, 28, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Takamori, E.R.; Cruz, R.; Gonçalvez, F.; Zanetti, R.V.; Zanetti, A.; Granjeiro, J.M. Effect of Roughness of Zirconia and Titanium on Fibroblast Adhesion. Artif. Organs 2008, 32, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Geurtsen, W.; Lehmann, F.; Spahl, W.; Leyhausen, G. Cytotoxicity of 35 dental resin composite monomers/additives in permanent 3T3 and three human primary fibroblast cultures. J. Biomed. Mater. Res. 1998, 41, 474–480. [Google Scholar] [CrossRef]
- Bohinc, K.; Dražić, G.; Abram, A.; Jevšnik, M.; Jeršek, B.; Nipič, D.; Kurinčič, M.; Raspor, P. Metal surface characteristics dictate bacterial adhesion capacity. Int. J. Adhes. Adhes. 2016, 68, 39–46. [Google Scholar] [CrossRef]
- Wu, Y.; Zitelli, J.P.; TenHuisen, K.S.; Yu, X.; Libera, M.R. Differential response of Staphylococci and osteoblasts to varying titanium surface roughness. Biomaterials 2011, 32, 951–960. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, B.; Liu, Y.; Suo, X.; Li, H. Influence of surface topography on bacterial adhesion: A review (Review). Biointerphases 2018, 13, 060801. [Google Scholar] [CrossRef]
- Andrukhov, O.; Behm, C.; Blufstein, A.; Wehner, C.; Gahn, J.; Pippenger, B.; Wagner, R.; Rausch-Fan, X. Effect of implant surface material and roughness to the susceptibility of primary gingival fibroblasts to inflammatory stimuli. Dent. Mater. 2020, 36, e194–e205. [Google Scholar] [CrossRef] [PubMed]
- Schünemann, F.H.; Galárraga-Vinueza, M.E.; Magini, R.; Fredel, M.; Silva, F.; Souza, J.C.M.; Zhang, Y.; Henriques, B. Zirconia surface modifications for implant dentistry. Mater. Sci. Eng. C 2019, 98, 1294–1305. [Google Scholar] [CrossRef]
- Wennerberg, A.; Albrektsson, T. Suggested guidelines for the topographic evaluation of implant sur-faces. Int. J. Oral Maxillofac. Surg. 2000, 15, 331–344. [Google Scholar]
- Noro, A.; Kaneko, M.; Murata, I.; Yoshinari, M. Influence of surface topography and surface physicochemistry on wettability of zirconia (tetragonal zirconia polycrystal). J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 101, 355–363. [Google Scholar] [CrossRef]
- Lopes, B.B.; Ayres, A.A.P.; Lopes, L.B.; Negreiros, W.M.; Giannini, M. The effect of atmospheric plasma treatment of dental zirconia ceramics on the contact angle of water. Appl. Adhes. Sci. 2014, 2, 17. [Google Scholar] [CrossRef]
- Yoshida, A.; Kuramitsu, H.K. Multiple Streptococcus mutans Genes Are Involved in Biofilm Formation. Appl. Environ. Microbiol. 2002, 68, 6283–6291. [Google Scholar] [CrossRef]
- Rigolin, M.S.M.; Barbugli, P.A.; Jorge, J.H.; Reis, M.R.D.; Adabo, G.L.; Casemiro, L.A.; Martins, C.H.G.; de Lima, O.J.; Junior, F.D.A.M. Effect of the aging of titanium and zirconia abutment surfaces on the viability, adhesion, and proliferation of cells and the adhesion of microorganisms. J. Prosthet. Dent. 2019, 122, 564.e1–564.e10. [Google Scholar] [CrossRef]
- Kurtulmus-Yilmaz, S.; Ulusoy, M. Comparison of the translucency of shaded zirconia all-ceramic systems. J. Adv. Prosthodont. 2014, 6, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Dongdong, Q.; Lei, Z.; Xiaoping, L.; Wenli, C. Effect of repeated sintering on the color and translucency of dental lithium disilicate-based glass ceramic. West China J. Stomatol. 2015, 33, 50–53. [Google Scholar]
- Walczak, K.; Meißner, H.; Range, U.; Sakkas, A.; Boening, K.; Wieckiewicz, M.; Konstantinidis, I. Translucency of Zirconia Ceramics before and after Artificial Aging. J. Prosthodont. 2018, 28, e319–e324. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.-C.; Aquilino, S.A.; Lund, P.S.; Vargas, M.A.; Gratton, D.G.; Qian, F.; Diaz-Arnold, A.M. Human Perception of Dental Porcelain Translucency Correlated to Spectrophotometric Measurements. J. Prosthodont. 2010, 19, 187–193. [Google Scholar] [CrossRef]
- Zeighami, S.; Mahgoli, H.; Farid, F.; Azari, A. The Effect of Multiple Firings on Microtensile Bond Strength of Core-Veneer Zirconia-Based All-Ceramic Restorations. J. Prosthodont. 2012, 22, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Dehoff, P.H.; Barrett, A.A.; Lee, R.B.; Anusavice, K.J. Thermal compatibility of dental ceramic systems using cylindrical and spherical geometries. Dent. Mater. 2008, 24, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Benetti, P.; Della Bona, A.; Kelly, J.R. Evaluation of thermal compatibility between core and veneer dental ceramics using shear bond strength test and contact angle measurement. Dent. Mater. 2010, 26, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Benetti, P.; Kelly, J.R.; Della Bona, A. Analysis of thermal distributions in veneered zirconia and metal restorations during firing. Dent. Mater. 2013, 29, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Witek, S.R.; Butler, E.P. Zirconia Particle Coarsening and the Effects of Zirconia Additions on the Mechanical Properties of Certain Commercial Aluminas. J. Am. Ceram. Soc. 1986, 69, 523–529. [Google Scholar] [CrossRef]
- Karakoca, S.; Yılmaz, H. Influence of surface treatments on surface roughness, phase transformation, and biaxial flexural strength of Y-TZP ceramics. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 91, 930–937. [Google Scholar] [CrossRef]
- Guazzato, M.; Quach, L.; Albakry, M.; Swain, M.V. Influence of surface and heat treatments on the flexural strength of Y-TZP dental ceramic. J. Dent. 2005, 33, 9–18. [Google Scholar] [CrossRef]
- Nakamura, K.; Adolfsson, E.; Milleding, P.; Kanno, T.; Örtengren, U. Influence of grain size and veneer firing process on the flexural strength of zirconia ceramics. Eur. J. Oral Sci. 2012, 120, 249–254. [Google Scholar] [CrossRef]
- Crespi, R.; Cappare, P.; Gherlone, E. Dental implants placed in extraction sites grafted with different bone substitutes: Radi-ographic evaluation at 24 months. J. Periodontol. 2009, 80, 1616–1621. [Google Scholar] [CrossRef] [PubMed]
- Crespi, R.; Cappare, P.; Gherlone, E. Comparison of magnesium-enriched hydroxyapatite and porcine bone in human ex-traction socket healing: A histologic and histomorphometric evaluation. Int. J. Oral Maxillofac. Implants 2011, 26, 1057–1062. [Google Scholar]
- Crespi, R.; Capparé, P.; Romanos, G.E.; Mariani, E.; Benasciutti, E.; Gherlone, E. Corticocancellous porcine bone in the healing of human extraction sockets: Combining histomorphometry with osteoblast gene expression profiles in vivo. Int. J. Oral Maxillofac. Implant. 2011, 26, 866–872. [Google Scholar]
- Gherlone, E.F.; Capparé, P.; Tecco, S.; Polizzi, E.; Pantaleo, G.; Gastaldi, G.; Grusovin, M.G. A Prospective Longitudinal Study on Implant Prosthetic Rehabilitation in Controlled HIV-Positive Patients with 1-Year Follow-Up: The Role of CD4+ Level, Smoking Habits, and Oral Hygiene. Clin. Implant. Dent. Relat. Res. 2015, 18, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Gherlone, E.F.; Capparé, P.; Tecco, S.; Polizzi, E.; Pantaleo, G.; Gastaldi, G.; Grusovin, M.G. Implant Prosthetic Rehabilitation in Controlled HIV-Positive Patients: A Prospective Longitudinal Study with 1-Year Follow-Up. Clin. Implant. Dent. Relat. Res. 2016, 18, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Capparé, P.; Teté, G.; Romanos, G.E.; Nagni, M.; Sannino, G.; Gherlone, E.F. The ‘All-on-four’ protocol in HIV-positive patients: A prospective, longitudinal 7-year clinical study. Int. J. Oral Implantol. 2019, 12, 501–510. [Google Scholar]
- Capparè, P.; Tetè, G.; Sberna, M.T.; Panina-Bordignon, P. The Emerging Role of Stem Cells in Regenerative Dentistry. Curr. Gene Ther. 2020, 20, 259–268. [Google Scholar] [CrossRef] [PubMed]

| Groups | |||||||
|---|---|---|---|---|---|---|---|
| F0 | F1 | F2 | F3 | F4 | F5 | F6 | |
| Ra (μm) | 0.16 A (0.15, 0.16) | 0.06 B (0.06, 0.06) | 0.04 C (0.04, 0.04) | 0.05 CD (0.05, 0.05) | 0.06 BE (0.06, 0.06) | 0.05 BDF (0.05, 0.06) | 0.05 BDG (0.05, 0.06) |
| Sa (μm) | 0.17 A (0.15, 0.18) | 0.07 B (0.06, 0.07) | 0.06 C (0.06, 0.06) | 0.06 BCD (0.06, 0.06) | 0.06 BCE (0.06, 0.07) | 0.06 BCF (0.06, 0.06) | 0.06 BCG (0.06, 0.07) |
| Sz (μm) | 2.25 A (1.95, 2.32) | 0.48 B (0.45, 0.49) | 0.45 BC (0.43, 0.46) | 0.47 BD (0.45, 0.49) | 0.48 BE (0.44, 0.48) | 0.55 F (0.55, 0.57) | 0.54 BFG (0.50, 0.59) |
| Sv (μm) | 2.09 A (2.05, 2.40) | 0.75 B (0.59, 0.75) | 0.98 BC (0.63, 1.30) | 0.51 BD (0.43, 0.63) | 0.28 BE (0.28, 0.63) | 0.83 CEF (0.83, 1.02) | 0.83 BEG (0.63, 0.87) |
| Groups | |||||||
|---|---|---|---|---|---|---|---|
| F0 | F1 | F2 | F3 | F4 | F5 | F6 | |
| TP | 5.05 A (5.05, 5.20) | 4.39 BCDEFG (4.34, 4.50) | 4.21 C (4.20, 4,25) | 4.43 D (4.38, 4.44) | 4.66 E (4.58, 4.68) | 4.53 EF (4.51, 4.57) | 4.79 DEG (4.57, 4.80) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, W.; Park, J.H.; Lee, H.-A.; Lim, B.-S.; Chung, S.H. Influence of Additive Firing on the Surface Characteristics, Streptococcus mutans Viability and Optical Properties of Zirconia. Materials 2021, 14, 1286. https://doi.org/10.3390/ma14051286
Moon W, Park JH, Lee H-A, Lim B-S, Chung SH. Influence of Additive Firing on the Surface Characteristics, Streptococcus mutans Viability and Optical Properties of Zirconia. Materials. 2021; 14(5):1286. https://doi.org/10.3390/ma14051286
Chicago/Turabian StyleMoon, Wonjoon, Joo Hyang Park, Han-Ah Lee, Bum-Soon Lim, and Shin Hye Chung. 2021. "Influence of Additive Firing on the Surface Characteristics, Streptococcus mutans Viability and Optical Properties of Zirconia" Materials 14, no. 5: 1286. https://doi.org/10.3390/ma14051286
APA StyleMoon, W., Park, J. H., Lee, H.-A., Lim, B.-S., & Chung, S. H. (2021). Influence of Additive Firing on the Surface Characteristics, Streptococcus mutans Viability and Optical Properties of Zirconia. Materials, 14(5), 1286. https://doi.org/10.3390/ma14051286






