Toxicity of Essential Oils Nanoemulsion Against Aphis Craccivora and Their Inhibitory Activity on Insect Enzymes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents, and Essential Oils
2.2. Preparation and Characterization of Nanoemulsions
2.3. Droplet Size Analysis
2.4. Analysis of Volatiles in Essential Ooils and Nanoemulsions
2.5. Aphid Rearing
2.6. Biochemical Aspects
2.7. Statistical Analysis
3. Results and Discussions
3.1. Characterization of the Nanoemulsion
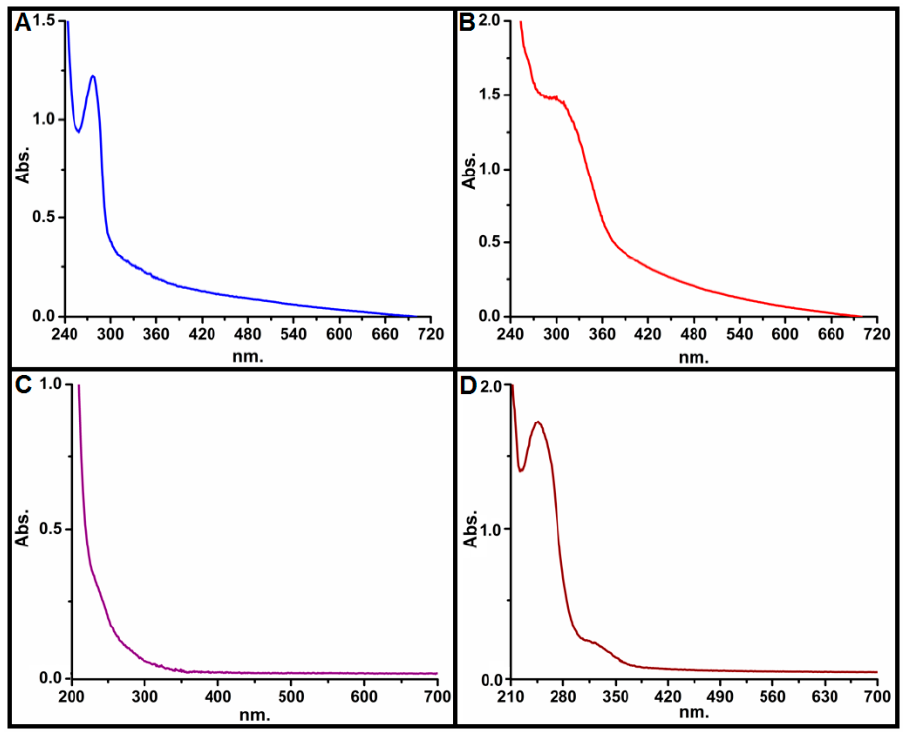
3.1.1. Electronic Spectrum
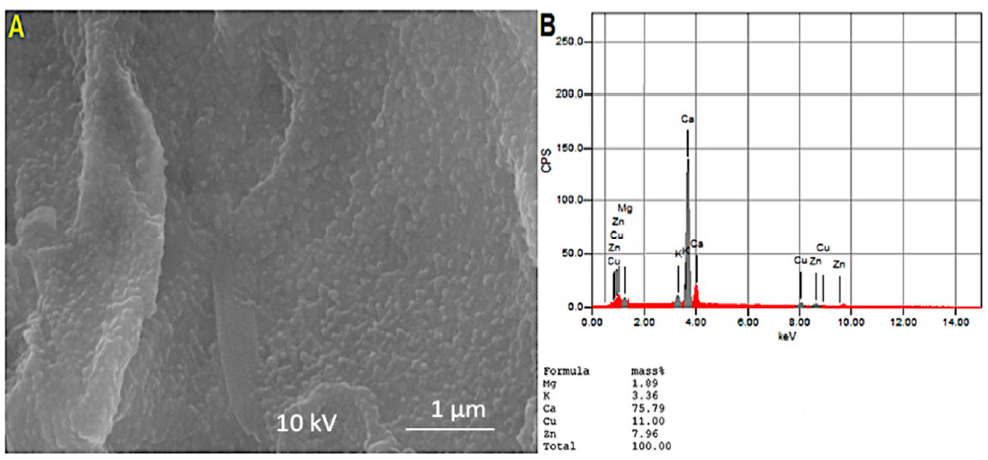
3.1.2. Morphological Structure
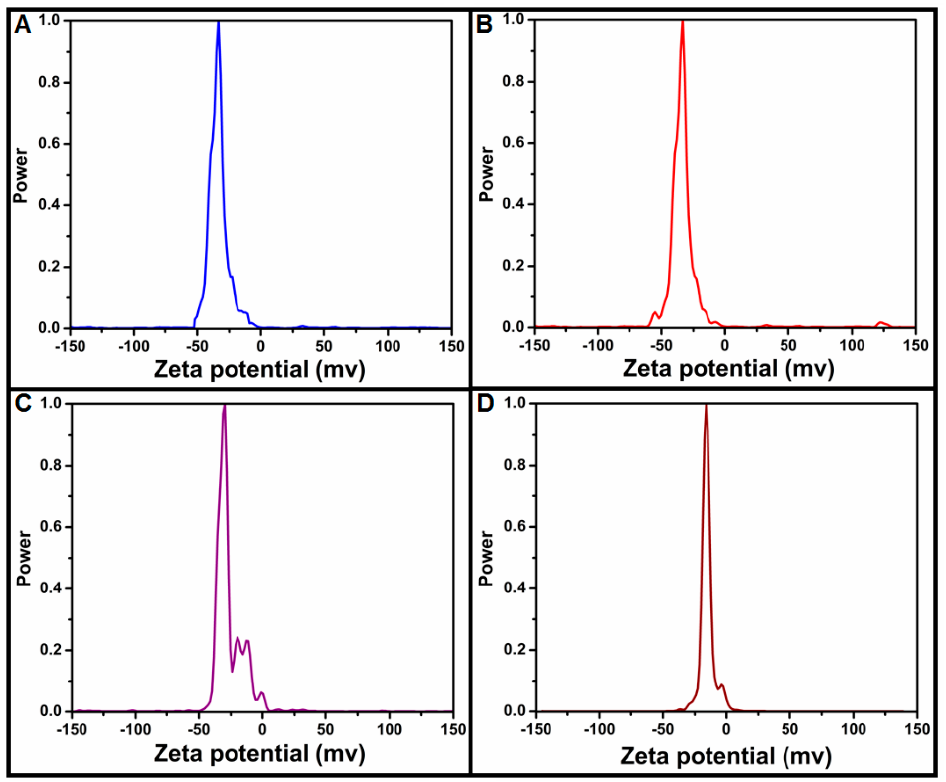
3.1.3. Size Distribution Analysis
3.1.4. GC-MS Analysis and Identification of Components
3.2. Susceptibility of A. Craccivora toTtested Insecticides
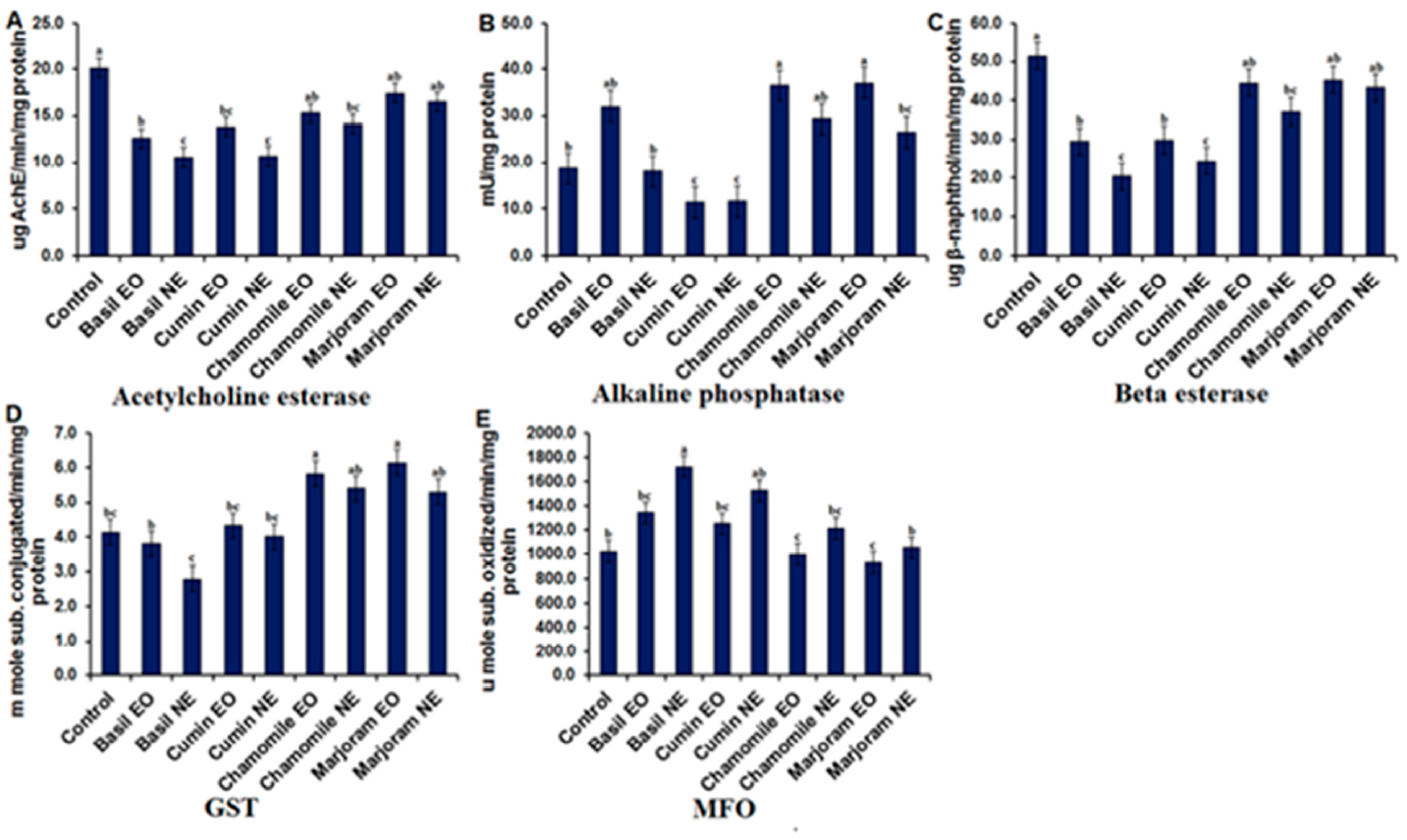
3.3. Inhibitory Activity Evaluation of Essential Oils and Synthesized Nanoemulsions on Insect Enzymes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Togola, A.; Boukar, O.; Belko, N.; Chamarthi, S.K.; Fatokun, C.; Tamo, M.; Oigiangbe, N. Host plant resistance to insect pests of cowpea (Vigna unguiculata L. Walp.): Achievements and future prospects. Euphytica 2017, 213, 239. [Google Scholar] [CrossRef]
- Van Emden, H.F.; Harrington, R. (Eds.) Aphids as Crop Pests; Cabi: Wallingford, Oxfordshire, UK; Boston, MA, USA, 2017. [Google Scholar]
- Li, F.; Han, Z. Mutations in acetylcholinesterase associated with insecticide resistance in the cotton aphid, Aphis gossypii Glov-er. Insect Biochem. Mol. Biol. 2004, 34, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Isman, M.B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Èntomol. 2006, 51, 45–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambethgar, V. Potential of entomopathogenic fungi in insecticide resistance management (IRM): A review. J. Biopestic. 2009, 2, 177–193. [Google Scholar]
- Govindarajan, M.; Mathivanan, T.; Elumalai, K.; Krishnappa, K.; Anandan, A. Ovicidal and repellent activities of botanical ex-tracts against Culex quinquefasciatus, Aedes aegypti and Anopheles stephensi (Diptera: Culicidae). Asian Pac. J. Trop. Biomed. 2011, 1, 43–48. [Google Scholar] [CrossRef] [Green Version]
- Ga’Al, H.; Fouad, H.; Mao, G.; Tian, J.; Jianchu, M. Larvicidal and pupicidal evaluation of silver nanoparticles synthesized using Aquilaria sinensis and Pogostemon cablin essential oils against dengue and zika viruses vector Aedes albopictus mosquito and its histopathological analysis. Artif. Cells Nanomed. Biotechnol. 2017, 46, 1171–1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fouad, H.; Hongjie, L.; Yanmei, D.; Baoting, Y.; El-Shakh, A.; Abbas, G.; Jianchu, M. Artificial Cells, Nanomedicine, and Bio-technology. Cocos Nucifera 2017, 1369–1378. [Google Scholar]
- Liu, Z.; Han, Z. Fitness costs of laboratory-selected imidacloprid resistance in the brown planthopper, Nilaparvata lugens Stål. Pest Manag. Sci. 2006, 62, 279–282. [Google Scholar] [CrossRef]
- Mokbel, E.M.S.; Swelam, E.S.H.; Radwan, E.M.M.; Kandil, M.E. Role of metabolic enzymes in resistance to chlorpyrifos-methyl in the cowpea aphid, Aphis craccivora (Koch). J. Plant Prot. Res. 2017, 57, 275–280. [Google Scholar] [CrossRef] [Green Version]
- Fouad, H.; Hongjie, L.; Hosni, D.; Wei, J.; Abbas, G.; Ga’Al, H.; Jianchu, M. Controlling Aedes albopictus and Culex pipiens pallens using silver nanoparticles synthesized from aqueous extract of Cassia fistula fruit pulp and its mode of action. Artif. Cells Nanomed. Biotechnol. 2018, 46, 558–567. [Google Scholar] [CrossRef] [Green Version]
- Ga’Al, H.; Yang, G.; Fouad, H.; Guo, M.; Mo, J. Mannosylerythritol Lipids Mediated Biosynthesis of Silver Nanoparticles: An Eco-friendly and Operative Approach against Chikungunya Vector Aedes albopictus. J. Clust. Sci. 2021, 32, 17–25. [Google Scholar] [CrossRef]
- Pandey, A.K.; Tripathi, S.; Singh, P. Plant essential oils: A substitute for conventional insecticides against Tribolium species (Coleoptera: Tenebrionidae)-achievements and challenges. Arch. Phytopathol. Plant Prot. 2018, 51, 696–728. [Google Scholar] [CrossRef]
- Pavela, R. History, presence and perspective of using plant extracts as commercial botanical insecticides and farm products for protection against insects–a review. Plant Prot. Sci. 2016, 52, 229–241. [Google Scholar]
- Benelli, G.; Pavela, R.; Giordani, C.; Casettari, L.; Curzi, G.; Cappellacci, L.; Petrelli, R.; Maggi, F. Acute and sub-lethal toxicity of eight essential oils of commercial interest against the filariasis mosquito Culex quinquefasciatus and the housefly Musca domestica. Ind. Crop. Prod. 2018, 112, 668–680. [Google Scholar] [CrossRef]
- Nathan, S.S.; Kalaivani, K.; Chung, P.G. The effects of azadirachtin and nucleopolyhedrovirus on midgut enzymatic profile of Spodoptera litura Fab. (Lepidoptera: Noctuidae). Pestic. Biochem. Physiol. 2005, 83, 46–57. [Google Scholar] [CrossRef]
- Pavela, R.; Žabka, M.; Vrchotová, N.; Tříska, J. Effect of foliar nutrition on the essential oil yield of Thyme (Thymus vulgaris L.). Ind. Crop. Prod. 2018, 112, 762–765. [Google Scholar] [CrossRef]
- Cespi, M.; Quassinti, L.; Perinelli, D.R.; Bramucci, M.; Iannarelli, R.; Papa, F.; Ricciutelli, M.; Bonacucina, G.; Palmieri, G.F.; Maggi, F. Microemulsions enhance the shelf-life and processability of Smyrnium olusatrum L. essential oil. Flavour Fragr. J. 2017, 32, 159–164. [Google Scholar] [CrossRef]
- Hamouda, T.; Hayes, M.M.; Cao, Z.; Tonda, R.; Johnson, K.; Wright, D.C.; Brisker, J.; Baker, J.R., Jr. A novel surfactant nanoemulsion with broad-spectrum sporicidal activity against Bacillus species. J. Infect. Dis. 1999, 180, 1939–1949. [Google Scholar] [CrossRef] [Green Version]
- Joe, M.M.; Bradeeba, K.; Parthasarathi, R.; Sivakumaar, P.K.; Chauhan, P.S.; Tipayno, S.; Benson, A.; Sa, T. Development of surfactin based nanoemulsion formulation from selected cooking oils: Evaluation for antimicrobial activity against selected food associ-ated microorganisms. J. Taiwan Inst. Chem. Eng. 2012, 43, 172–180. [Google Scholar] [CrossRef]
- Busvine, J.R. Recommended Methods for Measurement of Pest Resistance to Pesticides; Busvine, J.R., Ed.; FAO: Rome, Italy, 1980. [Google Scholar]
- Harlow, C.D.; Lampert, E.P. Resistance Mechanisms in Two Color Forms of the Tobacco Aphid (Homoptera: Aphididae). J. Econ. Èntomol. 1990, 83, 2130–2135. [Google Scholar] [CrossRef]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Finney, D.J. Probit Analysis; Cambridge University Press: Cambridge, UK, 1971. [Google Scholar]
- Van Asperen, K. A study of housefly esterases by means of a sensitive colorimetric method. J. Insect Physiol. 1962, 8, 401–416. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione Stransferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Powell, M.E.A.; Smith, M.J.H. The Determination of Serum Acid and Alkaline Phosphatase Activity with 4-Aminoantipyrine (A.A.P.). J. Clin. Pathol. 1954, 7, 245–248. [Google Scholar] [CrossRef] [Green Version]
- Hansen, L.G.; Hodgson, E. Biochemical characteristics of insect microsomes: N-and O-demethylation. Biochem. Pharmacol. 1971, 20, 1569–1978. [Google Scholar] [CrossRef]
- Amin, T.R. Biochemical and Physiological Studies of Some Insect Growth Regulators on the Cotton Leafworm, Spodoptera Littoralis (Boisd.). Ph.D. Thesis, Cairo University, Giza, Egypt, 1998. [Google Scholar]
- Solairaj, D.; Rameshthangam, P. Silver nanoparticle embedded α-chitin nanocomposite for enhanced antimicrobial and mos-quito larvicidal activity. J. Polym. Environ. 2017, 25, 435–452. [Google Scholar] [CrossRef]
- Yuan, C.-G.; Huo, C.; Gui, B.; Liu, P.; Zhang, C. Green Synthesis of Silver Nanoparticles Using Chenopodium aristatum L. Stem Extract and Their Catalytic/Antibacterial Activities. J. Clust. Sci. 2016, 28, 1319–1333. [Google Scholar] [CrossRef]
- Manjamadha, V.P.; Muthukumar, K. Ultrasound assisted green synthesis of silver nanoparticles using weed plant. Bioprocess Biosyst. Eng. 2016, 39, 401–411. [Google Scholar] [CrossRef]
- Deepak, P.; Sowmiya, R.; Ramkumar, R.; Balasubramani, G.; Aiswarya, D.; Perumal, P. Structural characterization and evaluation of mosquito-larvicidal property of silver nanoparticles synthesized from the seaweed, Turbinaria ornata (Turner) J. Agardh 1848. Artif. Cells Nanomed. Biotechnol. 2017, 45, 990–998. [Google Scholar] [CrossRef] [Green Version]
- Benelli, G.; Govindarajan, M.; Rajeswary, M.; Senthilmurugan, S.; Vijayan, P.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M. Larvicidal activity of Blumea eriantha essential oil and its components against six mosquito species, including Zika virus vectors: The promising potential of (4E,6Z)-allo-ocimene, carvotanacetone and dodecyl acetate. Parasitol. Res. 2017, 116, 1175–1188. [Google Scholar] [CrossRef]
- Bedini, S.; Flamini, G.; Cosci, F.; Ascrizzi, R.; Benelli, G.; Conti, B. Cannabis sativa and Humulus lupulus essential oils as novel con-trol tools against the invasive mosquito Aedes albopictus and fresh water snail Physella acuta. Ind. Crop. Prod. 2016, 85, 318–323. [Google Scholar] [CrossRef] [Green Version]
- Lett, A.M. Microstructure Engineering of Emulsion-Based Systems for the Control of Satiation, Satiety, Hedonic Acceptability and Sensory Quality. Ph.D. Thesis, University of Birmingham, Birmingham, UK, 2016. [Google Scholar]
- Bordes, P.; Pollet, E.; Avérous, L. Nano-biocomposites: Biodegradable polyester/nanoclay systems. Prog. Polym. Sci. 2009, 34, 125–155. [Google Scholar] [CrossRef]
- Marques, M.G.; Landim, T.N.; Silva, S.M.; Cunha, J.P.A.R.; Sampaio, M.V. Toxicidade do óleo essencial de Ocimum basilicum combinado ao inseticida tiametoxam para o pulgão-do-algodoeiro. Revista Brasileira de Ciências Agrárias (Agrária) 2019, 14, 5651. [Google Scholar]
- Pandey, A.K.; Singh, P.; Tripathi, N.N. Chemistry and bioactivities of essential oils of some Ocimum species: An overview. Asian Pac. J. Trop. Biomed. 2014, 4, 682–694. [Google Scholar] [CrossRef] [Green Version]
- Chenni, M.; El Abed, D.; Rakotomanomana, N.; Fernandez, X.; Chemat, F. Comparative Study of Essential Oils Extracted from Egyptian Basil Leaves (Ocimum basilicum L.) Using Hydro-Distillation and Solvent-Free Microwave Extraction. Molcules 2016, 21, 113. [Google Scholar] [CrossRef] [Green Version]
- Muráriková, A.; Ťažký, A.; Neugebauerová, J.; Planková, A.; Jampílek, J.; Mučaji, P.; Mikuš, P. Characterization of essential oil composition in different basil species and pot cultures by a GC-MS method. Molecules 2017, 22, 1221. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.; Qin, P.; Ji, M.; An, R.; Guo, H.; Shafi, J. Spinasterol, 22,23-Dihydrospinasterol and Fernenol from Citrullus colocynthis, L. with Aphicidal Activity against Cabbage Aphid Brevicoryne Brassicae, L. Molcules 2020, 25, 2184. [Google Scholar] [CrossRef]
- González, J.O.W.; Gutiérrez, M.M.; Ferrero, A.A.; Band, B.F. Essential oils nanoformulations for stored-product pest control—Characterization and biological properties. Chemosphere 2014, 100, 130–138. [Google Scholar] [CrossRef]
- Abdel-Aziz, N.F.; Abdou, W.L.; Abdel-Hakim, E.A.; El-Hawarya, F.M.; El-Bakry, A.M.; Sammour, E.A. The effect of some green insec-ticides from essential oils on Aphis craccivora, and their side effects. J. Entomol. Res. 2015, 39, 275–286. [Google Scholar] [CrossRef]
- Pradeepa, V.; Senthil-Nathan, S.; Sathish-Narayanan, S.; Selin-Rani, S.; Vasantha-Srinivasan, P.; Thanigaivel, A.; Ponsankar, A.; Edwin, E.S.; Sakthi-Bagavathy, M.; Kalaivani. K.; et al. Potential mode of action of a novel plumbagin as a mosquito re-pellent against the malarial vector Anopheles stephensi (Culicidae: Diptera). Pestic. Biochem. Physiol. 2016, 134, 84–93. [Google Scholar] [CrossRef]
- Selin-Rani, S.; Senthil-Nathan, S.; Revathi, K.; Chandrasekaran, R.; Thanigaivel, A.; Vasantha-Srinivasan, P.; Ponsankar, A.; Edwin, E.S.; Pradeepa, V. Toxicity of Alangium salvifolium Wang chemical constituents against the tobacco cutworm Spodoptera litu-ra Fab. Pestic. Biochem. Physiol. 2016, 126, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Amutha, C.; Bupesh, G.; Ramesh, R.; Kavitha, P.; Subramanian, P. Cytochrome P450-Dependent Mixed Function Oxidases (MFO) System Dynamics During the Poly Aromatic Hydrocarbon (PAH) Metabolism in Green Mussel Perna Viridis (Linnaeus, 1758). Environ. Bioindic. 2009, 4, 97–116. [Google Scholar] [CrossRef]
- Kehrer, J.; Robertson, J.; Smith, C. Free Radicals and Reactive Oxygen Species. Compr. Toxicol. 2010, 277–307. [Google Scholar] [CrossRef]

| Nanoemulsions | Particle Diameter (nm) | Polydispersity Index PDI | Conductivity (ms/cm) |
|---|---|---|---|
| B. ocimum | 129.5 ± 1.3 | 0.28 | 0.26 |
| M. chamomilla | 149.6 ± 1.7 | 0.54 | 0.28 |
| O. marjorana | 182.1 ± 1.8 | 0.22 | 0.28 |
| C. cyminum | 172.7 ± 1.5 | 0.37 | 0.29 |
| Compounds | Other Names | RT a (min) | Molecular Formula | Concentrations % | |
|---|---|---|---|---|---|
| Basil Oil | Cumin Oil | ||||
| Bicyclo[3.1.1]heptane,6,6-dimethyl-2-methylene-, (1s)- | L-β-Pinene | 6.55 | C10H16 | - | 21.63 |
| Benzene, methyl(1-methylethyl)- | β-Cymene | 7.46 | C10H14 | - | 8.78 |
| Eucalyptol | - | 7.68 | C10H18O | 19.18 | - |
| 2-oxabicyclo[2.2.2]octane, 1,3,3-trimethyl- | furan | 7.81 | C10H18O | 5.58 | - |
| 1,4-cyclohexadiene, 1-methyl-4-(1-methylet hyl)- | γ-Terpinen | 8.38 | C10H16 | - | 22.25 |
| Linalool | - | 9.93 | C10H18O | 22.91 | - |
| 1,6-octadien-3-ol,3,7-dimethyl- | Linalool | 10.27 | C10H18O | 6.56 | - |
| Benzaldehyde, 4-(1-methylethyl)- | Cumic aldehyde | 12.17 | C10H12O | - | 13.02 |
| 4-Isopropyl cyclohexa-1,3-dien ecarbaldehyde | α-Terpinene | 13.63 | C10H14O | - | 19.31 |
| Eugenol | - | 14.75 | C10H12O2 | 6.89 | - |
| Bicyclo[3.1.1]hept-2-ene, 2,6-dimethyl-6-(4-methyl-3-pentenyl)- | α-Bergamotene | 15.89 | C15H24 | 6.39 | - |
| (1R,2S,6S,7S,8S)-8-Isopropyl-1-methyl-3-methylenetricyclo[4.4.0.02,7]decane-rel- | β-Copaen-4α-ol | 16.66 | C15H24 | 1.58 | - |
| Naphthalene, 1,2,3,4,4a,5,6,8a-octahydro-7-m ethyl-4-methylene-1-(1-methyl ethyl)- | γ-Cadinene | 17.25 | C15H24 | 2.55 | - |
| tau.-Cadinol | Cadinol T | 19.25 | C15H26O | 2.95 | - |
| Compound | Other Names | RTa (min) | Molecular formula | Concentrations % | |
|---|---|---|---|---|---|
| Basil NE | Cumin NE | ||||
| Bicyclo[3.1.1]heptane,6,6-dimethyl-2-methylene- | β-Pinene | 6.28 | C10H16 | 2.65 | 5.53 |
| Benzene, methyl(1-methylethyl)- | β-Cymene | 7.25 | C10H14 | 1.12 | 18.23 |
| Eucalyptol | Eucalyptol | 7.38 | C10H18O | 2.65 | - |
| 1,4-cyclohexadiene, 1-methyl-4-(1-methylethyl) | γ-Terpinen | 7.88 | C10H16 | - | 2.20 |
| ç-Terpinene | ç-Terpinene | 7.91 | C10H16 | 1.29 | - |
| Linalool | Linalool | 9.03 | C10H18O | 10.12 | - |
| Benzaldehyde,4-(1-methylethyl)- | Cumic aldehyde | 11.81 | C10H12O | - | 57.28 |
| Bornyl acetate | Bornyl acetate | 12.46 | C12H20O2 | 1.06 | - |
| 4-Isopropyl cyclohexa-1,3-dienecarbaldehyde | α-Terpinene | 12.57 | C10H14O | - | 9.91 |
| p-Cymen-7-ol | p-Cymen-7-ol | 12.67 | C10H14O | - | 6.11 |
| Eugenol | Eugenol | 13.90 | C10H12O2 | 2.57 | - |
| Bicyclo[3.1.1]hept-2-ene, 2,6-dimethyl-6-(4-methyl-3-pentenyl)- | α-Bergamotene | 15.53 | C15H24 | 24.73 | - |
| Naphthalene,1,2,3,4,4a,5,6,8a-octahydro-7- methyl-4-methylene-1-(1-methylethyl)-, (1α,4aβ,8aα)- | γ-Cadinene | 16.93 | C15H24 | 9.67 | - |
| tau.-Cadinol | Cadinol T | 18.91 | C15H26O | 5.64 | - |
| Treatments | LC50 mg/L (95% LCL–UCL) | LC90 mg/L (95% LCL–UCL) | Slope ± SE | χ2 | PL | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| EO | NE | EO | NE | EO | NE | EO | NE | EO | NE | |
| O. basilicum | 992 (820–1163) | 45 (35–55) | 3998 (3358–4969) | 209 (176–256) | 2.1 ± 0.17 | 1.9 ± 0.17 | 2.9 | 1.8 | 3.2 | 69.5 |
| C. cyminum | 1710 (1462–1967) | 79 (65–94) | 7600.32 (6282–9650) | 425 (349–545) | 1.9 ± 0.15 | 1.8 ± 0.14 | 3.5 | 2.8 | 1.9 | 39.7 |
| M. chamomilla | 2327 (2024–2653) | 134 (114–155) | 9962.66 (8160–12821) | 726 (572–990) | 2.0 ± 0.15 | 1.9 ± 0.14 | 1.9 | 1.8 | 1.4 | 23.5 |
| O. marjorana | 3162 (2753–3624) | 188 (163–216) | 14862 (11736–20206) | 938 (734–1293) | 1.9 ± 0.15 | 1.8 ± 0.14 | 2.4 | 2.6 | 1.0 | 16.8 |
| Pymetrozine | 9.4 (8.1–10.9) | 44.0 (34.1–61.8) | 1.9 ± 0.16 | 1.9 | 335 | |||||
| Dinotefuran | 4.6 (3.8–5.6) | 34.0 (23.7–57.0) | 1.5 ± 0.15 | 3.3 | 679 | |||||
| Treatments | LC50 mg/L (95% LCL–UCL) | LC90 mg/L (95% LCL–UCL) | Slope ± SE | χ2 | PL | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| EO | NE | EO | NE | EO | NE | EO | NE | EO | NE | |
| O. basilicum | 1376 (1148–1607) | 54 (43–64) | 6778 (5628–8541) | 225 (191–273) | 1.9 ± 0.14 | 2.1 ± 0.16 | 2.1 | 1.5 | 2.6 | 67.6 |
| C. cyminum | 2263 (1963–2584) | 83 (67–99) | 9873 (8071–12744) | 535 (419–738) | 2.0 ± 0.15 | 1.6 ± 0.14 | 4.6 | 1.9 | 1.6 | 43.6 |
| M. chamomilla | 2857 (2455–3310) | 163 (140–188) | 15,727 (12,104–22,244) | 856 (670–1179) | 1.7 ± 0.14 | 1.8 ± 0.14 | 3.8 | 2.5 | 1.3 | 22.2 |
| O. marjorana | 3624 (3143–4191) | 221 (194–253) | 18,541 (14,202–26,419) | 962 (765–1293) | 1.8 ± 0.15 | 2.0 ± 0.15 | 2.4 | 1.9 | 1.0 | 16.3 |
| Pymetrozine | 12.5 (10.7–14.3) | 49.1 (40.0–64.0) | 2.2 ± 0.18 | 2.2 | 291.1 | |||||
| Dinotefuran | 27.4 (23.9–31.1) | 94.1 (78.1–119.4) | 2.4 ± 0.19 | 2.5 | 132.4 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelaal, K.; Essawy, M.; Quraytam, A.; Abdallah, F.; Mostafa, H.; Shoueir, K.; Fouad, H.; Hassan, F.A.S.; Hafez, Y. Toxicity of Essential Oils Nanoemulsion Against Aphis Craccivora and Their Inhibitory Activity on Insect Enzymes. Processes 2021, 9, 624. https://doi.org/10.3390/pr9040624
Abdelaal K, Essawy M, Quraytam A, Abdallah F, Mostafa H, Shoueir K, Fouad H, Hassan FAS, Hafez Y. Toxicity of Essential Oils Nanoemulsion Against Aphis Craccivora and Their Inhibitory Activity on Insect Enzymes. Processes. 2021; 9(4):624. https://doi.org/10.3390/pr9040624
Chicago/Turabian StyleAbdelaal, Khaled, Mamdouh Essawy, Attia Quraytam, Fahmy Abdallah, Heba Mostafa, Kamel Shoueir, Hatem Fouad, Fahmy A. S. Hassan, and Yaser Hafez. 2021. "Toxicity of Essential Oils Nanoemulsion Against Aphis Craccivora and Their Inhibitory Activity on Insect Enzymes" Processes 9, no. 4: 624. https://doi.org/10.3390/pr9040624
APA StyleAbdelaal, K., Essawy, M., Quraytam, A., Abdallah, F., Mostafa, H., Shoueir, K., Fouad, H., Hassan, F. A. S., & Hafez, Y. (2021). Toxicity of Essential Oils Nanoemulsion Against Aphis Craccivora and Their Inhibitory Activity on Insect Enzymes. Processes, 9(4), 624. https://doi.org/10.3390/pr9040624







