Massive Edema of the Lower Limbs in Patients after Spinal Cord Injury—One Picture, Different Diagnoses
Abstract
:1. Introduction
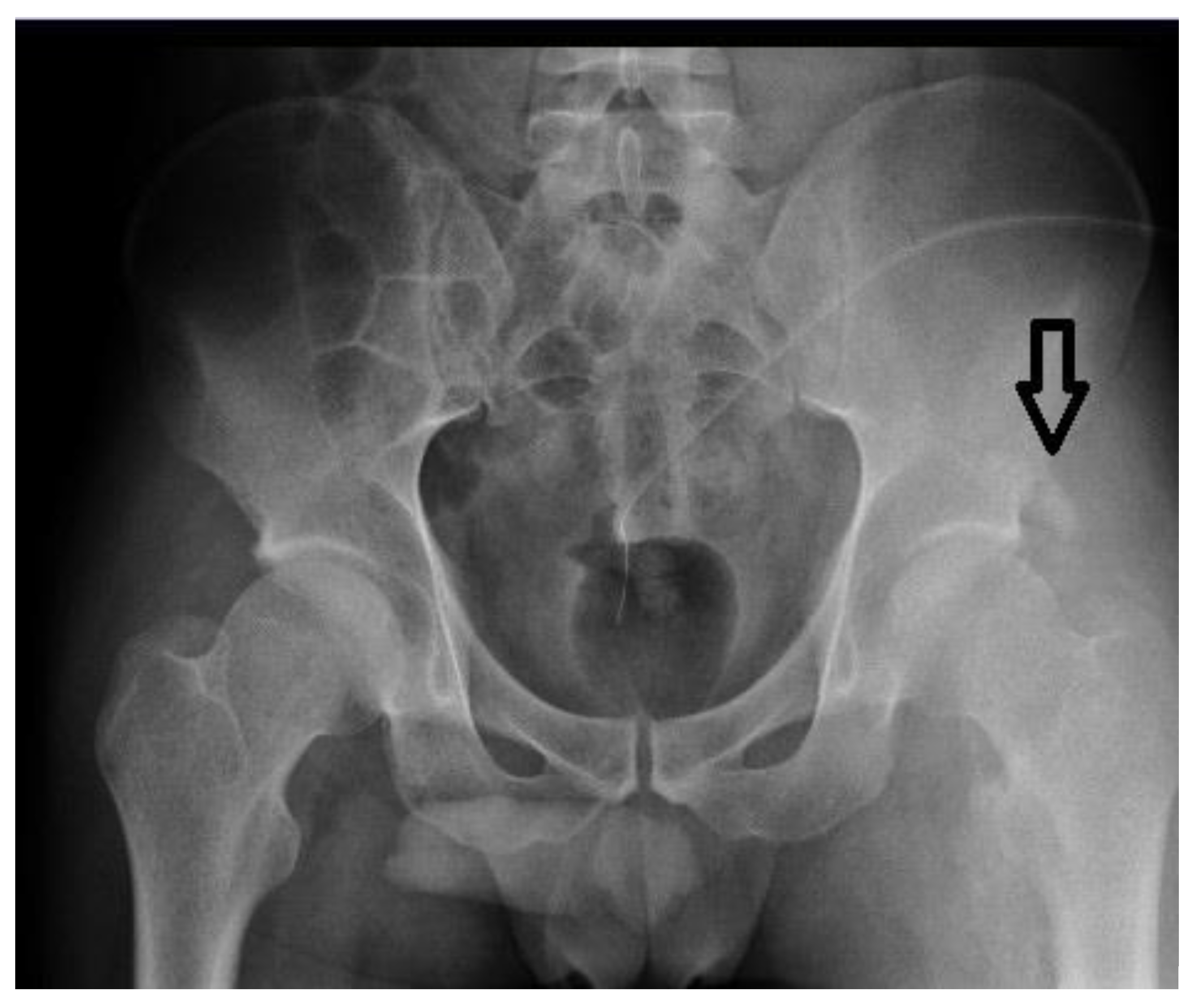
- Case No. 1
- Case No. 2
- Case No. 3
- Case No. 4
2. Discussion
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bang, J.H.; Cho, K.T.; Lee, H.J. Leg swelling caused by heterotopic ossification mimicking deep vein thrombosis in a paraplegic patient. Korean J. Neurotrauma 2015, 11, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Mackiewicz-Milewska, M.; Jung, S.; Kroszczyński, A.C.; Mackiewicz-Nartowicz, H.; Serafin, Z.; Cisowska-Adamiak, M.; Pyskir, J.; Szymkuć-Bukowska, I.; Hagner, W.; Rość, D. Deep venous thrombosis in patients with chronic spinal cord injury. J. Spinal Cord Med. 2016, 39, 400–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boudaoud, L.; Roussi, J.; Lortat-Jacob, S.; Bussel, B.; Dizien, O.; Drouet, L. Endothelial fibrinolytic reactivity and the risk of deep venous thrombosis after spinal cord injury. Spinal Cord 1997, 35, 151–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarek, A.Z.K.G. Significant reduction of the risk of venous thromboembolism in all long term immobile patients a few months after the onset of immobility. Med. Hypotheses 2005, 64, 1173–1176. [Google Scholar]
- Kim, S.W.; Charallel, J.T.; Park, K.W.; Bauerle, L.C.; Shang, C.C.; Gordon, S.K.; Bauman, W.A. Prevalence of deep venous thrombosis in patients with chronic spinal cord injury. Arch. Phys. Med. Rehabil. 1994, 75, 965–968. [Google Scholar] [CrossRef]
- Akman, M.N.; Cetin, N.; Bayramoglu, M.; Isiklar, I.; Kilinc, S. Value of the D-dimer test in diagnosing deep vein thrombosis in rehabilitation inpatients. Arch. Phys. Med. Rehabil. 2004, 85, 1091–1094. [Google Scholar] [CrossRef]
- Goodacre, S.; Sampson, F.C.; Sutton, A.J.; Mason, S.; Morris, F. Variation in the diagnostic performance of D-dimer for suspected deep vein thrombosis. QJM 2005, 98, 513–527. [Google Scholar] [CrossRef] [Green Version]
- Wells, P.h.S.; Anderson, D.R.; Rodger, M.; Forgie, M.; Kearon, C.; Dreyer, J. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N. Engl. J. Med. 2003, 349, 1227–1235. [Google Scholar] [CrossRef] [Green Version]
- Roussi, J.; Bentolila, S.; Boudaoud, L.; Casadevall, N.; Vallée, C.; Carlier, R.; Lortat-Jacob, S.; Dizien, O.; Bussel, B. Contribution of D-dimer determination in the exclusion of deep venous thrombosis in spinal cord injury patients. Spinal Cord 1999, 37, 548–552. [Google Scholar] [CrossRef] [Green Version]
- Stein, P.D.; Hull, R.D.; Patel, K.C.; Olson, R.E.; Ghali, W.A.; Brant, R.; Biel, R.K.; Bharadia, V.; Kalra, N.K. D-dimer for the exclusion of acute venous thrombosis and pulmonary embolism: A systematic review. Ann. Intern. Med. 2004, 140, 589–602. [Google Scholar] [CrossRef]
- Chen, Y.T.; Gershkoff, A.M. Lower extremity hemorrhage in spinal cord injured patients receiving therapeutic anticoagulation. Arch. Phys. Med. Rehabil. 1984, 65, 263–266. [Google Scholar] [PubMed]
- Giyanani, V.L.; Grozinger, K.T.; Gerlock, A.J., Jr.; Mirfakhraee, M.; Husbands, H.S. Calf hematoma mimicking thrombophlebitis: Sonographic and computed tomographic appearance. Radiology 1985, 154, 779–781. [Google Scholar] [CrossRef]
- Draghi, F.M.; Zacchino, M.; Canepari, P.N.; Alessandrino, F. Muscle injuries: Ultrasound evaluation in the acute phase. J. Ultrasound 2013, 16, 209–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, S.R.; Wiggs, L.L.; Ivanhoe, C.B. Forced use as a potential cause of gastrocnemius tears during neurologic rehabilitation: A report of 2 cases. Arch. Phys. Med. Rehabil. 2007, 88, 386–388. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, J.C.; Craven, B.C.; Moore, C.; Thabane, L.; Adachi, J.D.; Giangregorio, L.M. Muscle density and bone quality of the distal lower extremity among individuals with chronic spinal cord injury. Top. Spinal Cord Inj. Rehabil. 2015, 21, 282–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeung, V.; Formal, C. Lower extremity hemorrhage in patients with spinal cord injury receiving enoxaparin therapy. J. Spinal Cord Med. 2015, 38, 236–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mackiewicz-Milewska, M.; Jung, S.; Lach-Inszczak, S.; Borland, S.; Szymkuć, I.; Ciescinski, J.; Cisowaka Adamiak, M.; Hagner, W.; Lasek, W. Heterotopic ossification following cardiac arrest and hypoxic brain damage. Int. J. Ther. Rehabil. 2010, 17, 180–185. [Google Scholar] [CrossRef]
- Blankenship, L.D.; Strommen, J.A. 27-year-old man with a swollen leg. Mayo Clin. Proc. 2000, 75, 977–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simones, L.L.; Sonne-Holm, S.; Kraseninnikoff, M.; Engberg, A. Symptomatic heterotopic ossification after very sever traumatic brain injury in 114 patients. Incidence and risk factors. Int. J. Care Inj. 2007, 38, 1146–1150. [Google Scholar] [CrossRef] [PubMed]
- Bodley, R.; Jamous, A.; Short, D. Ultrasound in the early diagnosis of heterotopic ossification in patients with spinal injuries. Paraplegia 1993, 31, 500–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosteius, T.; Suero, E.M.; Grasmücke, D.; Aach, M.; Gisevius, A.; Ohlmeier, M.; Meindl, R.; Schildhauer, T.A.; Citak, M. The sensitivity of ultrasound screening examination in detecting heterotopic ossification following spinal cord injury. Spinal Cord 2017, 55, 71–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scola, F.H.; Parziale, J.R. Ultrasonographic diagnosis of heterotopic ossification mimicking deep vein thrombosis. J. Clin. Ultrasound 1991, 19, 55–57. [Google Scholar] [CrossRef] [PubMed]
- Lazo, M.G.; Shirazi, P.; Sam, M.; Giobbie-Hurder, A.; Blacconiere, M.J.; Muppidi, M. Osteoporosis and risk of fracture in men with spinal cord injury. Spinal Cord 2001, 39, 208–214. [Google Scholar] [CrossRef] [Green Version]
- Zehnder, Y.; Lüthi, M.; Michel, D.; Knecht, H.; Perrelet, R.; Neto, I.; Kraenzlin, M.; Zäch, G.; Lippuner, K. Long-term changes in bone metabolism, bone mineral density, quantitative ultrasound parameters, and fracture incidence after spinal cord injury: A cross-sectional observational study in 100 paraplegic men. Osteoporos. Int. 2004, 15, 180–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freehafer, A. Limb fractures in patients with spinal cord injury. Arch. Phys. Med. Rehabil. 1995, 76, 823–827. [Google Scholar] [CrossRef]

| Case | Age | Time from Injury | Level of Injury | Type of Paresis | Muscle Tension | D-Dimer Level (mg/L) | Cause of Edema |
|---|---|---|---|---|---|---|---|
| 1 | 57 | 1 month | Th12 | Paraplegia | Flaccid | 8035 | IH DVT |
| 2 | 44 | 1 month | Th10 | Paraplegia | Flaccid | 8159 | DVT IH |
| 3 | 28 | 2.5 months | C5–C7 | Paresis of upper limbs, paraplegia | Flaccid | 3534 | HO |
| 4 | 30 | 63 months | C5 | Paresis of upper limbs, paraplegia | Spastic (MAS 3/4) | 2900 | Fracture |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mackiewicz-Milewska, M.; Cisowska-Adamiak, M.; Sakwińska, K.; Szymkuć-Bukowska, I.; Głowacka-Mrotek, I. Massive Edema of the Lower Limbs in Patients after Spinal Cord Injury—One Picture, Different Diagnoses. Int. J. Environ. Res. Public Health 2021, 18, 4219. https://doi.org/10.3390/ijerph18084219
Mackiewicz-Milewska M, Cisowska-Adamiak M, Sakwińska K, Szymkuć-Bukowska I, Głowacka-Mrotek I. Massive Edema of the Lower Limbs in Patients after Spinal Cord Injury—One Picture, Different Diagnoses. International Journal of Environmental Research and Public Health. 2021; 18(8):4219. https://doi.org/10.3390/ijerph18084219
Chicago/Turabian StyleMackiewicz-Milewska, Magdalena, Małgorzata Cisowska-Adamiak, Katarzyna Sakwińska, Iwona Szymkuć-Bukowska, and Iwona Głowacka-Mrotek. 2021. "Massive Edema of the Lower Limbs in Patients after Spinal Cord Injury—One Picture, Different Diagnoses" International Journal of Environmental Research and Public Health 18, no. 8: 4219. https://doi.org/10.3390/ijerph18084219
APA StyleMackiewicz-Milewska, M., Cisowska-Adamiak, M., Sakwińska, K., Szymkuć-Bukowska, I., & Głowacka-Mrotek, I. (2021). Massive Edema of the Lower Limbs in Patients after Spinal Cord Injury—One Picture, Different Diagnoses. International Journal of Environmental Research and Public Health, 18(8), 4219. https://doi.org/10.3390/ijerph18084219






