Role of SIRT1 in Isoflurane Conditioning-Induced Neurovascular Protection against Delayed Cerebral Ischemia Secondary to Subarachnoid Hemorrhage
Abstract
:1. Introduction
2. Results
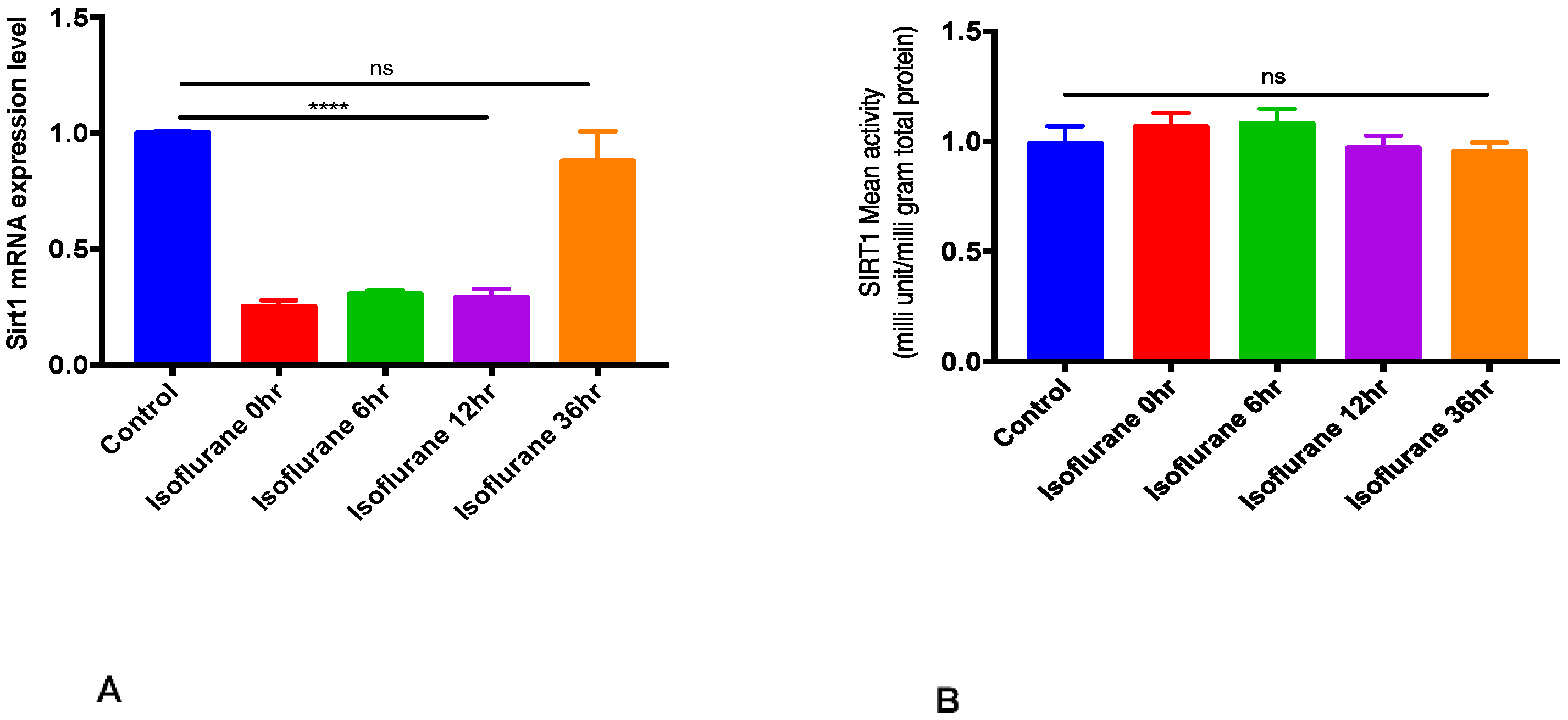
2.1. SIRT1 mRNA Expression and Activity after Isoflurane Conditioning in Naive Animals
2.2. Isoflurane Conditioning with or without EX-527 Administration Attenuated SAH-Induced Large Artery Vasospasm and Improved Neurological Deficits in Wild Type Mice
2.3. Isoflurane Conditioning with or without EX-527 Administration Attenuated SAH-Induced Microvessel Thrombosis in Wild-Type Mice
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Experimental SAH
4.3. Isoflurane Conditioning
4.4. EX-527 (a Selective SIRT1 Inhibitor) Treatment
4.5. Vasospasm Assessment
4.6. Neurobehavioral Assessment
4.7. Microvessel Thrombosis Assessment
4.8. Quantitative Polymerase Chain Reaction (qPCR) for SIRT1 Gene Expression
4.9. Enzyme-Linked Immunosorbent Assay (ELISA) for SIRT1 Protein Expression
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SAH | Subarachnoid hemorrhage |
| DCI | Delayed Cerebral Ischemia |
| SIRT1 | Silent mating type information regulation 2 homolog |
| NAD | Nicotinamide adenine dinucleotide |
| eNOS | Endothelial nitric oxide synthase |
| HIF-1α | Hypoxia inducible factor 1α |
| MCA | Middle cerebral artery |
| ICA | Internal carotid artery |
| ECA | External Carotid artery |
| ROX-SE | 5-(and-6)-Carboxy-X-rhodamine, succinimidyl ester |
| PBS | Phosphate-buffered saline |
| DMSO | Dimethyl Sulfoxide |
| TBS-T | Tris-Buffered Saline + Tween |
| BSA | Bovine Serum Albumin |
| Gapdh | Glyceraldehyde 3-phosphate dehydrogenase |
| ActB | Actin Beta |
References
- Broderick, J.P.; Brott, T.G.; Duldner, J.E.; Tomsick, T.; Leach, A. Initial and recurrent bleeding are the major causes of death following subarachnoid hemorrhage. Stroke 1994, 25, 1342–1347. [Google Scholar] [CrossRef] [Green Version]
- Brathwaite, S.; Macdonald, R.L. Current Management of Delayed Cerebral Ischemia: Update from Results of Recent Clinical Trials. Transl. Stroke Res. 2014, 5, 207–226. [Google Scholar] [CrossRef] [PubMed]
- van Gijn, J.; Kerr, R.S.; Rinkel, G.J. Subarachnoid haemorrhage. Lancet 2007, 369, 306–318. [Google Scholar] [CrossRef]
- Dernbach, P.D.; Little, J.R.; Jones, S.C.; Ebrahim, Z.Y. Altered Cerebral Autoregulation and CO2 Reactivity after Aneurysmal Subarachnoid Hemorrhage. Neurosurgery 1988, 22, 822–826. [Google Scholar] [CrossRef] [PubMed]
- Touho, H.; Ueda, H. Disturbance of autoregulation in patients with ruptured intracranial aneurysms: Mechanism of cortical and motor dysfunction. Surg. Neurol. 1994, 42, 57–64. [Google Scholar] [CrossRef]
- Sabri, M.; Ai, J.; Lakovic, K.; D’Abbondanza, J.; Ilodigwe, D.; Macdonald, R. Mechanisms of microthrombi formation after experimental subarachnoid hemorrhage. Neuroscience 2012, 224, 26–37. [Google Scholar] [CrossRef]
- Vergouwen, M.D.I.; Vermeulen, M.; Coert, B.A.; Stroes, E.S.G.; Roos, Y.B.W.E.M. Microthrombosis after Aneurysmal Subarachnoid Hemorrhage: An Additional Explanation for Delayed Cerebral Ischemia. Br. J. Pharmacol. 2008, 28, 1761–1770. [Google Scholar] [CrossRef] [PubMed]
- Gidday, J.M. Cerebral preconditioning and ischaemic tolerance. Nat. Rev. Neurosci. 2006, 7, 437–448. [Google Scholar] [CrossRef]
- Trendelenburg, G.; Dirnagl, U. Neuroprotective role of astrocytes in cerebral ischemia: Focus on ischemic preconditioning. Glia 2005, 50, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Bastide, M.; Gelé, P.; Pétrault, O.; Pu, Q.; Caliez, A.; Robin, E.; Deplanque, D.; Duriez, P.; Bordet, R. Delayed Cerebrovascular Protective Effect of Lipopolysaccharide in Parallel to Brain Ischemic Tolerance. Br. J. Pharmacol. 2003, 23, 399–405. [Google Scholar] [CrossRef] [Green Version]
- Vlasov, T.D.; Korzhevskii, D.E.; Polyakova, E.A. Ischemic Preconditioning of the Rat Brain as a Method of Endothelial Protection from Ischemic/Repercussion Injury. Neurosci. Behav. Physiol. 2005, 35, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Masada, T.; Hua, Y.; Xi, G.; Ennis, S.R.; Keep, R.F. Attenuation of Ischemic Brain EDEMA and Cerebrovascular Injury after Ischemic Preconditioning in the Rat. Br. J. Pharmacol. 2001, 21, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Stowe, A.M.; Altay, T.; Bs, A.B.F.; Gidday, J.M. Repetitive hypoxia extends endogenous neurovascular protection for stroke. Ann. Neurol. 2011, 69, 975–985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milner, E.; Johnson, A.W.; Nelson, J.W.; Harries, M.D.; Gidday, J.M.; Han, B.H.; Zipfel, G.J. HIF-1α Mediates Isoflurane-Induced Vascular Protection in Subarachnoid Hemorrhage. Ann. Clin. Transl. Neurol. 2015, 2, 325–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Athiraman, U.; Jayaraman, K.; Liu, M.; Giri, T.; Yuan, J.; Zipfel, G.J. Role of Endothelial Nitric Oxide Synthase in Isoflurane Conditioning-Induced Neurovascular Protection in Subarachnoid Hemorrhage. J. Am. Heart Assoc. 2020, 9, e017477. [Google Scholar] [CrossRef] [PubMed]
- Ramadori, G.; Lee, C.E.; Bookout, A.L.; Lee, S.; Williams, K.W.; Anderson, J.; Elmquist, J.K.; Coppari, R. Brain SIRT1: Anatomical Distribution and Regulation by Energy Availability. J. Neurosci. 2008, 28, 9989–9996. [Google Scholar] [CrossRef]
- Herskovits, A.Z.; Guarente, L. SIRT1 in Neurodevelopment and Brain Senescence. Neuron 2014, 81, 471–483. [Google Scholar] [CrossRef] [Green Version]
- Pallàs, M.; Pizarro, J.; Gutierrez-Cuesta, J.; Crespo-Biel, N.; Alvira, D.; Tajes, M.; Yeste-Velasco, M.; Folch, J.; Canudas, A.; Sureda, F.; et al. Modulation of SIRT1 expression in different neurodegenerative models and human pathologies. Neuroscience 2008, 154, 1388–1397. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.L. Sirt1′s Complex Roles in Neuroprotection. Cell. Mol. Neurobiol. 2009, 29, 1093–1103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, S.; Gan, L.; Vosler, P.S.; Gao, Y.; Zigmond, M.J.; Chen, J. Protective effects and mechanisms of sirtuins in the nervous system. Prog. Neurobiol. 2011, 95, 373–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vellimana, A.K.; Diwan, D.; Clarke, J.; Gidday, J.M.; Zipfel, G.J. SIRT1 Activation: A Potential Strategy for Harnessing Endogenous Protection Against Delayed Cerebral Ischemia After Subarachnoid Hemorrhage. Neurosurgery 2018, 65, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vellimana, A.K.; Aum, D.J.; Diwan, D.; Clarke, J.V.; Nelson, J.W.; Lawrence, M.; Han, B.H.; Gidday, J.M.; Zipfel, G.J. SIRT1 mediates hypoxic preconditioning induced attenuation of neurovascular dysfunction following subarachnoid hemorrhage. Exp. Neurol. 2020, 334, 113484. [Google Scholar] [CrossRef] [PubMed]
- Mattagajasingh, I.; Kim, C.-S.; Naqvi, A.; Yamamori, T.; Hoffman, T.A.; Jung, S.-B.; DeRicco, J.; Kasuno, K.; Irani, K. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc. Natl. Acad. Sci. USA 2007, 104, 14855–14860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rane, S.; He, M.; Sayed, D.; Vashistha, H.; Malhotra, A.; Sadoshima, J.; Vatner, D.E.; Vatner, S.F.; Abdellatif, M. Downregulation of MiR-199a Derepresses Hypoxia-Inducible Factor-1α and Sirtuin 1 and Recapitulates Hypoxia Preconditioning in Cardiac Myocytes. Circ. Res. 2009, 104, 879–886. [Google Scholar] [CrossRef]
- Rajamohan, S.B.; Pillai, V.B.; Gupta, M.; Sundaresan, N.R.; Birukov, K.G.; Samant, S.; Hottiger, M.O.; Gupta, M.P. SIRT1 Promotes Cell Survival under Stress by Deacetylation-Dependent Deactivation of Poly(ADP-Ribose) Polymerase 1. Mol. Cell. Biol. 2009, 29, 4116–4129. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.S.; Park, K.Y.; Min, H.G.; Lee, S.J.; Kim, J.; Choi, J.; Kim, W.; Cha, H. Negative regulation of stress-induced matrix metallopro-teinase-9 by Sirt1 in skin tissue. Exp. Dermatol. 2010, 19, 1060–1066. [Google Scholar] [CrossRef]
- Breitenstein, A.; Stein, S.; Holy, E.W.; Camici, G.G.; Lohmann, C.; Akhmedov, A.; Spescha, R.; Elliott, P.J.; Westphal, C.H.; Matter, C.M.; et al. Sirt1 inhibition promotes in vivo arterial thrombosis and tissue factor expression in stimulated cells. Cardiovasc. Res. 2010, 89, 464–472. [Google Scholar] [CrossRef]
- Chaitanya, G.V.; Alexander, J.S.; Babu, P.P. PARP-1 cleavage fragments: Signatures of cell-death proteases in neurodegeneration. Cell Commun. Signal. 2010, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Vellimana, A.K.; Milner, E.; Azad, T.D.; Harries, M.D.; Zhou, M.-L.; Gidday, J.M.; Han, B.H.; Zipfel, G.J. Endothelial Nitric Oxide Synthase Mediates Endogenous Protection Against Subarachnoid Hemorrhage-Induced Cerebral Vasospasm. Stroke 2011, 42, 776–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pillarisetti, S. A Review of Sirt1 and Sirt1 Modulators in Cardiovascular and Metabolic Diseases. Recent Pat. Cardiovasc. Drug Discov. 2008, 3, 156–164. [Google Scholar] [CrossRef]
- Satoh, A.; Stein, L.; Imai, S. The Role of Mammalian Sirtuins in the Regulation of Metabolism, Aging, and Longevity. Organotypic Models Drug Dev. 2011, 206, 125–162. [Google Scholar] [CrossRef] [Green Version]
- Raval, A.P.; Dave, K.R.; Perez-Pinzon, M.A. Resveratrol Mimics Ischemic Preconditioning in the Brain. Br. J. Pharmacol. 2005, 26, 1141–1147. [Google Scholar] [CrossRef] [Green Version]
- Perez-Pinzon, M.A.; Koronowski, K.B. Sirt1 in cerebral ischemia. Brain Circ. 2015, 1, 69–78. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.-S.; Wu, Q.; Wu, L.-Y.; Ye, Z.-N.; Jiang, T.-W.; Ling-Yun, W.; Zhuang, Z.; Zhou, M.-L.; Zhang, X.; Hang, C.-H. Sirtuin 1 activation protects against early brain injury after experimental subarachnoid hemorrhage in rats. Cell Death Dis. 2016, 7, e2416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, C.; Jin, J.; Chen, J.; Li, J.; Yu, X.; Mo, H.; Chen, G. SIRT1 activation by resveratrol reduces brain edema and neuronal apoptosis in an experimental rat subarachnoid hemorrhage model. Mol. Med. Rep. 2017, 16, 9627–9635. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Han, X. Resveratrol alleviates early brain injury following subarachnoid hemorrhage: Possible involvement of the AMPK/SIRT1/autophagy signaling pathway. Biol. Chem. 2018, 399, 1339–1350. [Google Scholar] [CrossRef]
- Athiraman, U.; Aum, D.; Vellimana, A.K.; Osbun, J.W.; Dhar, R.; Tempelhoff, R.; Zipfel, G.J. Evidence for a conditioning effect of inhalational anesthetics on angiographic vasospasm after aneurysmal subarachnoid hemorrhage. J. Neurosurg. 2020, 133, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Athiraman, U.; Dhar, R.; Jayaraman, K.; Karanikolas, M.; Helsten, D.; Yuan, J.; Lele, A.V.; Rath, G.P.; Tempelhoff, R.; Roth, S.; et al. Conditioning Effect of Inhalational Anesthetics on Delayed Cerebral Ischemia After Aneurysmal Subarachnoid Hemorrhage. Neurosurgery 2020, 88, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Athiraman, U.; Liu, M.; Jayaraman, K.; Yuan, J.; Mehla, J.; Zipfel, G.J. Anesthetic and subanesthetic doses of isoflurane conditioning provides strong protection against delayed cerebral ischemia in a mouse model of subarachnoid hemorrhage. Brain Res. 2021, 1750, 147169. [Google Scholar] [CrossRef]
- Athiraman, U.; Zipfel, G.J. Anesthetic Conditioning for Secondary Brain Injury After Aneurysmal Subarachnoid Hemorrhage. World Neurosurg. 2020, 143, 577–578. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Miguelez, P.; Lima-Cabello, E.; Martínez-Flórez, S.; Almar, M.; Cuevas, M.J.; González-Gallego, J. Hypoxia-inducible factor-1 modulates the expression of vascular endothelial growth factor and endothelial nitric oxide synthase induced by eccentric exercise. J. Appl. Physiol. 2015, 118, 1075–1083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alique, M.; Sánchez-López, E.; Bodega, G.; Giannarelli, C.; Carracedo, J.; Ramírez, R. Hypoxia-Inducible Factor-1α: The Master Regulator of Endothelial Cell Senescence in Vascular Aging. Cells 2020, 9, 195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagel, S.; Papadakis, M.; Chen, R.; Hoyte, L.C.; Brooks, K.J.; Gallichan, D.; Sibson, N.R.; Pugh, C.; Buchan, A.M. Neuroprotection by Dimethyloxalylglycine following Permanent and Transient Focal Cerebral Ischemia in Rats. Br. J. Pharmacol. 2010, 31, 132–143. [Google Scholar] [CrossRef] [Green Version]
- Mi, D.-H.; Fang, H.-J.; Zheng, G.-H.; Liang, X.-H.; Ding, Y.-R.; Liu, X.; Liu, L.-P. DPP-4 inhibitors promote proliferation and migration of rat brain microvascular endothelial cells under hypoxic/high-glucose conditions, potentially through the SIRT1/HIF-1/VEGF pathway. CNS Neurosci. Ther. 2018, 25, 323–332. [Google Scholar] [CrossRef]
- Hong-Qiang, H.; Mang-Qiao, S.; Fen, X.; Shan-Shan, L.; Hui-Juan, C.; Wu-Gang, H.; Wen-Jun, Y.; Zheng-Wu, P. Sirt1 mediates improvement of isoflurane-induced memory impairment following hyperbaric oxygen preconditioning in middle-aged mice. Physiol. Behav. 2018, 195, 1–8. [Google Scholar] [CrossRef]
- Fang, X.; Han, Q.; Li, S.; Zhao, Y.; Luo, A. Chikusetsu saponin IVa attenuates isoflurane-induced neurotoxicity and cognitive def-icits via SIRT1/ERK1/2 in developmental rats. Am. J. Transl. Res. 2017, 9, 4288–4299. [Google Scholar]
- Liu, L.; Liu, C.; Fang, L. AMPK-SIRT1 pathway dysfunction contributes to neuron apoptosis and cognitive impairment induced by sevoflurane. Mol. Med. Rep. 2020, 23, 1. [Google Scholar] [CrossRef]
- Yang, X.-Y.; Li, Q.-J.; Zhang, W.-C.; Zheng, S.-Q.; Qu, Z.-J.; Xi, Y.; Wang, G. AMPK-SIRT1-PGC1α Signal Pathway Influences the Cognitive Function of Aged Rats in Sevoflurane-Induced Anesthesia. J. Mol. Neurosci. 2020, 70, 2058–2067. [Google Scholar] [CrossRef]
- Peck, B.; Chen, C.-Y.; Ho, K.-K.; Di Fruscia, P.; Myatt, S.S.; Coombes, R.C.; Fuchter, M.J.; Hsiao, C.-D.; Lam, E.W.-F. SIRT Inhibitors Induce Cell Death and p53 Acetylation through Targeting Both SIRT1 and SIRT2. Mol. Cancer Ther. 2010, 9, 844–855. [Google Scholar] [CrossRef] [Green Version]
- Aum, D.J.; Vellimana, A.K.; Singh, I.; Milner, E.; Nelson, J.W.; Han, B.H.; Zipfel, G.J. A novel fluorescent imaging technique for assessment of cerebral vasospasm after experimental subarachnoid hemorrhage. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Jayaraman, K.; Giri, T.; Zipfel, G.J.; Athiraman, U. Role of SIRT1 in Isoflurane Conditioning-Induced Neurovascular Protection against Delayed Cerebral Ischemia Secondary to Subarachnoid Hemorrhage. Int. J. Mol. Sci. 2021, 22, 4291. https://doi.org/10.3390/ijms22084291
Liu M, Jayaraman K, Giri T, Zipfel GJ, Athiraman U. Role of SIRT1 in Isoflurane Conditioning-Induced Neurovascular Protection against Delayed Cerebral Ischemia Secondary to Subarachnoid Hemorrhage. International Journal of Molecular Sciences. 2021; 22(8):4291. https://doi.org/10.3390/ijms22084291
Chicago/Turabian StyleLiu, Meizi, Keshav Jayaraman, Tusar Giri, Gregory J. Zipfel, and Umeshkumar Athiraman. 2021. "Role of SIRT1 in Isoflurane Conditioning-Induced Neurovascular Protection against Delayed Cerebral Ischemia Secondary to Subarachnoid Hemorrhage" International Journal of Molecular Sciences 22, no. 8: 4291. https://doi.org/10.3390/ijms22084291
APA StyleLiu, M., Jayaraman, K., Giri, T., Zipfel, G. J., & Athiraman, U. (2021). Role of SIRT1 in Isoflurane Conditioning-Induced Neurovascular Protection against Delayed Cerebral Ischemia Secondary to Subarachnoid Hemorrhage. International Journal of Molecular Sciences, 22(8), 4291. https://doi.org/10.3390/ijms22084291





