Recent Advances in Effector-Triggered Immunity in Plants: New Pieces in the Puzzle Create a Different Paradigm
Abstract
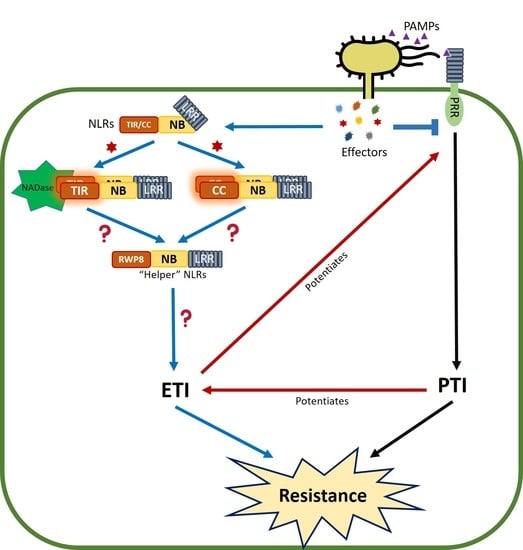
:1. Introduction
2. The Evolution of Pathogen Perception by NLRs
3. NLR Activation and Signaling Events Following Pathogen Recognition
3.1. Multi-Domain NLRs Act as Molecular Switches
3.2. Homo/Hetero-Complex Formation Is Necessary for NLR Signaling
3.3. Intramolecular Regulation of Guardee/Decoy Contributes to NLR-Mediated Resistance
3.4. News-Breaking: Enzyme Activity of Plant TIR in ETI Signaling
4. Helper NLR Cooperation beyond Genetically Linked Pairs
5. Transcriptional Control in ETI
5.1. NLRs Regulate Transcriptional Control
5.2. Effector-Regulated Transcriptional Control
6. PTI/ETI Unity Produces Full Plant Immunity
7. Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADR1 | ACTIVATED DISEASE RESISTANCE PROTEIN 1 |
| Avr | Avirulence |
| AvrPphB | Avirulence protein Pseudomonas phaseolicola B |
| bHLH84 | basic Helix-Loop-Helix 84 |
| BIK1 | BOTRYTIS-INDUCED KINASE 1 |
| CC | Coiled-Coil |
| CNL | Coiled-coil NLR |
| DAMP | Damage-Associated Molecular Pattern |
| EDS1 | ENHANCED DISEASE SUSCEPTIBILITY 1 |
| ETI | Effector-Triggered Immunity |
| Hop | Hrp-dependent outer protein |
| HR | Hypersensitive Response |
| ID | Integrated Decoy |
| LRR | Leucine-Rich Repeat |
| MAPK | Mitogen-Activated Protein Kinase |
| MLA10 | Mildew A 10 |
| MYB | Myeloblastosis |
| NAD | Nicotinamide Adenine Dinucleotide |
| NB | Nucleotide-Binding |
| NLR | Nucleotide-binding and Leucine-rich-repeat-containing Receptor |
| NRG1 | N REQUIREMENT GENE 1 |
| PAMP | Pathogen-Associated Molecular Pattern |
| PBS1 | AVRPPHB-SUSCEPTIBLE 1 |
| PopP2 | Pseudomonas-out-protein P2 |
| PRR | Pattern Recognition Receptor |
| PTI | Pattern-Triggered Immunity |
| R | Resistance |
| RBA1 | Response to the bacterial type III effector protein HopBA1 |
| RBOHD | RESPIRATORY BURST OXIDASE HOMOLOGUE D |
| RGA5 | R-GENE ANALOG 5 |
| RIN4 | RPM1-INTERACTING PROTEIN 4 |
| ROS | Reactive Oxygen Species |
| RPM1 | RESISTANCE TO PSEUDOMONAS SYRINGAE PV. MACULICOLA 1 |
| RPP1 | RECOGNITION OF PERONOSPORA PARASITICA 1 |
| RPS2 | RESISTANCE TO PSEUDOMONAS SYRINGAE 2 |
| RPW8 | Resistance to Powdery Mildew 8 |
| RRS1 | RESISTANCE TO RALSTONIA SOLANACEARUM 1 |
| SARM1 | Sterile Alpha and TIR Motif Containing 1 |
| SNC1 | SUPPRESSOR OF npr1-1, CONSTITUTIVE1 |
| SRFR1 | SUPPRESSOR OF rps4-RLD1 |
| TIR | Toll-interleukin 1-like Receptor |
| TNL | Toll interleukin-1-receptor NLR |
| TPR1 | TOPLESS-RELATED PROTEIN 1 |
| v-cADPR | Variant-cyclic ADP-ribose |
| XopQ | Xanthomonas outer protein Q |
References
- Dangl, J.L.; Horvath, D.M.; Staskawicz, B.J. Pivoting the plant immune system from dissection to deployment. Science 2013, 341, 746–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pascale, A.; Proietti, S.; Pantelides, I.S.; Stringlis, I.A. Modulation of the Root Microbiome by Plant Molecules: The Basis for Targeted Disease Suppression and Plant Growth Promotion. Front. Plant. Sci. 2019, 10, 1741. [Google Scholar] [CrossRef] [PubMed]
- Lapin, D.; Van den Ackerveken, G. Susceptibility to plant disease: More than a failure of host immunity. Trends Plant Sci. 2013, 18, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Staskawicz, B.J. Genetics of plant-pathogen interactions specifying plant disease resistance. Plant Physiol. 2001, 125, 73–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, J.D.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [Green Version]
- Saijo, Y.; Loo, E.P.; Yasuda, S. Pattern recognition receptors and signaling in plant-microbe interactions. Plant J. 2018, 93, 592–613. [Google Scholar] [CrossRef]
- He, Y.; Zhou, J.; Shan, L.; Meng, X. Plant cell surface receptor-mediated signaling—A common theme amid diversity. J. Cell Sci. 2018, 131. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Zhou, J.M. Receptor-like kinases in plant innate immunity. J. Integr. Plant Biol. 2013, 55, 1271–1286. [Google Scholar] [CrossRef] [PubMed]
- Zipfel, C. Pattern-recognition receptors in plant innate immunity. Curr. Opin. Immunol. 2008, 20, 10–16. [Google Scholar] [CrossRef]
- Bigeard, J.; Colcombet, J.; Hirt, H. Signaling mechanisms in pattern-triggered immunity (PTI). Mol. Plant 2015, 8, 521–539. [Google Scholar] [CrossRef] [Green Version]
- Jeworutzki, E.; Roelfsema, M.R.; Anschutz, U.; Krol, E.; Elzenga, J.T.; Felix, G.; Boller, T.; Hedrich, R.; Becker, D. Early signaling through the Arabidopsis pattern recognition receptors FLS2 and EFR involves Ca-associated opening of plasma membrane anion channels. Plant J. 2010, 62, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Ranf, S.; Eschen-Lippold, L.; Pecher, P.; Lee, J.; Scheel, D. Interplay between calcium signalling and early signalling elements during defence responses to microbe- or damage-associated molecular patterns. Plant J. 2011, 68, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Nomura, H.; Komori, T.; Uemura, S.; Kanda, Y.; Shimotani, K.; Nakai, K.; Furuichi, T.; Takebayashi, K.; Sugimoto, T.; Sano, S.; et al. Chloroplast-mediated activation of plant immune signalling in Arabidopsis. Nat. Commun. 2012, 3, 926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Shao, F.; Li, Y.; Cui, H.; Chen, L.; Li, H.; Zou, Y.; Long, C.; Lan, L.; Chai, J.; et al. A Pseudomonas syringae effector inactivates MAPKs to suppress PAMP-induced immunity in plants. Cell Host Microbe 2007, 1, 175–185. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Wu, Y.; Gao, M.; Zhang, J.; Kong, Q.; Liu, Y.; Ba, H.; Zhou, J.; Zhang, Y. Disruption of PAMP-induced MAP kinase cascade by a Pseudomonas syringae effector activates plant immunity mediated by the NB-LRR protein SUMM2. Cell Host Microbe 2012, 11, 253–263. [Google Scholar] [CrossRef] [Green Version]
- Chinchilla, D.; Zipfel, C.; Robatzek, S.; Kemmerling, B.; Nurnberger, T.; Jones, J.D.; Felix, G.; Boller, T. A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature 2007, 448, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Yun, B.W.; Feechan, A.; Yin, M.; Saidi, N.B.; Le Bihan, T.; Yu, M.; Moore, J.W.; Kang, J.G.; Kwon, E.; Spoel, S.H.; et al. S-nitrosylation of NADPH oxidase regulates cell death in plant immunity. Nature 2011, 478, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Scheler, C.; Durner, J.; Astier, J. Nitric oxide and reactive oxygen species in plant biotic interactions. Curr. Opin. Plant Biol. 2013, 16, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, Y.; Saito, K. Roles of lipids as signaling molecules and mitigators during stress response in plants. Plant J. 2014, 79, 584–596. [Google Scholar] [CrossRef]
- Wang, Z.; Li, X.; Wang, X.; Liu, N.; Xu, B.; Peng, Q.; Guo, Z.; Fan, B.; Zhu, C.; Chen, Z. Arabidopsis Endoplasmic Reticulum-Localized UBAC2 Proteins Interact with PAMP-INDUCED COILED-COIL to Regulate Pathogen-Induced Callose Deposition and Plant Immunity. Plant Cell 2019, 31, 153–171. [Google Scholar] [CrossRef] [Green Version]
- Kiba, A.; Nakano, M.; Hosokawa, M.; Galis, I.; Nakatani, H.; Shinya, T.; Ohnishi, K.; Hikichi, Y. Phosphatidylinositol-phospholipase C2 regulates pattern-triggered immunity in Nicotiana benthamiana. J. Exp. Bot. 2020, 71, 5027–5038. [Google Scholar] [CrossRef]
- Jin, L.; Mackey, D.M. Measuring Callose Deposition, an Indicator of Cell Wall Reinforcement, During Bacterial Infection in Arabidopsis. Methods Mol. Biol. 2017, 1578, 195–205. [Google Scholar] [CrossRef]
- Nakano, M.; Nishihara, M.; Yoshioka, H.; Takahashi, H.; Sawasaki, T.; Ohnishi, K.; Hikichi, Y.; Kiba, A. Suppression of DS1 phosphatidic acid phosphatase confirms resistance to Ralstonia solanacearum in Nicotiana benthamiana. PLoS ONE 2013, 8, e75124. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Wang, Y.; Li, X.; Zhang, Y. Biosynthesis and Regulation of Salicylic Acid and N-Hydroxypipecolic Acid in Plant Immunity. Mol. Plant 2020, 13, 31–41. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, F.; Jiang, L.; Chen, C.; Wu, L.; Liu, Z. Different Pathogen Defense Strategies in Arabidopsis: More than Pathogen Recognition. Cells 2018, 7, 252. [Google Scholar] [CrossRef] [Green Version]
- Bryksova, M.; Dabravolski, S.; Kucerova, Z.; Zavadil Kokas, F.; Spundova, M.; Plihalova, L.; Takac, T.; Gruz, J.; Hudecek, M.; Hlouskova, V.; et al. Aromatic Cytokinin Arabinosides Promote PAMP-like Responses and Positively Regulate Leaf Longevity. ACS Chem. Biol. 2020, 15, 1949–1963. [Google Scholar] [CrossRef]
- Jarad, M.; Mariappan, K.; Almeida-Trapp, M.; Mette, M.F.; Mithofer, A.; Rayapuram, N.; Hirt, H. The Lamin-Like LITTLE NUCLEI 1 (LINC1) Regulates Pattern-Triggered Immunity and Jasmonic Acid Signaling. Front. Plant Sci. 2019, 10, 1639. [Google Scholar] [CrossRef] [PubMed]
- Zavaliev, R.; Mohan, R.; Chen, T.; Dong, X. Formation of NPR1 Condensates Promotes Cell Survival during the Plant Immune Response. Cell 2020, 182, 1093–1108.e18. [Google Scholar] [CrossRef] [PubMed]
- Tada, Y.; Spoel, S.H.; Pajerowska-Mukhtar, K.; Mou, Z.; Song, J.; Wang, C.; Zuo, J.; Dong, X. Plant immunity requires conformational changes of NPR1 via S-nitrosylation and thioredoxins. Science 2008, 321, 952–956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, Y.; van Wersch, R.; Zhang, Y. Convergent and Divergent Signaling in PAMP-Triggered Immunity and Effector-Triggered Immunity. Mol. Plant Microbe Interact. 2018, 31, 403–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alhoraibi, H.; Bigeard, J.; Rayapuram, N.; Colcombet, J.; Hirt, H. Plant Immunity: The MTI-ETI Model and Beyond. Curr. Issues Mol. Biol. 2019, 30, 39–58. [Google Scholar] [CrossRef]
- Richard, M.M.S.; Gratias, A.; Meyers, B.C.; Geffroy, V. Molecular mechanisms that limit the costs of NLR-mediated resistance in plants. Mol. Plant Pathol. 2018, 19, 2516–2523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Wersch, S.; Li, X. Stronger When Together: Clustering of Plant NLR Disease resistance Genes. Trends Plant Sci. 2019, 24, 688–699. [Google Scholar] [CrossRef] [PubMed]
- Dangl, J.L.; Jones, J.D. Plant pathogens and integrated defence responses to infection. Nature 2001, 411, 826–833. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, Y.; Du, J.; Zhan, Y.; Sun, D.; Zhao, J.; Zhang, S.; Li, J.; He, K. Both Light-Induced SA Accumulation and ETI Mediators Contribute to the Cell Death Regulated by BAK1 and BKK1. Front. Plant Sci. 2017, 8, 622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, Y.; Xie, Z.; Chen, W.; Glazebrook, J.; Chang, H.S.; Han, B.; Zhu, T.; Zou, G.; Katagiri, F. Quantitative nature of Arabidopsis responses during compatible and incompatible interactions with the bacterial pathogen Pseudomonas syringae. Plant Cell 2003, 15, 317–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro, L.; Zipfel, C.; Rowland, O.; Keller, I.; Robatzek, S.; Boller, T.; Jones, J.D. The transcriptional innate immune response to flg22. Interplay and overlap with Avr gene-dependent defense responses and bacterial pathogenesis. Plant Physiol. 2004, 135, 1113–1128. [Google Scholar] [CrossRef] [Green Version]
- Ngou, B.P.M.; Ahn, H.K.; Ding, P.; Jones, J.D.G. Mutual potentiation of plant immunity by cell-surface and intracellular receptors. Nature 2021. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Jiang, Z.; Bi, G.; Nomura, K.; Liu, M.; Wang, Y.; Cai, B.; Zhou, J.M.; He, S.Y.; Xin, X.F. Pattern-recognition receptors are required for NLR-mediated plant immunity. Nature 2021. [Google Scholar] [CrossRef] [PubMed]
- Van der Biezen, E.A.; Jones, J.D. Plant disease-resistance proteins and the gene-for-gene concept. Trends Biochem. Sci. 1998, 23, 454–456. [Google Scholar] [CrossRef]
- Cesari, S.; Kanzaki, H.; Fujiwara, T.; Bernoux, M.; Chalvon, V.; Kawano, Y.; Shimamoto, K.; Dodds, P.; Terauchi, R.; Kroj, T. The NB-LRR proteins RGA4 and RGA5 interact functionally and physically to confer disease resistance. EMBO J. 2014, 33, 1941–1959. [Google Scholar] [CrossRef] [PubMed]
- Cesari, S. Multiple strategies for pathogen perception by plant immune receptors. New Phytol. 2018, 219, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Mackey, D.; Belkhadir, Y.; Alonso, J.M.; Ecker, J.R.; Dangl, J.L. Arabidopsis RIN4 is a target of the type III virulence effector AvrRpt2 and modulates RPS2-mediated resistance. Cell 2003, 112, 379–389. [Google Scholar] [CrossRef] [Green Version]
- Axtell, M.J.; Staskawicz, B.J. Initiation of RPS2-specified disease resistance in Arabidopsis is coupled to the AvrRpt2-directed elimination of RIN4. Cell 2003, 112, 369–377. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.S.; Desveaux, D.; Singer, A.U.; Patel, P.; Sondek, J.; Dangl, J.L. The Pseudomonas syringae effector AvrRpt2 cleaves its C-terminally acylated target, RIN4, from Arabidopsis membranes to block RPM1 activation. Proc. Natl. Acad. Sci. USA 2005, 102, 6496–6501. [Google Scholar] [CrossRef] [Green Version]
- Mackey, D.; Holt, B.F., 3rd; Wiig, A.; Dangl, J.L. RIN4 interacts with Pseudomonas syringae type III effector molecules and is required for RPM1-mediated resistance in Arabidopsis. Cell 2002, 108, 743–754. [Google Scholar] [CrossRef] [Green Version]
- Prokchorchik, M.; Choi, S.; Chung, E.H.; Won, K.; Dangl, J.L.; Sohn, K.H. A host target of a bacterial cysteine protease virulence effector plays a key role in convergent evolution of plant innate immune system receptors. New Phytol. 2020, 225, 1327–1342. [Google Scholar] [CrossRef]
- Su, J.; Spears, B.J.; Kim, S.H.; Gassmann, W. Constant vigilance: Plant functions guarded by resistance proteins. Plant J. 2018, 93, 637–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Li, W.; Xiang, T.; Liu, Z.; Laluk, K.; Ding, X.; Zou, Y.; Gao, M.; Zhang, X.; Chen, S.; et al. Receptor-like cytoplasmic kinases integrate signaling from multiple plant immune receptors and are targeted by a Pseudomonas syringae effector. Cell Host Microbe 2010, 7, 290–301. [Google Scholar] [CrossRef] [Green Version]
- DeYoung, B.J.; Qi, D.; Kim, S.H.; Burke, T.P.; Innes, R.W. Activation of a plant nucleotide binding-leucine rich repeat disease resistance protein by a modified self protein. Cell Microbiol. 2012, 14, 1071–1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.H.; Qi, D.; Ashfield, T.; Helm, M.; Innes, R.W. Using decoys to expand the recognition specificity of a plant disease resistance protein. Science 2016, 351, 684–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, D.; DeYoung, B.J.; Innes, R.W. Structure-function analysis of the coiled-coil and leucine-rich repeat domains of the RPS5 disease resistance protein. Plant Physiol. 2012, 158, 1819–1832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, F.; Golstein, C.; Ade, J.; Stoutemyer, M.; Dixon, J.E.; Innes, R.W. Cleavage of Arabidopsis PBS1 by a bacterial type III effector. Science 2003, 301, 1230–1233. [Google Scholar] [CrossRef]
- Birker, D.; Heidrich, K.; Takahara, H.; Narusaka, M.; Deslandes, L.; Narusaka, Y.; Reymond, M.; Parker, J.E.; O’Connell, R. A locus conferring resistance to Colletotrichum higginsianum is shared by four geographically distinct Arabidopsis accessions. Plant J. 2009, 60, 602–613. [Google Scholar] [CrossRef] [PubMed]
- Narusaka, M.; Shirasu, K.; Noutoshi, Y.; Kubo, Y.; Shiraishi, T.; Iwabuchi, M.; Narusaka, Y. RRS1 and RPS4 provide a dual Resistance-gene system against fungal and bacterial pathogens. Plant J. 2009, 60, 218–226. [Google Scholar] [CrossRef]
- Le Roux, C.; Huet, G.; Jauneau, A.; Camborde, L.; Tremousaygue, D.; Kraut, A.; Zhou, B.; Levaillant, M.; Adachi, H.; Yoshioka, H.; et al. A receptor pair with an integrated decoy converts pathogen disabling of transcription factors to immunity. Cell 2015, 161, 1074–1088. [Google Scholar] [CrossRef] [Green Version]
- Sarris, P.F.; Duxbury, Z.; Huh, S.U.; Ma, Y.; Segonzac, C.; Sklenar, J.; Derbyshire, P.; Cevik, V.; Rallapalli, G.; Saucet, S.B.; et al. A Plant Immune Receptor Detects Pathogen Effectors that Target WRKY Transcription Factors. Cell 2015, 161, 1089–1100. [Google Scholar] [CrossRef] [Green Version]
- Cesari, S.; Bernoux, M.; Moncuquet, P.; Kroj, T.; Dodds, P.N. A novel conserved mechanism for plant NLR protein pairs: The “integrated decoy” hypothesis. Front. Plant Sci. 2014, 5, 606. [Google Scholar] [CrossRef] [Green Version]
- Griebel, T.; Maekawa, T.; Parker, J.E. NOD-like receptor cooperativity in effector-triggered immunity. Trends Immunol. 2014, 35, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Kapos, P.; Devendrakumar, K.T.; Li, X. Plant NLRs: From discovery to application. Plant Sci. 2019, 279, 3–18. [Google Scholar] [CrossRef]
- Monteiro, F.; Nishimura, M.T. Structural, Functional, and Genomic Diversity of Plant NLR Proteins: An Evolved Resource for Rational Engineering of Plant Immunity. Annu. Rev. Phytopathol. 2018, 56, 243–267. [Google Scholar] [CrossRef] [Green Version]
- Cesari, S.; Thilliez, G.; Ribot, C.; Chalvon, V.; Michel, C.; Jauneau, A.; Rivas, S.; Alaux, L.; Kanzaki, H.; Okuyama, Y.; et al. The rice resistance protein pair RGA4/RGA5 recognizes the Magnaporthe oryzae effectors AVR-Pia and AVR1-CO39 by direct binding. Plant Cell 2013, 25, 1463–1481. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.C.; Gassmann, W. RPS4-mediated disease resistance requires the combined presence of RPS4 transcripts with full-length and truncated open reading frames. Plant Cell 2003, 15, 2333–2342. [Google Scholar] [CrossRef]
- Yang, S.; Tang, F.; Zhu, H. Alternative splicing in plant immunity. Int. J. Mol. Sci. 2014, 15, 10424–10445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casey, L.W.; Lavrencic, P.; Bentham, A.R.; Cesari, S.; Ericsson, D.J.; Croll, T.; Turk, D.; Anderson, P.A.; Mark, A.E.; Dodds, P.N.; et al. The CC domain structure from the wheat stem rust resistance protein Sr33 challenges paradigms for dimerization in plant NLR proteins. Proc. Natl. Acad. Sci. USA 2016, 113, 12856–12861. [Google Scholar] [CrossRef] [Green Version]
- Cesari, S.; Moore, J.; Chen, C.; Webb, D.; Periyannan, S.; Mago, R.; Bernoux, M.; Lagudah, E.S.; Dodds, P.N. Cytosolic activation of cell death and stem rust resistance by cereal MLA-family CC-NLR proteins. Proc. Natl. Acad. Sci. USA 2016, 113, 10204–10209. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Hu, M.; Wang, J.; Qi, J.; Han, Z.; Wang, G.; Qi, Y.; Wang, H.W.; Zhou, J.M.; Chai, J. Reconstitution and structure of a plant NLR resistosome conferring immunity. Science 2019, 364. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bernoux, M.; Bentham, A.R.; Newman, T.E.; Ve, T.; Casey, L.W.; Raaymakers, T.M.; Hu, J.; Croll, T.I.; Schreiber, K.J.; et al. Multiple functional self-association interfaces in plant TIR domains. Proc. Natl. Acad. Sci. USA 2017, 114, E2046–E2052. [Google Scholar] [CrossRef] [Green Version]
- Ma, S.; Lapin, D.; Liu, L.; Sun, Y.; Song, W.; Zhang, X.; Logemann, E.; Yu, D.; Wang, J.; Jirschitzka, J.; et al. Direct pathogen-induced assembly of an NLR immune receptor complex to form a holoenzyme. Science 2020, 370. [Google Scholar] [CrossRef] [PubMed]
- Bernoux, M.; Burdett, H.; Williams, S.J.; Zhang, X.; Chen, C.; Newell, K.; Lawrence, G.J.; Kobe, B.; Ellis, J.G.; Anderson, P.A.; et al. Comparative Analysis of the Flax Immune Receptors L6 and L7 Suggests an Equilibrium-Based Switch Activation Model. Plant Cell 2016, 28, 146–159. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Ahn, H.K.; Sklenar, J.; Huang, J.; Ma, Y.; Ding, P.; Menke, F.L.H.; Jones, J.D.G. Phosphorylation-Regulated Activation of the Arabidopsis RRS1-R/RPS4 Immune Receptor Complex Reveals Two Distinct Effector Recognition Mechanisms. Cell Host Microbe 2020, 27, 769–781.e6. [Google Scholar] [CrossRef]
- Huh, S.U.; Cevik, V.; Ding, P.; Duxbury, Z.; Ma, Y.; Tomlinson, L.; Sarris, P.F.; Jones, J.D.G. Protein-protein interactions in the RPS4/RRS1 immune receptor complex. PLoS Pathog. 2017, 13, e1006376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, R.; Qi, T.; Zhang, H.; Liu, F.; King, M.; Toth, C.; Nogales, E.; Staskawicz, B.J. Structure of the activated ROQ1 resistosome directly recognizing the pathogen effector XopQ. Science 2020, 370. [Google Scholar] [CrossRef]
- Sinapidou, E.; Williams, K.; Nott, L.; Bahkt, S.; Tor, M.; Crute, I.; Bittner-Eddy, P.; Beynon, J. Two TIR:NB:LRR genes are required to specify resistance to Peronospora parasitica isolate Cala2 in Arabidopsis. Plant J. 2004, 38, 898–909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saucet, S.B.; Ma, Y.; Sarris, P.F.; Furzer, O.J.; Sohn, K.H.; Jones, J.D. Two linked pairs of Arabidopsis TNL resistance genes independently confer recognition of bacterial effector AvrRps4. Nat. Commun. 2015, 6, 6338. [Google Scholar] [CrossRef]
- Williams, S.J.; Sohn, K.H.; Wan, L.; Bernoux, M.; Sarris, P.F.; Segonzac, C.; Ve, T.; Ma, Y.; Saucet, S.B.; Ericsson, D.J.; et al. Structural basis for assembly and function of a heterodimeric plant immune receptor. Science 2014, 344, 299–303. [Google Scholar] [CrossRef]
- Nishimura, M.T.; Anderson, R.G.; Cherkis, K.A.; Law, T.F.; Liu, Q.L.; Machius, M.; Nimchuk, Z.L.; Yang, L.; Chung, E.H.; El Kasmi, F.; et al. TIR-only protein RBA1 recognizes a pathogen effector to regulate cell death in Arabidopsis. Proc. Natl. Acad. Sci. USA 2017, 114, E2053–E2062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, E.H.; El-Kasmi, F.; He, Y.; Loehr, A.; Dangl, J.L. A plant phosphoswitch platform repeatedly targeted by type III effector proteins regulates the output of both tiers of plant immune receptors. Cell Host Microbe 2014, 16, 484–494. [Google Scholar] [CrossRef] [Green Version]
- Redditt, T.J.; Chung, E.H.; Karimi, H.Z.; Rodibaugh, N.; Zhang, Y.; Trinidad, J.C.; Kim, J.H.; Zhou, Q.; Shen, M.; Dangl, J.L.; et al. AvrRpm1 Functions as an ADP-Ribosyl Transferase to Modify NOI Domain-Containing Proteins, Including Arabidopsis and Soybean RPM1-Interacting Protein4. Plant Cell 2019, 31, 2664–2681. [Google Scholar] [CrossRef]
- Takeda, K.; Akira, S. Toll-like receptors. Curr. Protoc. Immunol. 2015, 109, 14.12.1–14.12.10. [Google Scholar] [CrossRef]
- Kumar, H.; Kawai, T.; Akira, S. Toll-like receptors and innate immunity. Biochem. Biophys. Res. Commun. 2009, 388, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Horsefield, S.; Burdett, H.; Zhang, X.; Manik, M.K.; Shi, Y.; Chen, J.; Qi, T.; Gilley, J.; Lai, J.S.; Rank, M.X.; et al. NAD(+) cleavage activity by animal and plant TIR domains in cell death pathways. Science 2019, 365, 793–799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerdts, J.; Brace, E.J.; Sasaki, Y.; DiAntonio, A.; Milbrandt, J. SARM1 activation triggers axon degeneration locally via NAD(+) destruction. Science 2015, 348, 453–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Essuman, K.; Summers, D.W.; Sasaki, Y.; Mao, X.; Yim, A.K.Y.; DiAntonio, A.; Milbrandt, J. TIR Domain Proteins Are an Ancient Family of NAD(+)-Consuming Enzymes. Curr. Biol. 2018, 28, 421–430.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayless, A.M.; Nishimura, M.T. Enzymatic Functions for Toll/Interleukin-1 Receptor Domain Proteins in the Plant Immune System. Front. Genet. 2020, 11, 539. [Google Scholar] [CrossRef]
- Wan, L.; Essuman, K.; Anderson, R.G.; Sasaki, Y.; Monteiro, F.; Chung, E.H.; Osborne Nishimura, E.; DiAntonio, A.; Milbrandt, J.; Dangl, J.L.; et al. TIR domains of plant immune receptors are NAD(+)-cleaving enzymes that promote cell death. Science 2019, 365, 799–803. [Google Scholar] [CrossRef]
- Duxbury, Z.; Wang, S.; MacKenzie, C.I.; Tenthorey, J.L.; Zhang, X.; Huh, S.U.; Hu, L.; Hill, L.; Ngou, P.M.; Ding, P.; et al. Induced proximity of a TIR signaling domain on a plant-mammalian NLR chimera activates defense in plants. Proc. Natl. Acad. Sci. USA 2020, 117, 18832–18839. [Google Scholar] [CrossRef]
- Marcec, M.J.; Gilroy, S.; Poovaiah, B.W.; Tanaka, K. Mutual interplay of Ca(2+) and ROS signaling in plant immune response. Plant Sci. 2019, 283, 343–354. [Google Scholar] [CrossRef]
- Guse, A.H. Calcium mobilizing second messengers derived from NAD. Biochim. Biophys. Acta 2015, 1854, 1132–1137. [Google Scholar] [CrossRef] [PubMed]
- Collier, S.M.; Hamel, L.P.; Moffett, P. Cell death mediated by the N-terminal domains of a unique and highly conserved class of NB-LRR protein. Mol. Plant Microbe Interact. 2011, 24, 918–931. [Google Scholar] [CrossRef] [Green Version]
- Gabriels, S.H.; Vossen, J.H.; Ekengren, S.K.; van Ooijen, G.; Abd-El-Haliem, A.M.; van den Berg, G.C.; Rainey, D.Y.; Martin, G.B.; Takken, F.L.; de Wit, P.J.; et al. An NB-LRR protein required for HR signalling mediated by both extra- and intracellular resistance proteins. Plant J. 2007, 50, 14–28. [Google Scholar] [CrossRef]
- Xiao, S.; Calis, O.; Patrick, E.; Zhang, G.; Charoenwattana, P.; Muskett, P.; Parker, J.E.; Turner, J.G. The atypical resistance gene, RPW8, recruits components of basal defence for powdery mildew resistance in Arabidopsis. Plant J. 2005, 42, 95–110. [Google Scholar] [CrossRef] [PubMed]
- Bonardi, V.; Tang, S.; Stallmann, A.; Roberts, M.; Cherkis, K.; Dangl, J.L. Expanded functions for a family of plant intracellular immune receptors beyond specific recognition of pathogen effectors. Proc. Natl. Acad. Sci. USA 2011, 108, 16463–16468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, O.X.; Tong, M.; Bonardi, V.; El Kasmi, F.; Woloshen, V.; Wunsch, L.K.; Dangl, J.L.; Li, X. TNL-mediated immunity in Arabidopsis requires complex regulation of the redundant ADR1 gene family. New Phytol. 2016, 210, 960–973. [Google Scholar] [CrossRef]
- Wu, Z.; Li, M.; Dong, O.X.; Xia, S.; Liang, W.; Bao, Y.; Wasteneys, G.; Li, X. Differential regulation of TNL-mediated immune signaling by redundant helper CNLs. New Phytol. 2019, 222, 938–953. [Google Scholar] [CrossRef] [PubMed]
- Peart, J.R.; Mestre, P.; Lu, R.; Malcuit, I.; Baulcombe, D.C. NRG1, a CC-NB-LRR protein, together with N, a TIR-NB-LRR protein, mediates resistance against tobacco mosaic virus. Curr. Biol. 2005, 15, 968–973. [Google Scholar] [CrossRef] [Green Version]
- Qi, T.; Seong, K.; Thomazella, D.P.T.; Kim, J.R.; Pham, J.; Seo, E.; Cho, M.J.; Schultink, A.; Staskawicz, B.J. NRG1 functions downstream of EDS1 to regulate TIR-NLR-mediated plant immunity in Nicotiana benthamiana. Proc. Natl. Acad. Sci. USA 2018, 115, E10979–E10987. [Google Scholar] [CrossRef] [Green Version]
- Castel, B.; Ngou, P.M.; Cevik, V.; Redkar, A.; Kim, D.S.; Yang, Y.; Ding, P.; Jones, J.D.G. Diverse NLR immune receptors activate defence via the RPW8-NLR NRG1. New Phytol. 2019, 222, 966–980. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Garner, C.M.; Gassmann, W. New clues in the nucleus: Transcriptional reprogramming in effector-triggered immunity. Front. Plant Sci. 2013, 4, 364. [Google Scholar] [CrossRef] [Green Version]
- Garner, C.M.; Kim, S.H.; Spears, B.J.; Gassmann, W. Express yourself: Transcriptional regulation of plant innate immunity. Semin. Cell Dev. Biol. 2016, 56, 150–162. [Google Scholar] [CrossRef]
- Zhu, Z.; Xu, F.; Zhang, Y.; Cheng, Y.T.; Wiermer, M.; Li, X.; Zhang, Y. Arabidopsis resistance protein SNC1 activates immune responses through association with a transcriptional corepressor. Proc. Natl. Acad. Sci. USA 2010, 107, 13960–13965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, F.; Kapos, P.; Cheng, Y.T.; Li, M.; Zhang, Y.; Li, X. NLR-associating transcription factor bHLH84 and its paralogs function redundantly in plant immunity. PLoS Pathog. 2014, 10, e1004312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, S.I.; Kim, S.H.; Bhattacharjee, S.; Noh, J.J.; Gassmann, W. SRFR1, a suppressor of effector-triggered immunity, encodes a conserved tetratricopeptide repeat protein with similarity to transcriptional repressors. Plant J. 2009, 57, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kwon, S.I.; Saha, D.; Anyanwu, N.C.; Gassmann, W. Resistance to the Pseudomonas syringae effector HopA1 is governed by the TIR-NBS-LRR protein RPS6 and is enhanced by mutations in SRFR1. Plant Physiol. 2009, 150, 1723–1732. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Gao, F.; Bhattacharjee, S.; Adiasor, J.A.; Nam, J.C.; Gassmann, W. The Arabidopsis resistance-like gene SNC1 is activated by mutations in SRFR1 and contributes to resistance to the bacterial effector AvrRps4. PLoS Pathog. 2010, 6, e1001172. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S.; Halane, M.K.; Kim, S.H.; Gassmann, W. Pathogen effectors target Arabidopsis EDS1 and alter its interactions with immune regulators. Science 2011, 334, 1405–1408. [Google Scholar] [CrossRef]
- Kim, S.H.; Kwon, S.I.; Bhattacharjee, S.; Gassmann, W. Regulation of defense gene expression by Arabidopsis SRFR1. Plant Signal. Behav. 2009, 4, 149–150. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Son, G.H.; Bhattacharjee, S.; Kim, H.J.; Nam, J.C.; Nguyen, P.D.; Hong, J.C.; Gassmann, W. The Arabidopsis immune adaptor SRFR1 interacts with TCP transcription factors that redundantly contribute to effector-triggered immunity. Plant J. 2014, 78, 978–989. [Google Scholar] [CrossRef]
- Garner, C.M.; Spears, B.J.; Su, J.; Cseke, L.J.; Smith, S.N.; Rogan, C.J.; Gassmann, W. Opposing functions of the plant TOPLESS gene family during SNC1-mediated autoimmunity. PLoS Genet. 2021, 17, e1009026. [Google Scholar] [CrossRef]
- Caldo, R.A.; Nettleton, D.; Wise, R.P. Interaction-dependent gene expression in Mla-specified response to barley powdery mildew. Plant Cell 2004, 16, 2514–2528. [Google Scholar] [CrossRef] [Green Version]
- Hatsugai, N.; Igarashi, D.; Mase, K.; Lu, Y.; Tsuda, Y.; Chakravarthy, S.; Wei, H.L.; Foley, J.W.; Collmer, A.; Glazebrook, J.; et al. A plant effector-triggered immunity signaling sector is inhibited by pattern-triggered immunity. EMBO J. 2017, 36, 2758–2769. [Google Scholar] [CrossRef] [PubMed]
- Block, A.; Alfano, J.R. Plant targets for Pseudomonas syringae type III effectors: Virulence targets or guarded decoys? Curr. Opin. Microbiol. 2011, 14, 39–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacombe, S.; Rougon-Cardoso, A.; Sherwood, E.; Peeters, N.; Dahlbeck, D.; van Esse, H.P.; Smoker, M.; Rallapalli, G.; Thomma, B.P.; Staskawicz, B.; et al. Interfamily transfer of a plant pattern-recognition receptor confers broad-spectrum bacterial resistance. Nat. Biotechnol. 2010, 28, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Helm, M.; Qi, M.; Sarkar, S.; Yu, H.; Whitham, S.A.; Innes, R.W. Engineering a Decoy Substrate in Soybean to Enable Recognition of the Soybean Mosaic Virus NIa Protease. Mol. Plant Microbe Interact. 2019, 32, 760–769. [Google Scholar] [CrossRef]
- Li, L.; Kim, P.; Yu, L.; Cai, G.; Chen, S.; Alfano, J.R.; Zhou, J.M. Activation-Dependent Destruction of a Co-receptor by a Pseudomonas syringae Effector Dampens Plant Immunity. Cell Host Microbe 2016, 20, 504–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, G.; Yuan, M.; Ai, C.; Liu, L.; Zhuang, E.; Karapetyan, S.; Wang, S.; Dong, X. uORF-mediated translation allows engineered plant disease resistance without fitness costs. Nature 2017, 545, 491–494. [Google Scholar] [CrossRef]
- Pompili, V.; Dalla Costa, L.; Piazza, S.; Pindo, M.; Malnoy, M. Reduced fire blight susceptibility in apple cultivars using a high-efficiency CRISPR/Cas9-FLP/FRT-based gene editing system. Plant Biotechnol. J. 2020, 18, 845–858. [Google Scholar] [CrossRef]
- Oliva, R.; Ji, C.; Atienza-Grande, G.; Huguet-Tapia, J.C.; Perez-Quintero, A.; Li, T.; Eom, J.S.; Li, C.; Nguyen, H.; Liu, B.; et al. Broad-spectrum resistance to bacterial blight in rice using genome editing. Nat. Biotechnol. 2019, 37, 1344–1350. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, Q.-M.; Iswanto, A.B.B.; Son, G.H.; Kim, S.H. Recent Advances in Effector-Triggered Immunity in Plants: New Pieces in the Puzzle Create a Different Paradigm. Int. J. Mol. Sci. 2021, 22, 4709. https://doi.org/10.3390/ijms22094709
Nguyen Q-M, Iswanto ABB, Son GH, Kim SH. Recent Advances in Effector-Triggered Immunity in Plants: New Pieces in the Puzzle Create a Different Paradigm. International Journal of Molecular Sciences. 2021; 22(9):4709. https://doi.org/10.3390/ijms22094709
Chicago/Turabian StyleNguyen, Quang-Minh, Arya Bagus Boedi Iswanto, Geon Hui Son, and Sang Hee Kim. 2021. "Recent Advances in Effector-Triggered Immunity in Plants: New Pieces in the Puzzle Create a Different Paradigm" International Journal of Molecular Sciences 22, no. 9: 4709. https://doi.org/10.3390/ijms22094709
APA StyleNguyen, Q.-M., Iswanto, A. B. B., Son, G. H., & Kim, S. H. (2021). Recent Advances in Effector-Triggered Immunity in Plants: New Pieces in the Puzzle Create a Different Paradigm. International Journal of Molecular Sciences, 22(9), 4709. https://doi.org/10.3390/ijms22094709