Direct Bioelectrocatalytic Oxidation of Glucose by Gluconobacter oxydans Membrane Fractions in PEDOT:PSS/TEG-Modified Biosensors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Production of Gluconobacter oxydans Membrane Fractions
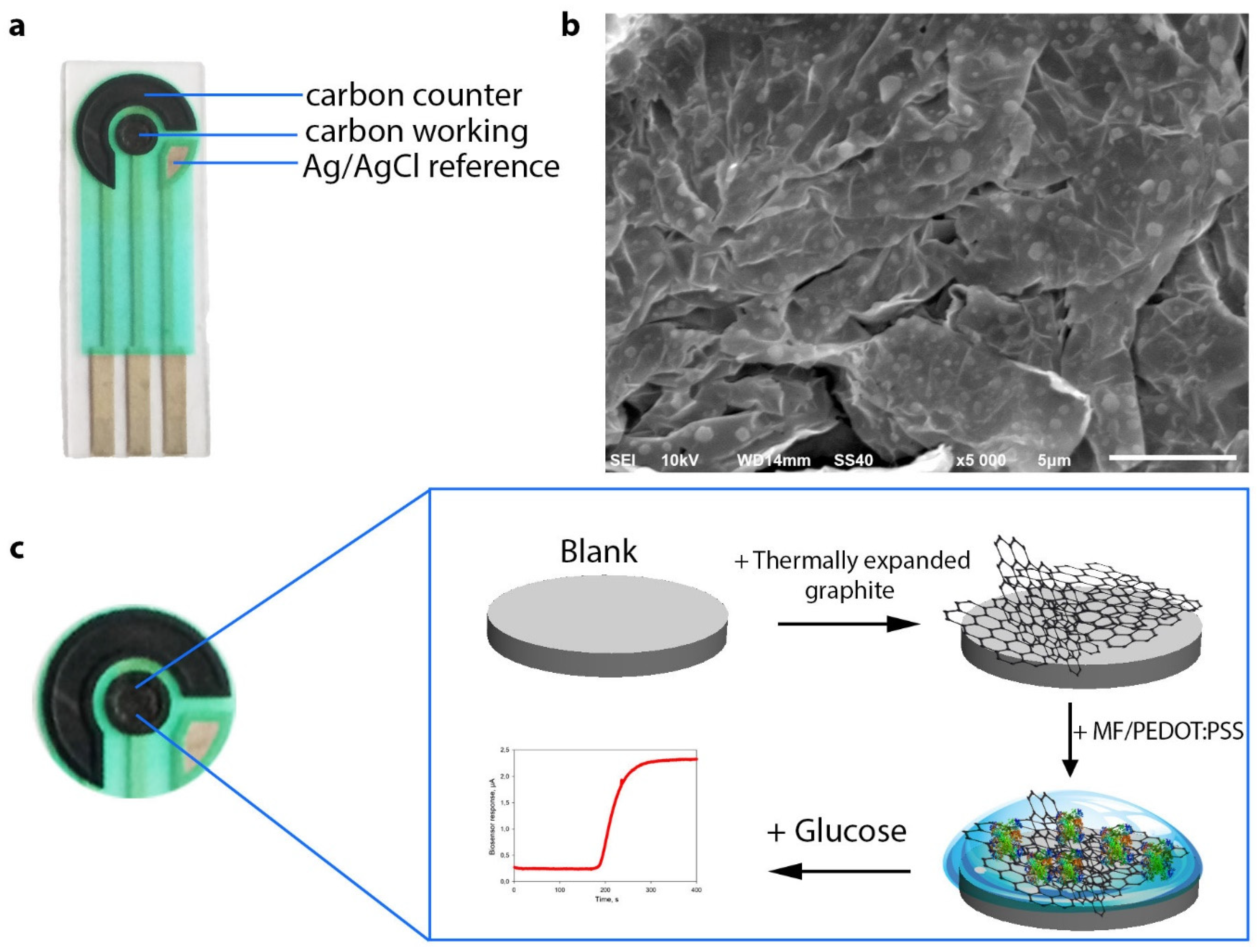
2.3. Preparation and Characterization of Biosensors
3. Results and Discussion
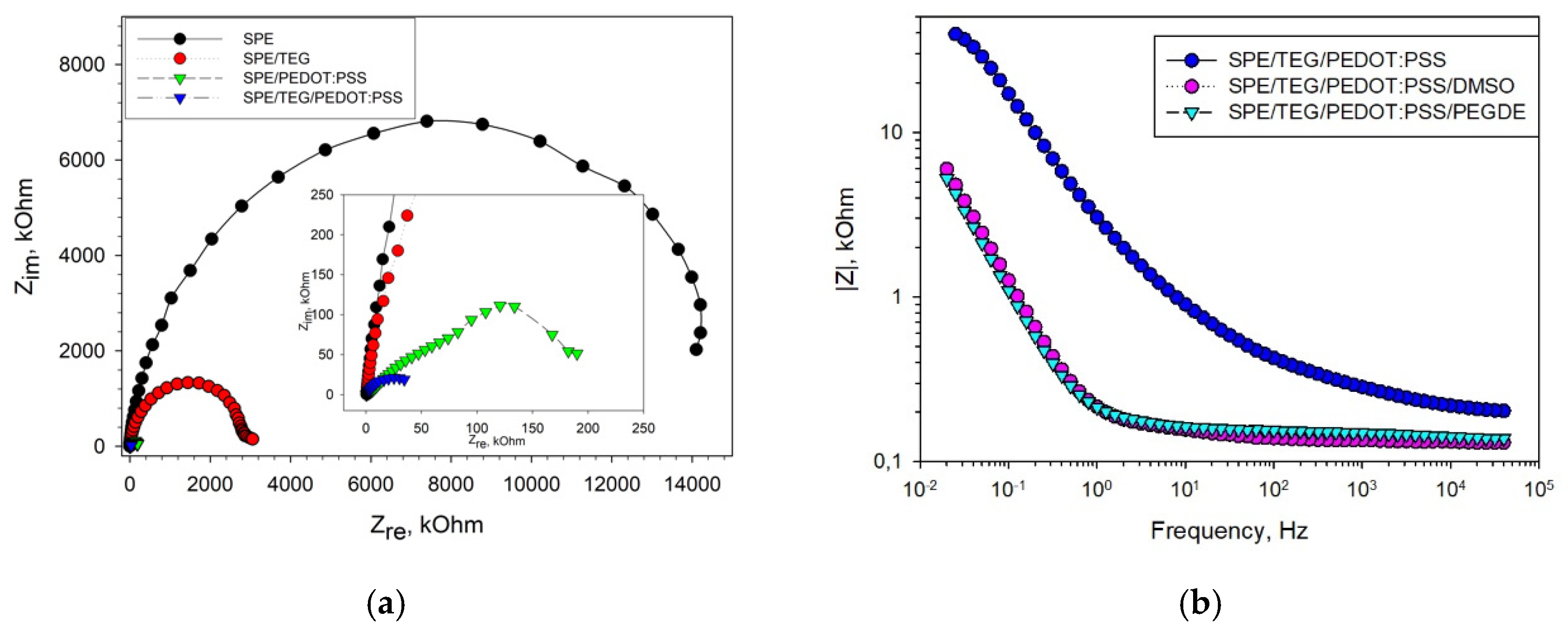
3.1. Optimization of Detection Electrodes
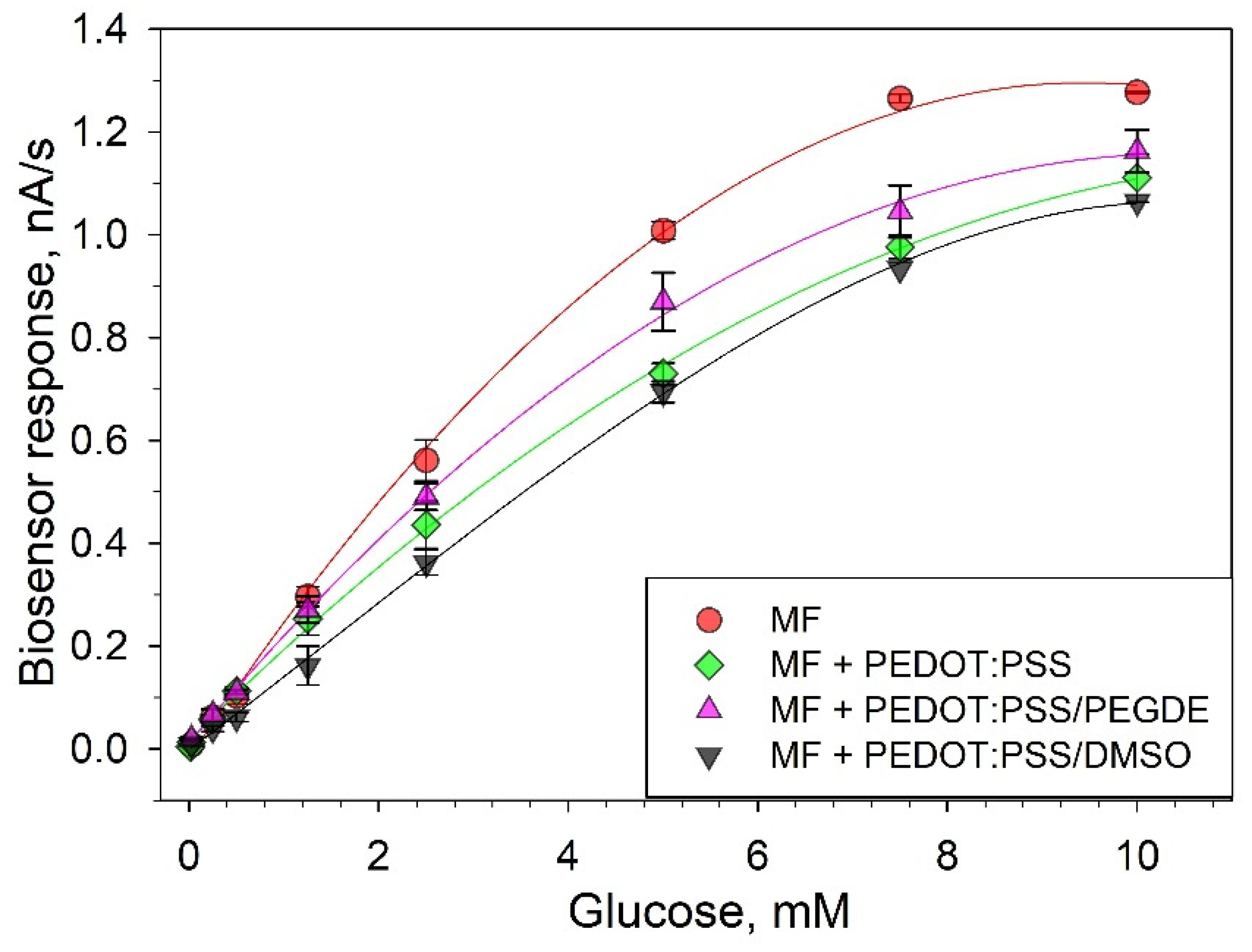
3.2. Respiratory Activity of G. oxydans Membrane Fractions
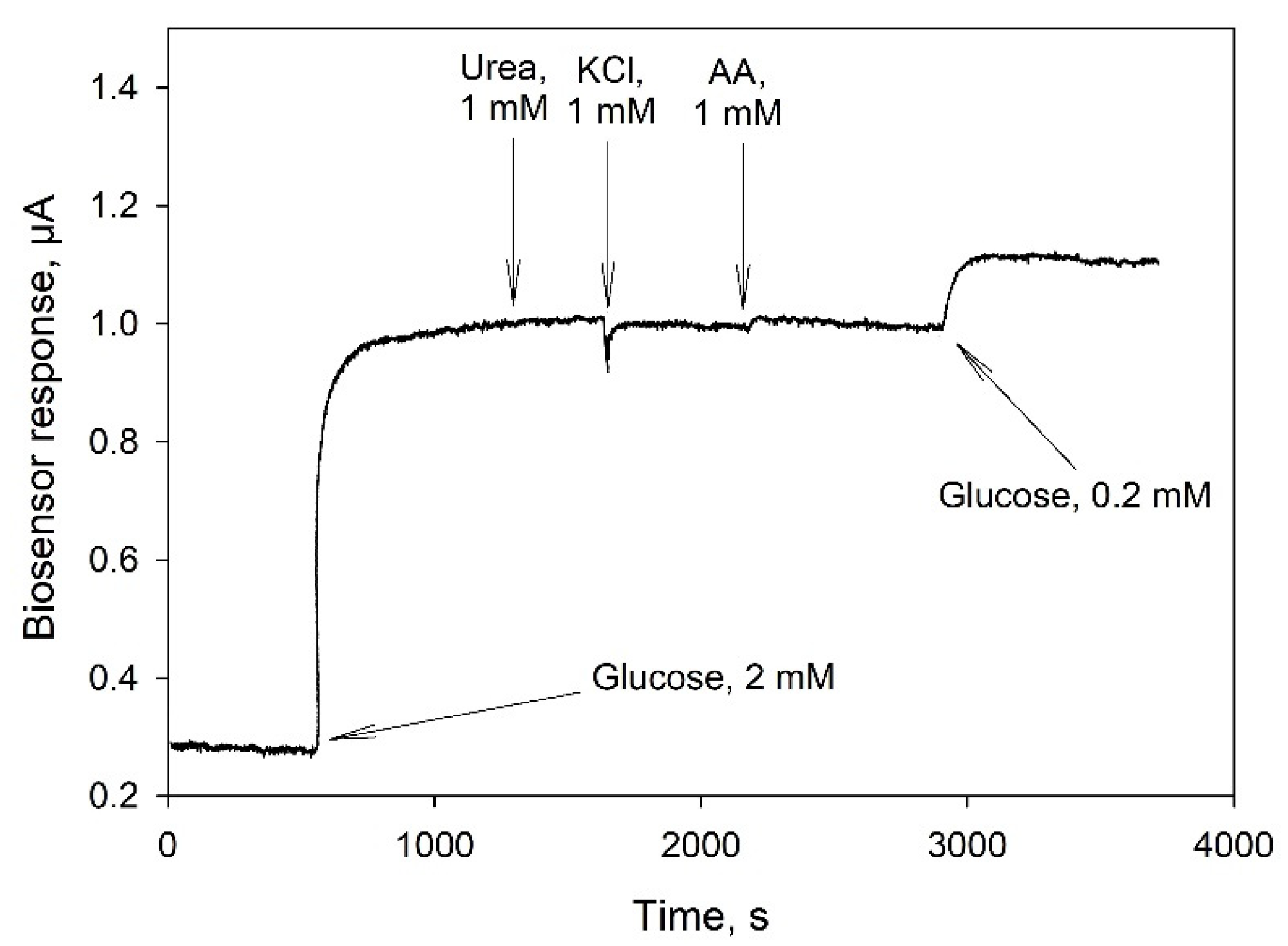
3.3. Electrochemical Parameters of Bioelectrodes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berezin, I.V.; Bogdanovskaya, V.A.; Varfolomeyev, S.D.; Tarasevich, M.R.; Yaropolov, A.I. Bioelectrocatalysis. Equilibrium oxygen potential under the action of laccase. Dokl. AN SSSR 1978, 240, 615–619. [Google Scholar]
- Yaropolov, A.I.; Malovik, V.; Varfolomeyev, S.D.; Berezin, I.V. Electroreduction of oxygen peroxide on an immobilized peroxidase electrode. Dokl. AN SSSR 1979, 249, 1399–1401. [Google Scholar]
- Bogdanovskaya, V.A.; Varfolomeyev, S.D.; Tarasevich, M.R.; Yaropolov, A.I. Electrocatalysis – activation of the hydrogen reaction of immobilized hydrogenase. Sov. Electrochem. 1980, 6, 657–661. [Google Scholar]
- Yu, S.; Myung Nosang, V. Recent advances in the direct electron transfer-enabled enzymatic fuel cells. Front. Chem. 2021, 8, 1243. [Google Scholar] [CrossRef]
- Bollella, P.; Katz, E. Enzyme-based biosensors: Tackling electron transfer issues. Sensors 2020, 20, 3517. [Google Scholar] [CrossRef] [PubMed]
- Kawai, S.; Yakushi, T.; Matsushita, K.; Kitazumi, Y.; Shirai, O.; Kano, K. The electron transfer pathway in direct electrochemical communication of fructose dehydrogenase with electrodes. Electrochem. Commun. 2014, 38, 28–31. [Google Scholar] [CrossRef]
- Adachi, T.; Kitazumi, Y.; Shirai, O.; Kano, K. Direct electron transfer-type bioelectrocatalysis by membrane-bound aldehyde dehydrogenase from Gluconobacter oxydans and cyanide effects on its bioelectrocatalytic properties. Electrochem. Commun. 2021, 123, 106911. [Google Scholar] [CrossRef]
- Torimura, M.; Kano, K.; Ikeda, T.; Ueda, T. Spectroelectrochemical characterization of quinohemoprotein alcohol dehydrogenase from Gluconobacter suboxydans. Chem. Lett. 1997, 26, 525–526. [Google Scholar] [CrossRef]
- Kaida, Y.; Hibino, Y.; Kitazumi, Y.; Shirai, O.; Kano, K. Ultimate downsizing of d-fructose dehydrogenase for improving the performance of direct electron transfer-type bioelectrocatalysis. Electrochem. Commun. 2019, 98, 101–105. [Google Scholar] [CrossRef]
- Tominaga, M.; Nomura, S.; Taniguchi, I. d-Fructose detection based on the direct heterogeneous electron transfer reaction of fructose dehydrogenase adsorbed onto multi-walled carbon nanotubes synthesized on platinum electrode. Biosens. Bioelectron. 2009, 24, 1184–1188. [Google Scholar] [CrossRef] [PubMed]
- Tsujimura, S.; Abo, T.; Ano, Y.; Matsushita, K.; Kano, K. Electrochemistry of D-gluconate 2-dehydrogenase from Gluconobacter frateurii on indium tin oxide electrodes. Chem. Lett. 2007, 36, 1164–1165. [Google Scholar] [CrossRef]
- Treu, B.L.; Minteer, S.D. Isolation and purification of PQQ-dependent lactate dehydrogenase from Gluconobacter and use for direct electron transfer at carbon and gold electrodes. Bioelectrochemistry 2008, 74, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Plekhanova, Y.V.; Tarasov, S.E.; Bykov, A.G.; Prisyazhnaya, N.V.; Tenchurin, T.K.; Chvalun, S.V.; Orekhov, A.S.; Shepelev, A.D.; Gotovtsev, P.M.; Reshetilov, A.N. Carbon fiber anode of biofuel cell with immobilized bacteria and membrane fractions. Nanotechnol. Russia 2018, 13, 531–538. [Google Scholar] [CrossRef]
- Reshetilov, A.N.; Kitova, A.E.; Kolesov, V.V.; Yaropolov, A.I. Mediator-free bioelectrocatalytic oxidation of ethanol on an electrode from thermally expanded graphite modified by Gluconobacter oxydans membrane fractions. Electroanalysis 2015, 27, 1443–1448. [Google Scholar] [CrossRef]
- Teymourian, H.; Barfidokht, A.; Wang, J. Electrochemical glucose sensors in diabetes management: An updated review (2010–2020). Chem. Soc. Rev. 2020, 49, 7671–7709. [Google Scholar] [CrossRef] [PubMed]
- Prust, C.; Hoffmeister, M.; Liesegang, H.; Wiezer, A.; Fricke, W.F.; Ehrenreich, A.; Gottschalk, G.; Deppenmeier, U. Complete genome sequence of the acetic acid bacterium Gluconobacter oxydans. Nat. Biotechnol. 2005, 23, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, J.; Cheng, Y.; Jiang, S.P. Effect of carbon nanotubes on direct electron transfer and electrocatalytic activity of immobilized glucose oxidase. ACS Omega 2018, 3, 667–676. [Google Scholar] [CrossRef]
- Algov, I.; Grushka, J.; Zarivach, R.; Alfonta, L. Highly efficient flavin-adenine dinucleotide glucose dehydrogenase fused to a minimal cytochrome C domain. J. Am. Chem. Soc. 2017, 139, 17217–17220. [Google Scholar] [CrossRef] [PubMed]
- Okuda-Shimazaki, J.; Yoshida, H.; Sode, K. FAD dependent glucose dehydrogenases – Discovery and engineering of representative glucose sensing enzymes. Bioelectrochemistry 2020, 132, 107414. [Google Scholar] [CrossRef]
- Yakovlev, A.V.; Finaenov, A.I.; Zabud’kov, S.L.; Yakovleva, E.V. Thermally expanded graphite: Synthesis, properties, and prospects for use. Russ. J. Appl. Chem. 2006, 79, 1741–1751. [Google Scholar] [CrossRef]
- Reshetilov, A.N.; Kitova, A.E.; Tarasov, S.E.; Plekhanova, Y.V.; Bykov, A.G.; Sundramoorthy, A.K.; Kuznetsova, I.E.; Kolesov, V.V.; Gotovtsev, P.M. Highly conductive polymer PEDOT:PSS—Application in biomedical and bioelectrochemical systems. RENSIT 2020, 12, 471–482. [Google Scholar] [CrossRef]
- Jeong, W.; Gwon, G.; Ha, J.-H.; Kim, D.; Eom, K.-J.; Park, J.H.; Kang, S.J.; Kwak, B.; Hong, J.-I.; Lee, S.; et al. Enhancing the conductivity of PEDOT:PSS films for biomedical applications via hydrothermal treatment. Biosens. Bioelectron. 2020, 112717. [Google Scholar] [CrossRef]
- Yang, Y.; Deng, H.; Fu, Q. Recent progress on PEDOT:PSS based polymer blends and composites for flexible electronics and thermoelectric devices. Mater. Chem. Front. 2020, 4, 3130–3152. [Google Scholar] [CrossRef]
- Wisitsoraat, A.; Pakapongpan, S.; Sriprachuabwong, C.; Phokharatkul, D.; Sritongkham, P.; Lomas, T.; Tuantranont, A. Graphene–PEDOT:PSS on screen printed carbon electrode for enzymatic biosensing. J. Electroanal. Chem. 2013, 704, 208–213. [Google Scholar] [CrossRef]
- David, M.; Barsan, M.M.; Brett, C.M.A.; Florescu, M. Improved glucose label-free biosensor with layer-by-layer architecture and conducting polymer poly(3,4-ethylenedioxythiophene). Sens. Actuators B Chem. 2018, 255, 3227–3234. [Google Scholar] [CrossRef]
- Gorshenev, V.N. Microwave-assisted and thermal stepwise expansion of oxidized graphites. Russ. J. Phys. Chem. B. 2011, 5, 780–786. [Google Scholar] [CrossRef]
- Reshetilov, A.N.; Plekhanova, J.V.; Tarasov, S.E.; Bykov, A.G.; Gutorov, M.A.; Alferov, S.V.; Tenchurin, T.K.; Chvalun, S.V.; Orekhov, A.S.; Shepelev, A.D.; et al. Evaluation properties of bioelectrodes based on carbon superfine materials containing model microorganisms Gluconobacter. Nanotechnol. Russia 2017, 12, 107–115. [Google Scholar] [CrossRef]
- Indzhgiya, E.; Ponamoreva, O.; Alferov, V.; Reshetilov, A.; Gorton, L. Interaction of ferrocene mediators with Gluconobacter oxydans immobilized whole cells and membrane fractions in oxidation of ethanol. Electroanalysis 2012, 24, 924–930. [Google Scholar] [CrossRef]
- Dong, J.; Portale, G. Role of the processing solvent on the electrical conductivity of PEDOT:PSS. Adv. Mater. Interfaces 2020, 7, 2000641. [Google Scholar] [CrossRef]
- Schubart, I.W.; Göbel, G.; Lisdat, F. A pyrroloquinolinequinone-dependent glucose dehydrogenase (PQQ-GDH)-electrode with direct electron transfer based on polyaniline modified carbon nanotubes for biofuel cell application. Electrochim. Acta 2012, 82, 224–232. [Google Scholar] [CrossRef]
- Boehm, R.; Donovan, J.; Sheth, D.; Durfor, A.; Roberts, J.; Isayeva, I. In vitro sugar interference testing with amperometric glucose oxidase sensors. J. Diabetes Sci. Technol. 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
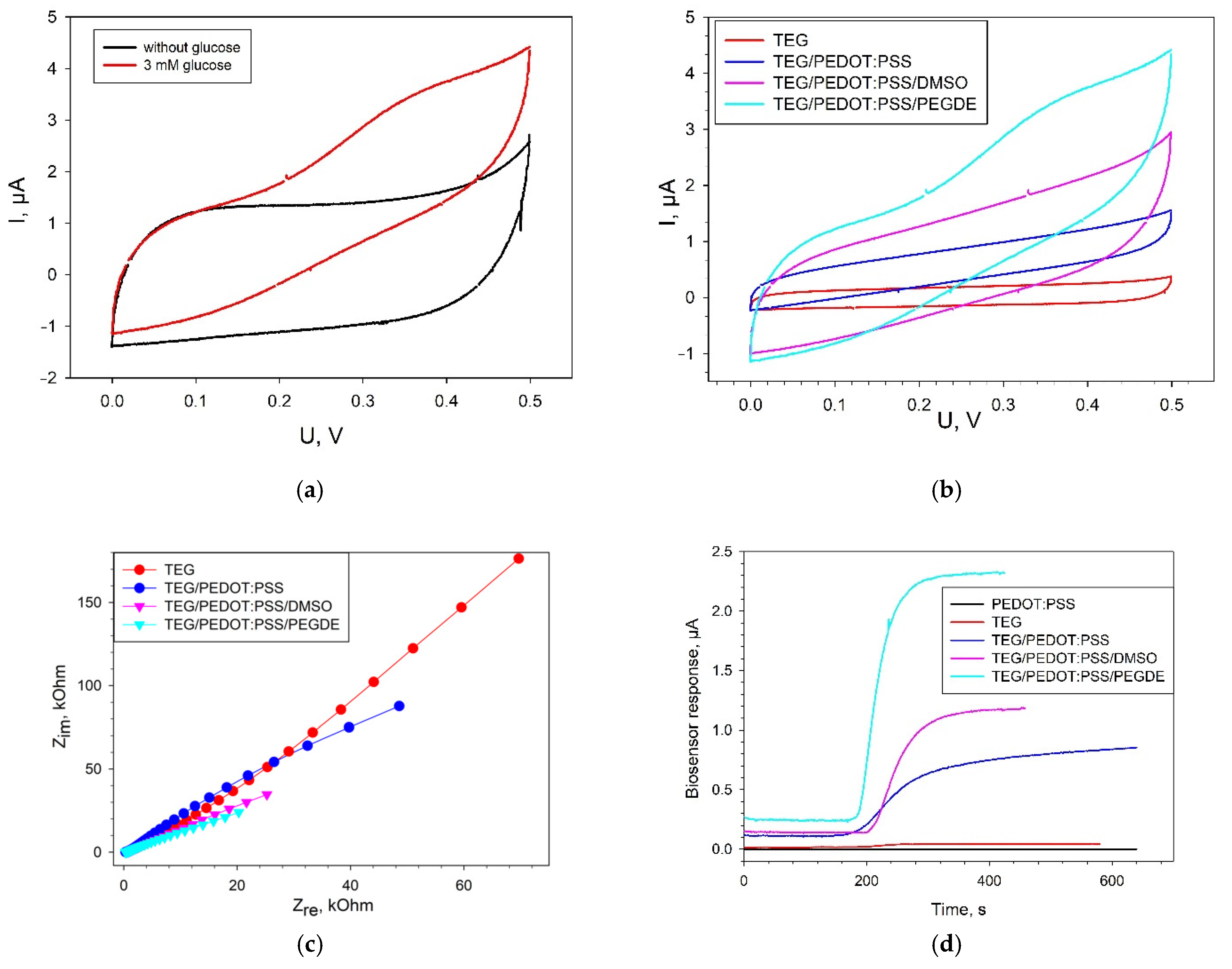
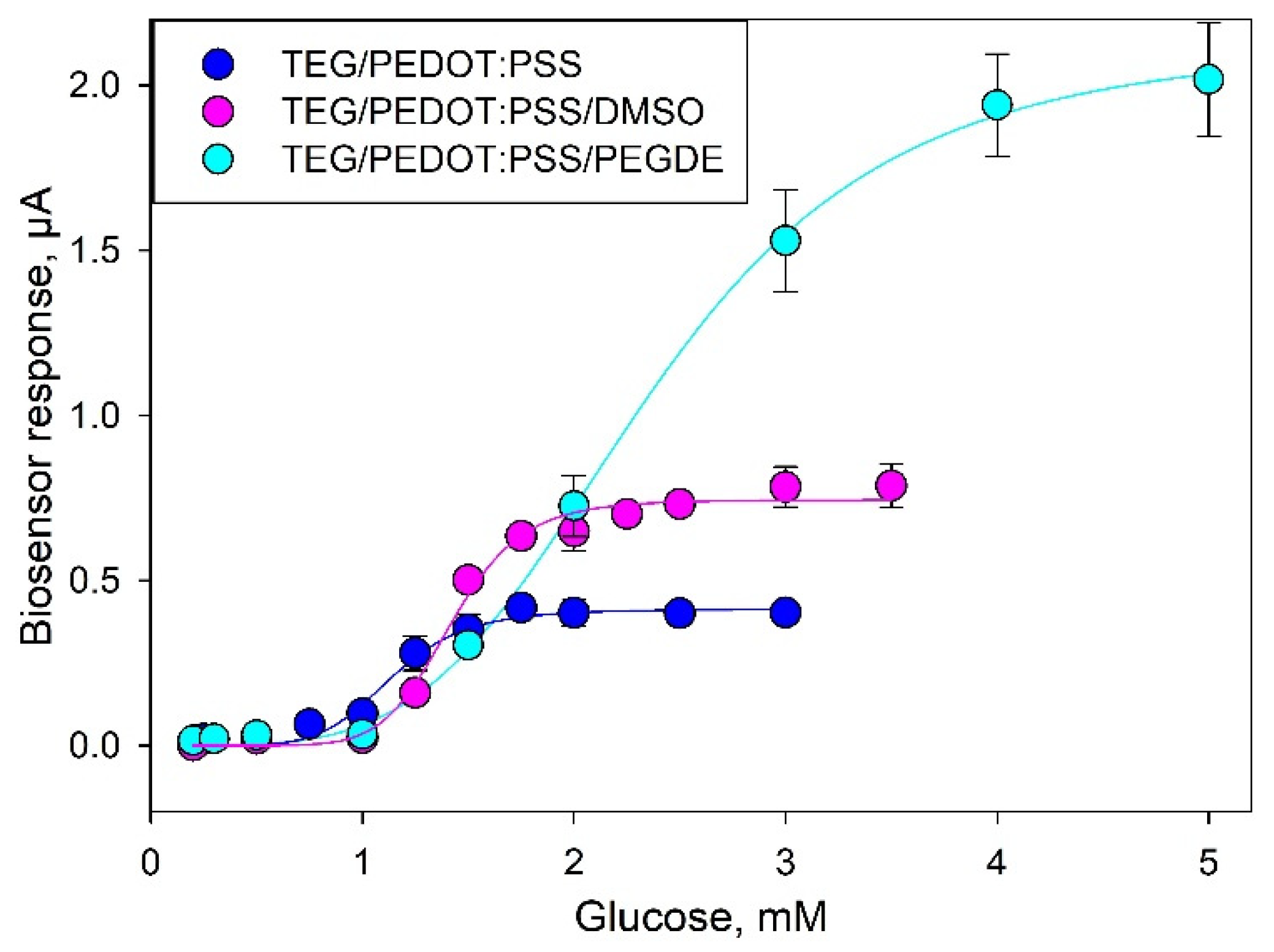
| Modification | TEG/PEDOT:PSS | TEG/PEDOT:PSS/DMSO | TEG/PEDOT:PSS/PEGDE | |
|---|---|---|---|---|
| Parameter | ||||
| I, μА | 0.41 | 0.74 | 2.13 | |
| Km, mM | 1.14 | 1.42 | 2.36 | |
| h | 6.48 | 8.48 | 4.08 | |
| Linear range of detection, mM | 0.81–1.59 | 0.90–1.90 | 1.03–3.01 | |
| Regression equation for the linear segment | y = 0.46x − 0.33 | y = 0.80x − 0.77 | y = 0.82x − 0.89 | |
| Correlation coefficient, R2 | 0.98 | 0.98 | 0.99 | |
| Sensitivity coefficient, μA/mM | 0.46 | 0.80 | 0.82 | |
| Single measurement time, min | 5–6 | 4–5 | 2–2.5 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kitova, A.; Tarasov, S.; Plekhanova, Y.; Bykov, A.; Reshetilov, A. Direct Bioelectrocatalytic Oxidation of Glucose by Gluconobacter oxydans Membrane Fractions in PEDOT:PSS/TEG-Modified Biosensors. Biosensors 2021, 11, 144. https://doi.org/10.3390/bios11050144
Kitova A, Tarasov S, Plekhanova Y, Bykov A, Reshetilov A. Direct Bioelectrocatalytic Oxidation of Glucose by Gluconobacter oxydans Membrane Fractions in PEDOT:PSS/TEG-Modified Biosensors. Biosensors. 2021; 11(5):144. https://doi.org/10.3390/bios11050144
Chicago/Turabian StyleKitova, Anna, Sergei Tarasov, Yulia Plekhanova, Aleksandr Bykov, and Anatoly Reshetilov. 2021. "Direct Bioelectrocatalytic Oxidation of Glucose by Gluconobacter oxydans Membrane Fractions in PEDOT:PSS/TEG-Modified Biosensors" Biosensors 11, no. 5: 144. https://doi.org/10.3390/bios11050144
APA StyleKitova, A., Tarasov, S., Plekhanova, Y., Bykov, A., & Reshetilov, A. (2021). Direct Bioelectrocatalytic Oxidation of Glucose by Gluconobacter oxydans Membrane Fractions in PEDOT:PSS/TEG-Modified Biosensors. Biosensors, 11(5), 144. https://doi.org/10.3390/bios11050144





