Effect of Co-Inoculation of Bacillus sp. Strain with Bacterial Endophytes on Plant Growth and Colonization in Tomato Plant (Solanum lycopersicum)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Plant Growth Promotion and Colonization of Bacillus sp. Strains in Tomato Plant
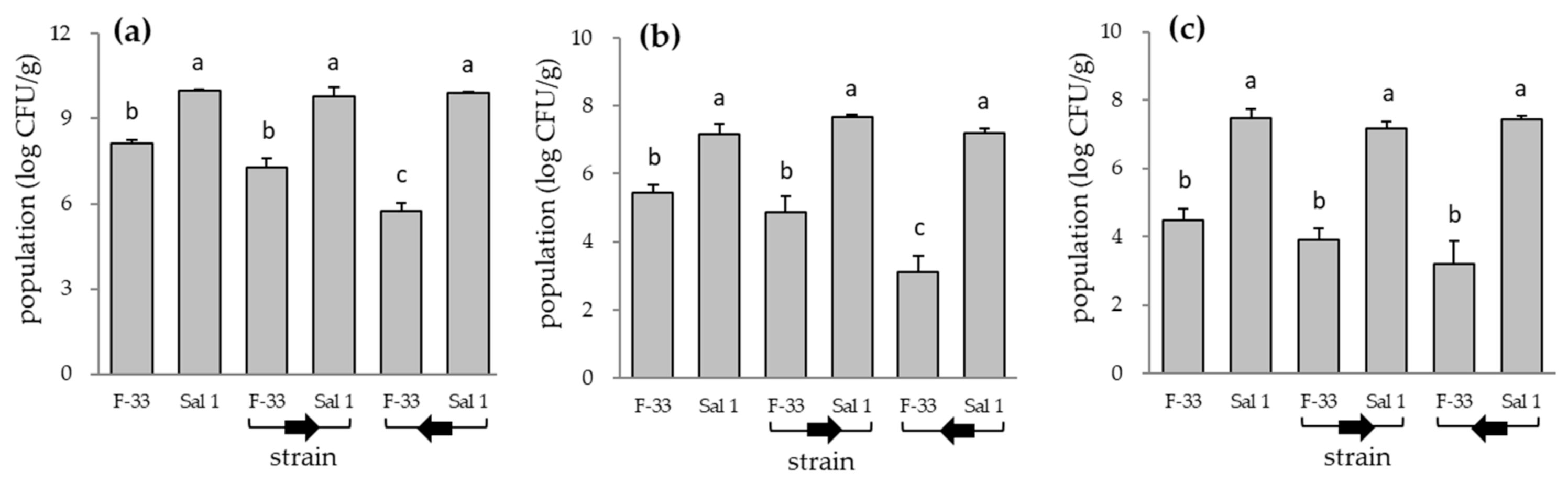
2.3. Effect of Co-Inoculation on Plant Growth Promotion and Colonization of Bacillus sp. F-33 with the Other Endophytic Strains in Tomato Plant
2.4. Effect of Time Interval Inoculation on Plant Growth Promotion and Colonization of Bacillus sp. F-33 and Klebsiella sp. Sal 1 in Tomato Plant
2.5. Statistical Analysis
3. Results
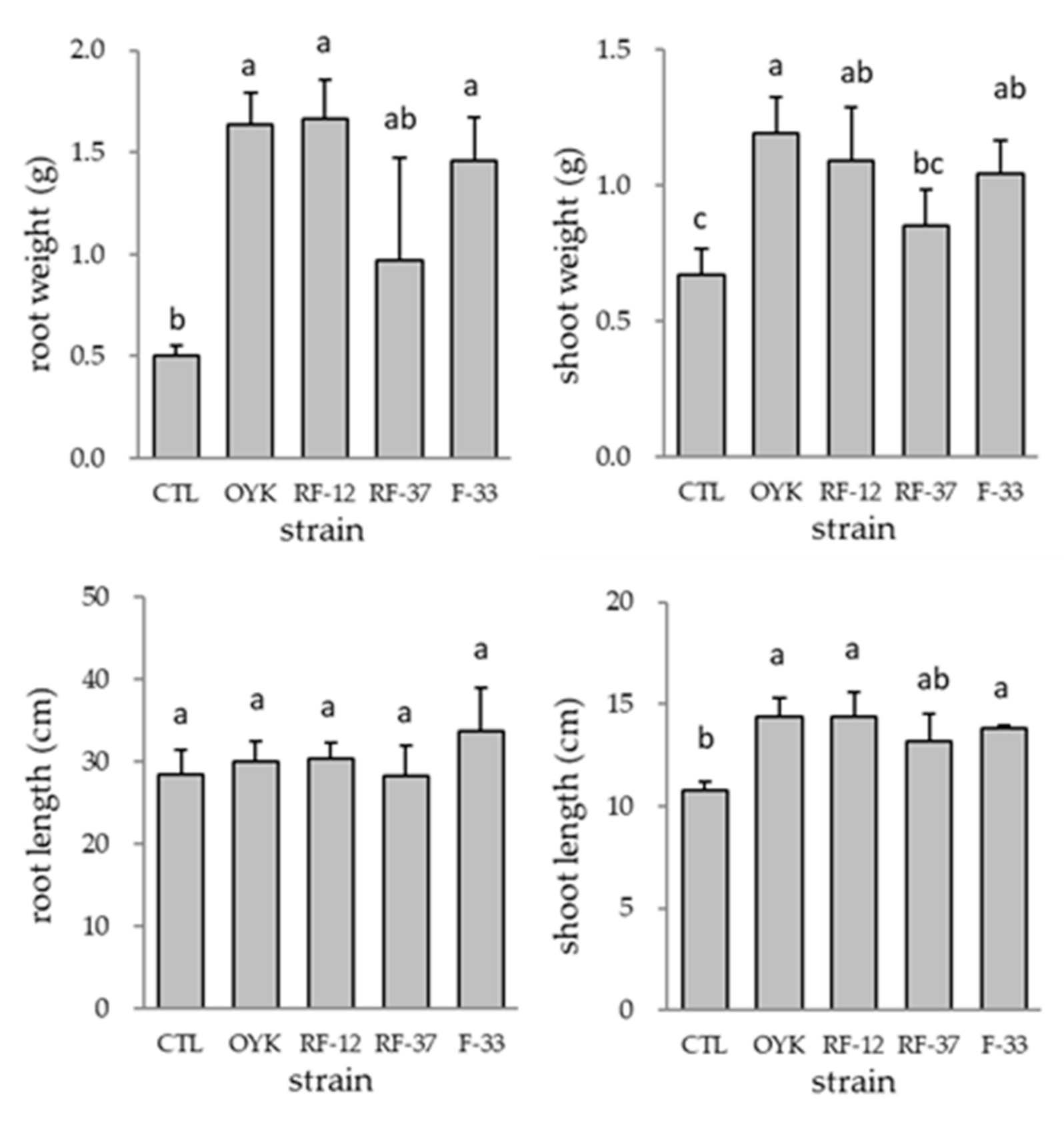
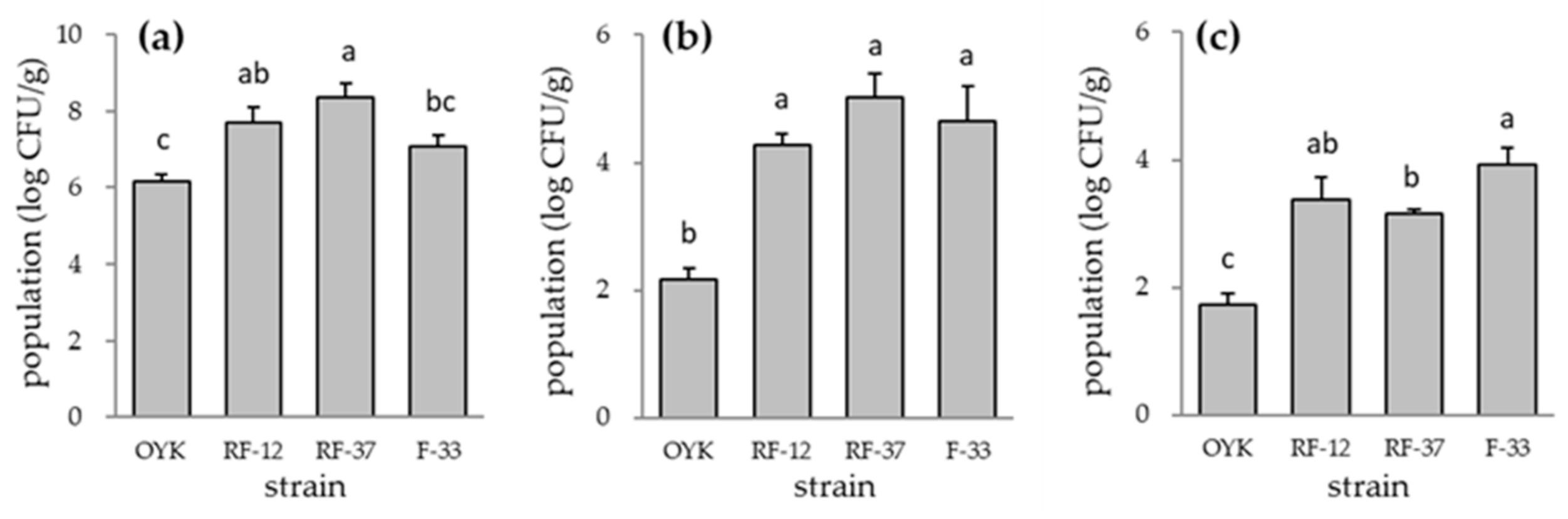
3.1. Plant Growth Promotion and Colonization of Bacillus sp. Strains in Tomato Plant
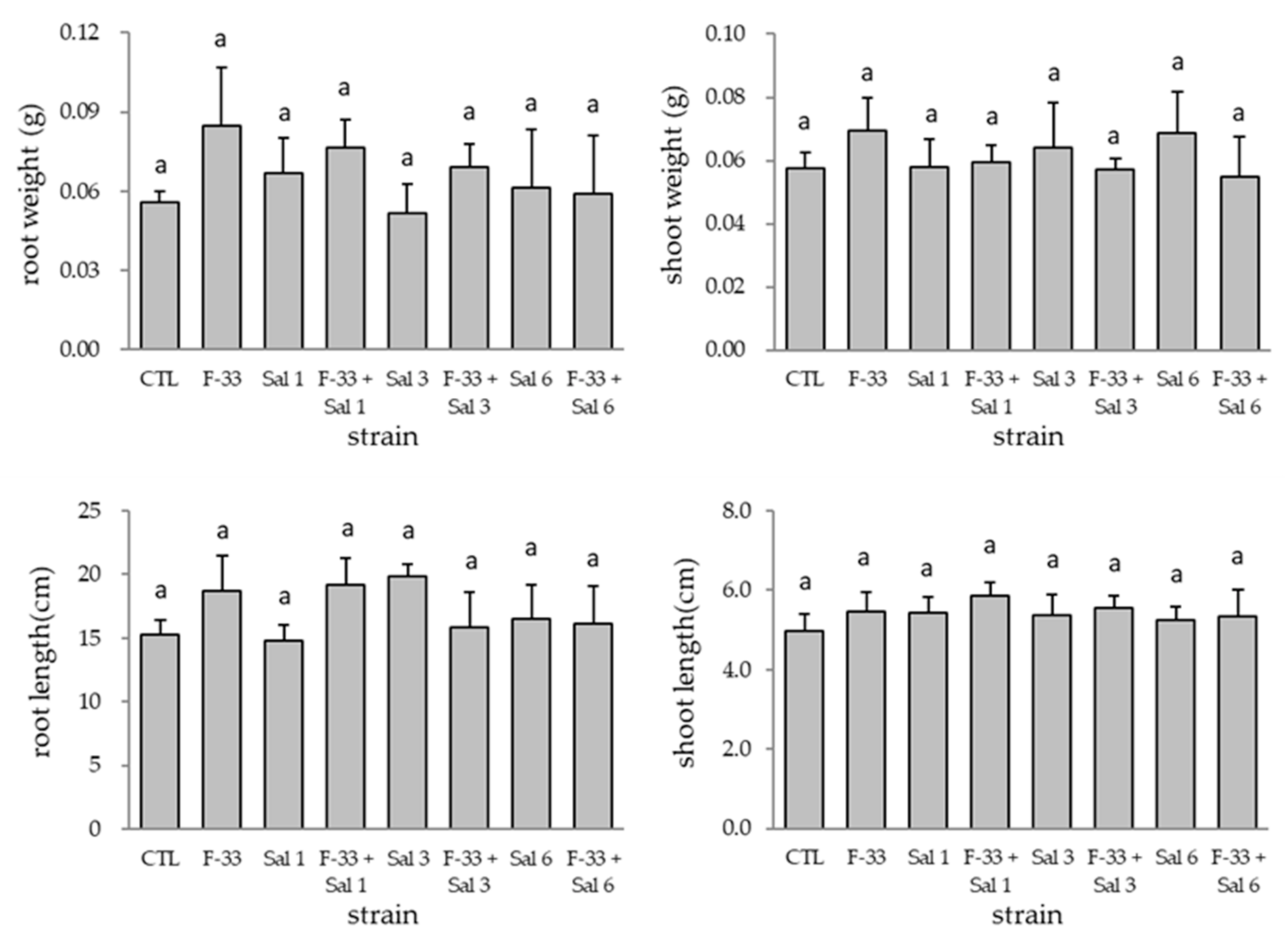
3.2. Effect of Co-Inoculation on Plant Growth Promotion and Colonization of Bacillus sp. F-33 with the Other Endophytic Strains in Tomato Plant
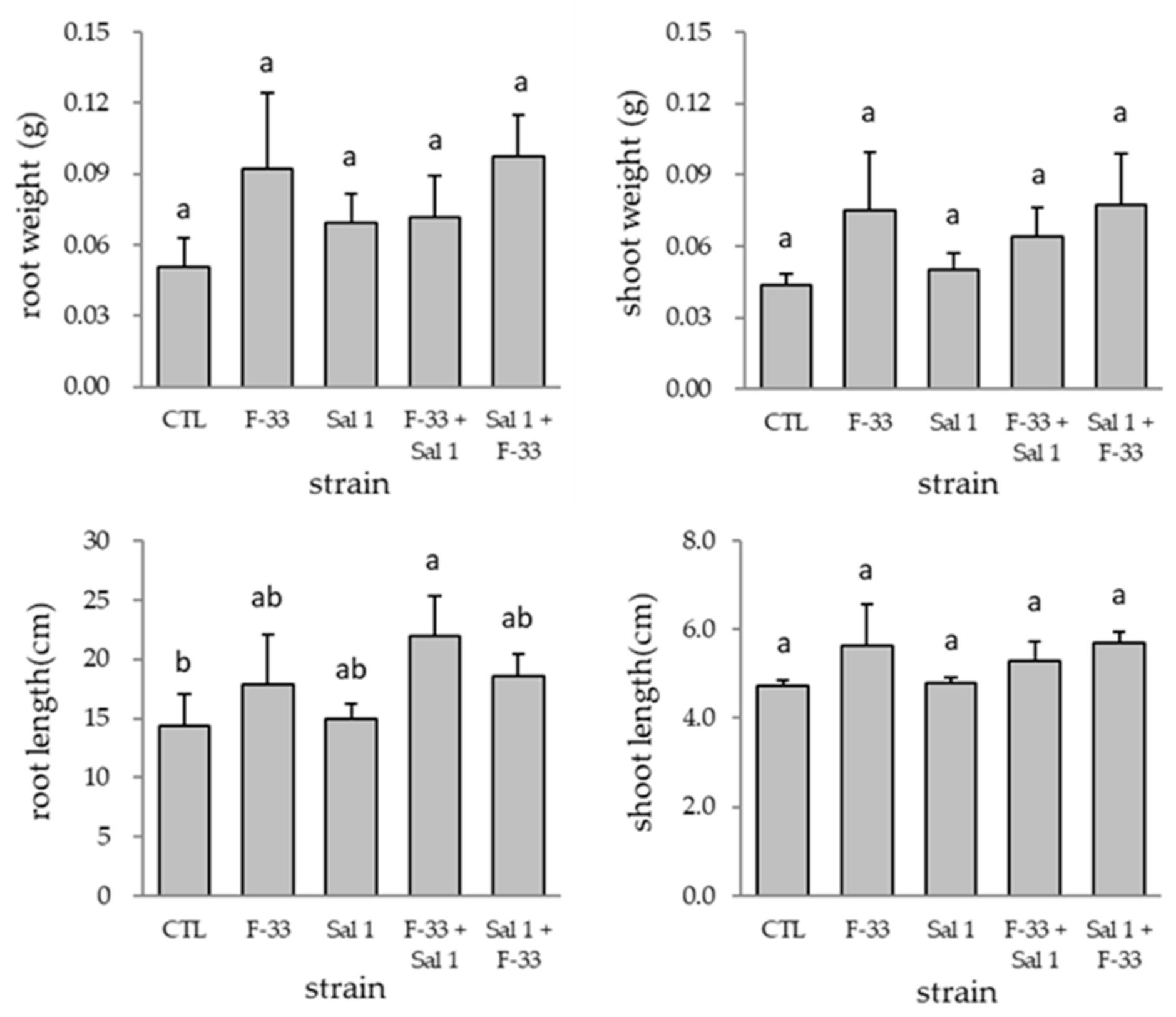
3.3. Effect of Time Interval Inoculation on Plant Growth Promotion and Colonization of Bacillus sp. F-33 and Klebsiella sp. Sal 1 in Tomato Plant
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kaymak, H.C. Potential of PGPR in agricultural innovations. In Plant Growth and Health Promoting Bacteria; Maheshwari, D., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 18, pp. 45–79. [Google Scholar]
- Glick, B.R. The enhancement of plant growth by free living bacteria. Can. J. Microbiol. 1995, 41, 109–117. [Google Scholar] [CrossRef]
- Zakria, M.; Udonishi, K.; Ogawa, T.; Yamamoto, A.; Saeki, Y.; Akao, S. Influence of inoculation technique on the endophytic colonization of rice by Pantoea sp. isolated from sweet potato and by Enterobacter sp. isolated from sugarcane. Soil Sci. Plant Nutr. 2008, 54, 224–236. [Google Scholar] [CrossRef]
- Vessey, J.K. Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 2003, 255, 571–586. [Google Scholar] [CrossRef]
- Miljaković, D.; Marinković, J.; Balešević-Tubić, S. The significance of Bacillus spp. in disease suppression and growth promotion of field and vegetable crops. Microorganisms 2020, 8, 1037. [Google Scholar] [CrossRef]
- Kumar, A.; Prakash, A.; Johri, B.N. Bacillus as PGPR in crop ecosystem. In Bacteria in Agrobiology: Crop Ecosystems; Maheshwari, D.K., Ed.; Springer-Verlag: Berlin/Heidelberg, Germany, 2011; pp. 37–59. [Google Scholar]
- Govindasamy, V.; Senthilkumar, M.; Magheshwaran, V.; Kumar, U.; Bose, P.; Sharma, V.; Annapurna, K. Bacillus and Paenibacillus spp.: Potential PGPR for Sustainable Agriculture. In Plant Growth and Health Promoting Bacteria; Maheshwari, D., Ed.; Springer-Verlag: Berlin/Heidelberg, Germany, 2010; Volume 18, pp. 333–364. [Google Scholar]
- Govindasamy, V.; Senthilkumar, M.; Kumar, U.; Annapurna, K. PGPR-biotechnology for management of abiotic and biotic stresses in crop plants. In Potential Microorganisms for Sustainable Agriculture; Maheshwari, D.K., Dubey, R.C., Eds.; IK International Publishing: New Delhi, India, 2008; pp. 26–48. [Google Scholar]
- Emmert, E.A.B.; Handelsman, J. Biocontrol of plant disease: A (Gram-) positive perspective. FEMS Microbiol. Lett. 1999, 171, 1–9. [Google Scholar] [CrossRef]
- Nain, L.; Yadav, R.C.; Saxena, J. Characterization of multifaceted Bacillus sp. RM-2 for its use as plant growth promoting bioinoculant for crops grown in semi-arid deserts. Appl. Soil Ecol. 2012, 59, 124–135. [Google Scholar]
- Kloepper, J.W.; Ryu, C.-M.; Zhang, S. Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology 2004, 94, 1259–1266. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, D.K.; Johri, B.N. Interactions of Bacillus spp. and plants—with special reference to induced systemic resistance (ISR). Microbiol. Res. 2009, 164, 493–513. [Google Scholar] [CrossRef]
- Chen, Y.; Yan, F.; Chai, Y.; Liu, H.; Kolter, R.; Losick, R.; Guo, J. Biocontrol of tomato wilt disease by Bacillus subtilis isolates from natural environments depends on conserved genes mediating biofilm formation. Environ. Microbiol. 2013, 15, 916–927. [Google Scholar] [CrossRef] [Green Version]
- Salehin, A.; Hafiz, M.H.R.; Hayashi, S.; Adachi, F.; Itoh, K. Effects of the biofertilizer OYK (Bacillus sp.) inoculation on endophytic microbial community in sweet potato. Horticulturae 2020, 6, 81. [Google Scholar] [CrossRef]
- Puri, R.R.; Dangi, S.; Dhungana, S.A.; Itoh, K. Diversity and plant growth promoting ability of culturable endophytic bacteria in Nepalese sweet potato. Adv. Microbiol. 2018, 8, 734–761. [Google Scholar] [CrossRef] [Green Version]
- Puri, R.R.; Adachi, F.; Omichi, M.; Saeki, Y.; Yamamoto, A.; Hayashi, S.; Itoh, K. Culture-dependent analysis of endophytic bacterial community of sweet potato (Ipomoea batatas) in different soils and climates. J. Adv. Microbiol. 2018, 13, 1–12. [Google Scholar] [CrossRef]
- Marques, J.M.; Da Silva, T.F.; Vollu, R.E.; Blank, A.F.; Ding, G.-C.; Seldin, L.; Smalla, K. Plant age and genotype affect the bacterial community composition in the tuber rhizosphere of field-grown sweet potato plants. FEMS Microbiol. Ecol. 2014, 88, 424–435. [Google Scholar] [CrossRef]
- Germida, J.J.; Siciliano, S.D.; Renato, D.F.J.; Seib, A.M. Diversity of root-associated bacteria associated with field-grown canola (Brassica napus L.) and wheat (Triticum aestivum L.). FEMS Microbiol. Ecol. 1998, 26, 43–50. [Google Scholar] [CrossRef]
- Tian, B.; Zhang, C.; Ye, Y.; Wen, J.; Wu, Y.; Wang, H.; Li, H.; Cai, S.; Cai, W.; Cheng, Z.; et al. Beneficial traits of bacterial endophytes belonging to the core communities of the tomato root microbiome. Agric. Ecosyst. Environ. 2017, 247, 149–156. [Google Scholar] [CrossRef]
- Souza, S.A.; Xavier, A.A.; Costa, M.R.; Cardoso, A.M.S.; Pereira, M.C.T.; Nietsche, S. Endophytic bacterial diversity in banana “Prata Anã” (Musa spp.) roots. Genet. Mol. Biol. 2013, 36, 252–264. [Google Scholar] [CrossRef]
- Xia, Y.; Greissworth, E.; Mucci, C.; Williams, M.A.; Bolt, S.D. Characterization of culturable bacterial endophytes of switchgrass (Panicum virgatum L.) and their capacity to influence plant growth. GCB Bioenergy 2013, 5, 674–682. [Google Scholar] [CrossRef]
- Liu, B.; Qiao, H.; Huang, L.; Buchenauer, H.; Han, Q.; Kang, Z.; Gong, Y. Biological control of take-all in wheat by endophytic Bacillus subtilis E1R-j and potential mode of action. Biol. Control 2009, 49, 277–285. [Google Scholar] [CrossRef]
- Molina-Romero, D.; Baez, A.; Quintero-Hernández, V.; Castañeda-Lucio, M.; Fuentes-Ramírez, L.E.; del Rocio Bustillos-Cristales, M.; Rodríguez-Andrade, O.; Morales-García, Y.E.; Munive, A.; Muñoz-Rojas, J. Compatible bacterial mixture, tolerant to desiccation, improves maize plant growth. PLoS ONE 2017, 12, 1–21. [Google Scholar] [CrossRef]
- Marimuthu, S.; Subbian, P.; Ramamoorthy, V.; Samiyappan, R. Synergistic effect of combined application of Azospirillum and Pseudomonas fluorescens with inorganic fertilizers on root rot incidence and yield of cotton. J. Plant Dis. Prot. 2002, 109, 569–577. [Google Scholar]
- Castanheira, N.L.; Dourado, A.C.; Pais, I.; Semedo, J.; Scotti-Campos, P.; Borges, N.; Carvalho, G.; Barreto Crespo, M.T.; Fareleira, P. Colonization and beneficial effects on annual ryegrass by mixed inoculation with plant growth promoting bacteria. Microbiol. Res. 2017, 198, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Vestberg, M.; Kukkonen, S.; Saari, K.; Prikka, P.; Huttunen, J.; Tainio, L.; Devos, N.; Weekers, F.; Kevers, C.; Thonart, P.; et al. Microbial inoculation for improving the growth and health of micropropagated strawberry. Appl. Soil Ecol. 2004, 27, 243–258. [Google Scholar] [CrossRef]
- Raupach, G.S.; Kloepper, J.W. Mixtures of plant growth-promoting rhizobacteria enhance biological control of multiple cucumber pathogens. Phytopathology 1998, 88, 1158–1164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, A.L.M.; Stoffels, M.; Schmid, M.; Reis, V.M.; Baldani, J.I.; Hartmann, A. Colonization of sugarcane plantlets by mixed inoculations with diazotrophic bacteria. Eur. J. Soil Biol. 2008, 45, 106–113. [Google Scholar] [CrossRef]
- Felici, C.; Vettori, L.; Giraldi, E.; Forino, L.M.C.; Toffanin, A.; Tagliasacchi, A.M.; Nuti, M. Single and co-inoculation of Bacillus subtilis and Azospirillum brasilense on Lycopersicon esculentum: Effects on plant growth and rhizosphere microbial community. Appl. Soil Ecol. 2008, 40, 260–270. [Google Scholar] [CrossRef]
- Dhungana, S.A.; Itoh, K. Effects of co-inoculation of indole-3-acetic acid-producing and -degrading bacterial endophytes on plant growth. Horticulturae 2019, 5, 17. [Google Scholar] [CrossRef]
- Elbeltagy, A.; Nishioka, K.; Sato, T.; Suzuki, H.; Ye, B.; Hamada, T.; Isawa, T.; Mitsui, H.; Minamisawa, K. Endophytic colonization and in planta nitrogen fixation by a Herbaspirillum sp. isolated from wild rice species. Appl. Environ. Microbiol. 2001, 67, 5285–5293. [Google Scholar] [CrossRef] [Green Version]
- Leonard, L.T. A simple assembly for use in the testing of cultures of rhizobia. J. Bacteriol. 1943, 45, 523–527. [Google Scholar] [CrossRef] [Green Version]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Calif. Agric. Exp. Stn. Circ. 1950, 347, 1–32. [Google Scholar]
- Freed, R. MSTAT: A software program for plant breeder. In Principles of Plant Genetics and Breeding, 2nd ed.; Acquaah, G., Ed.; Blackwell Publishing: Malden, MA, USA, 2007; Volume 1, pp. 426–431. [Google Scholar]
- Saharan, B.S.; Nehra, V. Plant growth promoting rhizobacteria: A critical review. Life Sci. Med. Res. 2011, 21, 1–30. [Google Scholar]
- Shen, M.; Kang, Y.J.; Wang, H.L.; Zhang, X.S.; Zhao, Q.X. Effect of plant growth-promoting rhizobacteria (PGPRs) on plant growth, yield, and quality of tomato (Lycopersicon esculentum Mill.) under simulated seawater irrigation. J. Gen. Appl. Microbiol. 2012, 58, 253–262. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.; Sheng, J.; Chen, L.; Men, Y.; Gan, L.; Guo, S.; Shen, L. Bacterial community compositions of tomato (Lycopersicum esculentum Mill.) seeds and plant growth promoting activity of ACC deaminase producing Bacillus subtilis (HYT-12-1) on tomato seedlings. World J. Microbiol. Biotechnol. 2014, 30, 835–845. [Google Scholar] [CrossRef]
- Batista, B.D.; Lacava, P.T.; Ferrari, A.; Teixeira-Silva, N.S.; Bonatelli, M.L.; Tsui, S.; Mondin, M.; Kitajima, E.W.; Pereira, J.O.; Azevedo, J.L. Screening of tropically derived, multi-trait plant growth-promoting rhizobacteria and evaluation of corn and soybean colonization ability. Microbiol. Res. 2018, 206, 33–42. [Google Scholar] [CrossRef]
- Egamberdieva, D. Indole-acetic acid production by root associated bacteria and its role in plant growth and development. In Auxins: Structure, Biosynthesis and Functions; Keller, A.H., Fallon, M.D., Eds.; Nova Science Publishers: Hauppauge, NY, USA, 2012; pp. 103–122. [Google Scholar]
- Chowdappa, P.; Kumar, S.M.; Lakshmi, M.J.; Mohan, S.P.; Upreti, K.K. Growth stimulation and induction of systemic resistance in tomato against early and late blight by Bacillus subtilis OTPB1 or Trichoderma harzianum OTPB3. Biol. Control 2013, 65, 109–117. [Google Scholar] [CrossRef]
- Bahadir, P.S.; Liaqat, F.; Eltem, R. Plant growth promoting properties of phosphate solubilizing Bacillus species isolated from the Aegean Region of Turkey. Turk. J. Bot. 2018, 42, 1–14. [Google Scholar] [CrossRef]
- Abbamondi, G.R.; Tommonaro, G.; Weyens, N.; Thijs, S.; Sillen, W.; Gkorezis, P.; Iodice, C.; de Melo Rangel, W.; Nicolaus, B.; Vangronsveld, J. Plant growth-promoting effects of rhizospheric and endophytic bacteria associated with different tomato cultivars and new tomato hybrids. Chem. Biol. Technol. Agric. 2016, 3, 1. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, C.S.; Agostini, F.; Simon, A.M.; Whyte, J.; Townend, J.; Lifert, C.; Killham, K.; Mullins, C. Influence of soil type and pH on the colonization of sugar beet seedlings by antagonistic Pseudomonas and Bacillus strains, and on their control of Pythium damping-off. Eur. J. Plant Pathol. 2004, 110, 1025–1046. [Google Scholar] [CrossRef]
- Dandurand, L.; Knudsen, G. Influence of Pseudomonas fluorescens on hyphal growth and biocontrol activity of Trichoderma harzianum in the spermosphere and rhizosphere of pea. Phytopathology 1993, 83, 265–270. [Google Scholar] [CrossRef]
- García, L.J.A.; Probanza, A.; Ramos, B.; Barriuso, J.; Gutierrez Mañero, F.J. Effects of inoculation with plant growth promoting rhizobacteria (PGPRs) and Sinorhizobium fredii on biological nitrogen fixation, nodulation and growth of Glycine max cv. Osumi. Plant Soil 2004, 267, 143–153. [Google Scholar] [CrossRef]

| Strain | Most Similar Genus a | Class | Origin | Accession Number |
|---|---|---|---|---|
| OYK | Bacillus sp. | Bacilli | Soil | LC590219 |
| RF-12 | Bacillus sp. | Bacilli | Rhizosphere | LC593252 |
| RF-37 | Bacillus sp. | Bacilli | Rhizosphere | LC593253 |
| F-33 | Bacillus sp. | Bacilli | Endophytic | LC430058 |
| Sal 1 | Klebsiella sp. | γ-Proteobacteria | Endophytic | LC389410 |
| Sal 3 | Enterobacter sp. | γ-Proteobacteria | Endophytic | LC389433 |
| Sal 6 | Herbaspirillum sp. | β-Proteobacteria | Endophytic | LC389442 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salehin, A.; Puri, R.R.; Hafiz, M.H.R.; Itoh, K. Effect of Co-Inoculation of Bacillus sp. Strain with Bacterial Endophytes on Plant Growth and Colonization in Tomato Plant (Solanum lycopersicum). Microbiol. Res. 2021, 12, 480-490. https://doi.org/10.3390/microbiolres12020032
Salehin A, Puri RR, Hafiz MHR, Itoh K. Effect of Co-Inoculation of Bacillus sp. Strain with Bacterial Endophytes on Plant Growth and Colonization in Tomato Plant (Solanum lycopersicum). Microbiology Research. 2021; 12(2):480-490. https://doi.org/10.3390/microbiolres12020032
Chicago/Turabian StyleSalehin, Ahsanul, Ramesh Raj Puri, Md Hafizur Rahman Hafiz, and Kazuhito Itoh. 2021. "Effect of Co-Inoculation of Bacillus sp. Strain with Bacterial Endophytes on Plant Growth and Colonization in Tomato Plant (Solanum lycopersicum)" Microbiology Research 12, no. 2: 480-490. https://doi.org/10.3390/microbiolres12020032






