Methane Production from H2 + CO2 Reaction: An Open Molecular Science Case for Computational and Experimental Studies
Abstract
:1. Introduction
2. Materials and Methods
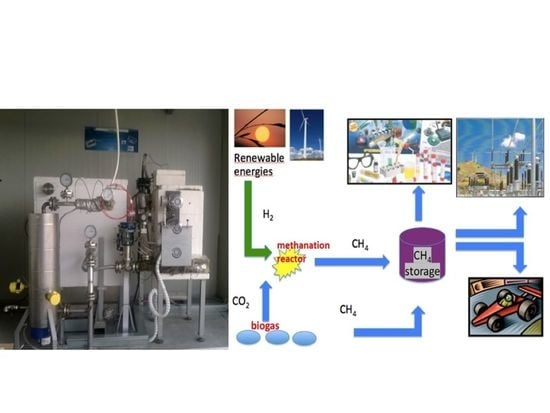
2.1. The PROGEO Apparatus
2.2. The Key Computational Outcomes
3. Results and Discussion
3.1. The Homogeneous Gas-Phase Plasma Assisted Catalysis
3.2. Open Science, Circular Economy and Demo Applications
3.2.1. Open Science and Circular Economy
3.2.2. Demo Applications
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Cook, J.; Nuccitelli, D.; Green, S.A.; Richardson, M.; Winkler, B.; Painting, R.; Way, R.; Jacobs, P.; Skuce, A. Quantifying the consensus on anthropogenic global warming in the scientific literature. Environ. Res. Lett. 2013, 8, 2. [Google Scholar] [CrossRef] [Green Version]
- Oreskes, N. Beyond the Ivory Tower: The Scientific Consensus on Climate Change. Science 2004, 306, 1686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falcinelli, S.; Capriccioli, A.; Pirani, F.; Vecchiocattivi, F.; Stranges, S.; Martì, C.; Nicoziani, A.; Topini, E.; Laganà, A. Methane production by CO2 hydrogenation reaction with and without solid phase catalysis. Fuel 2017, 209, 802–811. [Google Scholar] [CrossRef]
- Laganà, A.; Riganelli, A. Reaction and Molecular Dynamics; Springer: Berlin/Heidelberg, Germany, 1999; ISBN 3-540-41202-6. [Google Scholar]
- Laganà, A.; Parker, G.A. Chemical Reactions Basic Theory and Computing; Springer International Publishing: New York, NY, USA, 2018; ISBN 978-3-319-62355-9. [Google Scholar]
- Falcinelli, S.; Pirani, F.; Vecchiocattivi, F. The Possible role of Penning Ionization Processes in Planetary Atmospheres. Atmosphere 2015, 6, 299–317. [Google Scholar] [CrossRef] [Green Version]
- Falcinelli, S.; Bartocci, A.; Cavalli, S.; Pirani, F.; Vecchiocattivi, F. Stereo-dynamics in collisional autoionization of water, ammonia, and hydrogen sulfide with metastable rare gas atoms: Competition between intermolecular halogen and hydrogen bonds. Chem. Eur. J. 2016, 22, 764–771. [Google Scholar] [CrossRef]
- Falcinelli, S.; Rosi, M.; Cavalli, S.; Pirani, F.; Vecchiocattivi, F. Stereoselectivity in Autoionization Reactions of Hydrogenated Molecules by Metastable Noble Gas Atoms: The Role of Electronic Couplings. Chem. Eur. J. 2016, 22, 12518–12526. [Google Scholar] [CrossRef]
- Zhong, J.; Yang, X.; Wu, Z.; Liang, B.; Huang, Y.; Zhang, T. State of the art and perspectives in heterogeneous catalysis of CO2 hydrogenation to methanol. Chem. Soc. Rev. 2020, 49, 1385–1413. [Google Scholar] [CrossRef]
- PROGEO by PLC System. Available online: https://www.plc-spa.com/en/plc-system-progeo.php (accessed on 11 March 2021).
- Laboratoire de Chimie et Physique Quantiques—UMR5626. Available online: http://www.lcpq.ups-tlse.fr/?lang=fr (accessed on 11 March 2021).
- Rampino, S.; Skouteris, D.; Laganà, A.; Garcia, E.; Saracibar, A. A comparison of the quantum state-specific efficiency of N+N2 reaction computed on different potential energy surfaces. Phys. Chem. Chem. Phys. 2009, 11, 1752–1757. [Google Scholar] [CrossRef]
- Prats, H.; Gamallo, P.; Illas, F.; Sayós, R. Comparing the catalytic activity of the water gas shift reaction on Cu(321) and Cu(111) surfaces: Step sites do not always enhance the overall reactivity. J. Catal. 2016, 342, 75–83. [Google Scholar] [CrossRef] [Green Version]
- L’Energia Che Crea il tuo Futuro by PLC System. Available online: https://www.plc-spa.com/it/index.php (accessed on 11 March 2021).
- RadioAstroLab. Available online: https://www.radioastrolab.it/ (accessed on 11 March 2021).
- Chemical Processes by Johnson Matthey. Available online: http://www.jmprotech.com/methanation-catalysts-for-hydrogen-production-katalco (accessed on 16 March 2021).
- Martì Aliod, C. Networked Computing for Ab Initio Modelling the Chemical Storage of Alternative Energy, ITN-EJD-TCCM. Ph.D. Thesis, Università degli Studi di Perugia, Perugia, Italy, Universitè P. Sabatier de Toulouse, Toulouse, France, 14 December 2018. [Google Scholar]
- Pei, L.; Carrascosa, E.; Yang, N.; Falcinelli, S.; Farrar, J.M. Velocity Map Imaging Study of Charge-Transfer and Proton-Transfer Reactions of CH3 Radicals with H3+. J. Phys. Chem. Lett. 2015, 6, 1684–1689. [Google Scholar] [CrossRef]
- Brunetti, B.; Candori, P.; Cappelletti, D.; Falcinelli, S.; Pirani, F.; Stranges, D.; Vecchiocattivi, F. Penning Ionization Electron Spectroscopy of water molecules by metastable neon atoms. Chem. Phys. Lett. 2012, 539–540, 19–23. [Google Scholar] [CrossRef]
- Falcinelli, S.; Vecchiocattivi, F.; Pirani, F. Adiabatic and nonadiabatic effects in the transition states of state to state autoionization processes. Phys. Rev. Lett. 2018, 121, 163403. [Google Scholar] [CrossRef]
- Balucani, N.; Bartocci, A.; Brunetti, B.; Candori, P.; Falcinelli, S.; Pirani, F.; Palazzetti, F.; Vecchiocattivi, F. Collisional autoionization dynamics of Ne*(3P2,0)-H2O. Chem. Phys. Lett. 2012, 546, 34–39. [Google Scholar] [CrossRef]
- Leonori, F.; Petrucci, R.; Balucani, N.; Casavecchia, P.; Rosi, M.; Berteloite, C.; Le Picard, S.D.; Canosa, A.; Sims, I.R. Observation of organosulfur products (thiovinoxy, thioketene and thioformyl) in crossed-beam experiments and low temperature rate coefficients for reaction S(1D) + C2H4. Phys. Chem. Chem. Phys. 2009, 11, 4701–4706. [Google Scholar] [CrossRef]
- Dobrea, S.; Mihaila, I.; Popa, G. Carbon dioxide dissociation in a 2.45 GHz microwave discharge. In Proceedings of the 1st ICPIG, Granada, Spain, 14–19 July 2013; p. 14. [Google Scholar]
- Dobrea, S.; Mihaila, I.; Tiron, V.; Popa, G. Optical and mass spectrometry diagnosis of a CO2 microwave plasma discharge. Rom. Rep. Phys. 2014, 66, 1147–1154. [Google Scholar]
- de la Fuente, J.F.; Moreno, S.H.; Stankiewicz, A.I.; Stefanidis, G.D. Reduction of CO2 with hydrogen in a non-equilibrium microwave plasma reactor. Int. J. Hydrog. Energy 2016, 41, 21067–21077. [Google Scholar] [CrossRef]
- Tortora, C.; Mai, C.; Cascella, F.; Mauksch, M.; Seidel-Morgenstern, A.; Lorenz, H.; Tsogoeva, S.B. Speeding up Viedma Deracemization through Watercatalyzed and Reactant Self-catalyzed Racemization. ChemPhysChem 2020, 21, 1775–1787. [Google Scholar] [CrossRef]
- Falcinelli, S. Fuel production from waste CO2 using renewable energies. Catal. Today 2020, 348, 95–101. [Google Scholar] [CrossRef]
- Hayashi, N.; Yamakawa, T.; Baba, S. Effect of additive gases on synthesis of organic compounds from carbon dioxide using non-thermal plasma produced by atmospheric surface discharges. Vacuum 2006, 80, 1299–1304. [Google Scholar] [CrossRef]
- Gervasi, O.; Lagana’, A. SIMBEX: A portal for the a priori simulation of crossed beam experiments. Future Gener. Comput. Syst. 2004, 20, 703–716. [Google Scholar] [CrossRef]
- Laganà, A.; Rampino, S.; Storchi, L.; Garcia, E.; Coletti, C.; Blurock, E.; Bo, C.; Mariotti, M.; Vitillaro, G. GEMS Expression of Interest for the EGI, EUDAT and INDIGO-Datacloud H2020 Project Proposal EINFRA12 (A). Virt&l-Comm.10.2016.6. Available online: http://services.chm.unipg.it/ojs/index.php/virtlcomm/article/view/151 (accessed on 16 March 2021).
- Laganà, A.; Riganelli, A.; Gervasi, O. On the structuring of the computational chemistry virtual organization COMPCHEM. Lect. Notes Comput. Sci. 2006, 3980, 665–674. [Google Scholar]
- Towards a CMMST VRC Team Project Report. Available online: https://wiki.egi.eu/wiki/Towards_a_CMMST_VRC (accessed on 16 March 2021).
- European Cost Action D23: Metalaboratories for Complex Computational Applications in Chemistry. Available online: https://www.cost.eu/actions/D23/#tabs|Name:overview/ (accessed on 16 March 2021).
- European Cost Action D37: Grid Computing in Chemistry. Available online: https://www.cost.eu/actions/D37/#tabs|Name:overview/ (accessed on 16 March 2021).
- European Chemistry Thematic Network. Available online: http://ectn.eu/ (accessed on 16 March 2021).
- Enabling Grids for E-sciencE III (EGEE III). Available online: https://cordis.europa.eu/project/rcn/87264/factsheet/en (accessed on 16 March 2021).
- EGI-Inspire. Available online: https://wiki.egi.eu/wiki/EGI-InSPIRE:Main_Page (accessed on 16 March 2021).
- Laganà, A. Towards Open Molecular Science Cloud Services. Virt&l-Comm.16.2019.5. Available online: http://services.chm.unipg.it/ojs/index.php/virtlcomm/article/view/210 (accessed on 16 March 2021).
- European Open Science Cloud. Available online: https://ec.europa.eu/research/openscience/index.cfm?pg=open-science-cloud (accessed on 16 March 2021).
- EOSCpilot. Available online: https://eoscpilot.eu/ (accessed on 16 March 2021).
- Vitillaro, G.; Laganà, A. MOSEX: Molecular Open Science Enabled. Cloud Services. Virt&l-Comm.20.2020.7. Available online: http://services.chm.unipg.it/ojs/index.php/virtlcomm/article/view/248 (accessed on 2 April 2021).
- Laganà, A.; Garcia, E. Astrochemistry: An Open Molecular Science Cloud Approach to Low Temperature Reactivity. Virt&l-Comm.18.2019.3. Available online: http://services.chm.unipg.it/ojs/index.php/virtlcomm/article/view/219 (accessed on 16 March 2021).
- Skouteris, D.; Balucani, N.; Faginas-Lago, N.; Falcinelli, S.; Rosi, M. Dimerization of methanimine and its charged species in the atmosphere of Titan and interstellar/cometary ice analogs. Astron. Astrophys. 2015, 584, A76. [Google Scholar] [CrossRef] [Green Version]
- EChemTest by ECTN. Available online: http://ectn.eu/committees/virtual-education-community/echemtest/ (accessed on 16 March 2021).
- Laganà, A.; di Giorgio, L. A Circular Economy proposal on CO2 reuse to produce methane using energy from renewable sources. Lect. Notes Comput. Sci. 2018, 10962, 549–562. [Google Scholar]
- QCArchive. Available online: https://qcarchive.molssi.org/ (accessed on 16 March 2021).
- NIST Chemical Kinetics Database. Available online: http://kinetics.nist.gov (accessed on 16 March 2021).
- Wakelam, V.; Herbst, E.; Loison, J.-C.; Smith, I.W.M.; Chandrasekaran, V.; Pavone, B.; Adams, N.G.; Bacchus-Montabonel, M.-C.; Bergeat, A.; Beroff, K.; et al. A kinetic Database for Astrochemistry (KIDA). AstroPhys. J. Suppl. Ser. 2012, 199, 21. [Google Scholar] [CrossRef]
- McElroy, D.; Walsh, C.; Markwick, A.J.; Cordiner, M.A.; Smith, K.; Millar, T.J. The UMIST database for astrochemistry 2012. Astron. Astrophys. 2013, 550, A36. [Google Scholar] [CrossRef] [Green Version]
- Laganà, A.; Gervasi, O.; Tasso, S.; Perri, D.; Franciosa, F. The ECTN Virtual Education Community prosumer model for promoting and assessing chemical knowledge. Lect. Notes Comput. Sci. 2018, 10964, 533–548. [Google Scholar]
- Álvarez-Moreno, M.; de Graaf, C.; López, N.; Maseras, F.; Poblet, J.M.; Bo, C. Managing the computational chemistry big data problem: The ioChem-BD platform. J. Chem. Inf. Model. 2015, 55, 95–103. [Google Scholar] [CrossRef]
- I.S.FORM. by CNR, Perugia. Available online: http://www.iro.pg.cnr.it/ (accessed on 16 March 2021).
- 3A Parco Tecnologico Agroalimentare Dell’Umbria, Pantalla Todi (Italy). Available online: http://www.parco3a.org (accessed on 16 March 2021).
- Falcinelli, S.; Vecchiocattivi, F.; Pirani, F. General treatment for stereo-dynamics of state-to-state chemi-ionization reactions. Commun. Chem. 2020, 3, 64. [Google Scholar] [CrossRef]
- Leonori, F.; Petrucci, R.; Balucani, N.; Hickson, K.M.; Hamberg, M.; Geppert, W.D.; Casavecchia, P.; Rosi, M. Crossed-Beam and Theoretical Studies of the S(1D) + C2H2 Reaction. J. Phys. Chem. A 2009, 113, 4330–4339. [Google Scholar] [CrossRef]
- De Petris, G.; Cartoni, A.; Rosi, M.; Barone, V.; Puzzarini, C.; Troiani, A. The Proton Affinity and Gas-Phase Basicity of Sulfur Dioxide. ChemPhysChem 2011, 12, 112–115. [Google Scholar] [CrossRef]
- Alagia, M.; Balucani, N.; Candori, P.; Falcinelli, S.; Pirani, F.; Richter, R.; Rosi, M.; Stranges, S.; Vecchiocattivi, F. Production of ions at high energy and its role in extraterrestrial environments. Rend. Lincei Sci. Fis. Nat. 2013, 24, 53–65. [Google Scholar] [CrossRef]
- Falcinelli, S.; Farrar, J.M.; Vecchiocattivi, F.; Pirani, F. Quantum-state controlled reaction channels in chemiionization processes: Radiative (optical—physical) and exchange (oxidative—chemical) mechanisms. Acc. Chem. Res. 2020, 53, 2248–2260. [Google Scholar] [CrossRef]
- Podio, L.; Codella, C.; Lefloch, B.; Balucani, N.; Ceccarelli, C.; Bachiller, R.; Benedettini, M.; Cernicharo, J.; Faginas-Lago, N.; Fontani, F.; et al. Silicon-bearing molecules in the shock L1157-B1: First detection of SiS around a Sun-like protostar. Mon. Not. R. Astron. Soc. Lett. 2017, 470, L16–L20. [Google Scholar] [CrossRef]
- Candori, P.; Falcinelli, S.; Pirani, F.; Tarantelli, F.; Vecchiocattivi, F. Interaction components in the hydrogen halide dication. Chem. Phys. Lett. 2007, 436, 322–326. [Google Scholar] [CrossRef]






| Step | Ea Forward (kJ/mol) | Ea Reverse (kJ/mol) |
|---|---|---|
| CO2 + * ↔ CO2* | 0.0 | 8.3 |
| H2 + 2* ↔ 2H* | 4.0 | 77.1 |
| CO + * ↔ CO* | 0.0 | 127.7 |
| H2O + * ↔ H2O* | 0.0 | 49.0 |
| CO2* + H* ↔ COOH* + * | 113.1 | 155.6 |
| CO2* + 2H* ↔ C(OH)2* + 2* | 292.3 | 217.8 |
| CO2* + * ↔ CO* + O* | 93.7 | 169.3 |
| COOH* + * ↔ CO* + OH* | 306.8 | 308.7 |
| C(OH)2* + H* ↔ CH2O* + OH* | 98.7 | 125.7 |
| CH2O* + H* ↔ CH2* + OH* | 163.7 | 154.1 |
| CO* + * ↔ C* + O* | 237.4 | 111.8 |
| CO* + 2H* ↔ CH* + OH* + * | 221.4 | 146.1 |
| 2CO* ↔ CO2* + C* | 339.6 | 109.0 |
| C* + H* ↔ CH* + * | 69.2 | 154.1 |
| CH* + H* ↔ CH2* + * | 68.2 | 61.9 |
| CH2* + H* ↔ CH3* + * | 71.4 | 105.6 |
| CH3* + H* ↔ CH4 + 2* | 137.4 | 178.7 |
| O* + H* ↔ OH* + * | 137.9 | 116.0 |
| OH* + H* ↔ H2O* + * | 137.9 | 99.9 |
| H* + * ↔ * + H* | 13.0 | 13.0 |
| CO* + * ↔ * + CO* | 10.0 | 10.0 |
| O* + * ↔ * + O* | 48.0 | 48.0 |
| OH* + * ↔ * + OH* | 21.0 | 21.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falcinelli, S.; Capriccioli, A.; Rosi, M.; Martì, C.; Parriani, M.; Laganà, A. Methane Production from H2 + CO2 Reaction: An Open Molecular Science Case for Computational and Experimental Studies. Physchem 2021, 1, 82-94. https://doi.org/10.3390/physchem1010006
Falcinelli S, Capriccioli A, Rosi M, Martì C, Parriani M, Laganà A. Methane Production from H2 + CO2 Reaction: An Open Molecular Science Case for Computational and Experimental Studies. Physchem. 2021; 1(1):82-94. https://doi.org/10.3390/physchem1010006
Chicago/Turabian StyleFalcinelli, Stefano, Andrea Capriccioli, Marzio Rosi, Carles Martì, Marco Parriani, and Antonio Laganà. 2021. "Methane Production from H2 + CO2 Reaction: An Open Molecular Science Case for Computational and Experimental Studies" Physchem 1, no. 1: 82-94. https://doi.org/10.3390/physchem1010006
APA StyleFalcinelli, S., Capriccioli, A., Rosi, M., Martì, C., Parriani, M., & Laganà, A. (2021). Methane Production from H2 + CO2 Reaction: An Open Molecular Science Case for Computational and Experimental Studies. Physchem, 1(1), 82-94. https://doi.org/10.3390/physchem1010006