ASSURED Point-of-Need Food Safety Screening: A Critical Assessment of Portable Food Analyzers
Abstract
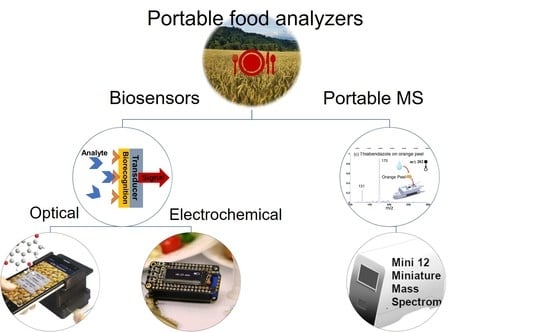
:1. Introduction
- The biorecognition element, in general, an antibody, aptamer, or enzyme that binds specifically to the target analyte;
- The transducer converts resulting signals, which can be optical, electrochemical, magnetic, calorimetric, etc.;
- The readout system is used to visualizes the result.
2. Portable Optical Food Analyzers
2.1. Paper-Based Optical Food Analyzers
2.2. Microfluidic, Chip-Based Optical Food Analyzers
2.3. Smartphone-Based Optical Food Analyzers
2.4. Raman and IR-Based Portable Food Analyzers
3. Portable Electrochemical Food Analyzers
3.1. Paper-Based Electrochemical Food Analyzers
3.2. Microfluidic Chip-Based Electrochemical Food Analyzers
3.3. Smartphone-Based Electrochemical Food Analyzers
4. Portable Mass Spectrometry for Food Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- World Health Organization (WHO). WHO Estimates of the Global Burden of Foodborne Diseases: Executive Summary; WHO: Genenva, Switzerland, 2015. [Google Scholar]
- Haughey, S.A.; Chevallier, O.P.; McVey, C.; Elliott, C.T. Laboratory Investigations into the Cause of Multiple Serious and Fatal Food Poisoning Incidents in Uganda during 2019. Food Control 2021, 121, 107648. [Google Scholar] [CrossRef]
- Wang, X.; Wang, S.; Cai, Z. The Latest Developments and Applications of Mass Spectrometry in Food-Safety and Quality Analysis. TrAC Trends Anal. Chem. 2013, 52, 170–185. [Google Scholar] [CrossRef]
- Griesche, C.; Baeumner, A.J. Biosensors to support sustainable agriculture and food safety. TrAC Trends Anal. Chem. 2020, 128, 115906. [Google Scholar] [CrossRef]
- Lu, Y.; Yang, Q.; Wu, J. Recent Advances in Biosensor-integrated enrichment methods for preconcentrating and detecting the low-abundant analytes in agriculture and food samples. TrAC Trends Anal. Chem. 2020, 128, 115914. [Google Scholar] [CrossRef]
- European Commisdion. Commission Decision of 12 August 2002 Implementing Council Directive 96/23/EC Concerning the Performance of Analytical Methods and the Interpretation of Results; European Commission: Brussels, Belgium, 2002. [Google Scholar]
- Ma, X.; Ouyang, Z. Ambient ionization and miniature mass spectrometry system for chemical and biological analysis. TrAC Trends Anal. Chem. 2016, 85, 10–19. [Google Scholar] [CrossRef] [Green Version]
- Kosack, C.S.; Page, A.L.; Klatser, P.R. A guide to aid the selection of diagnostic tests. Bull. World Health Organ. 2017, 95, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, J. Optical Biosensors: An exhaustive and comprehensive review. Analyst 2020, 145, 1605–1628. [Google Scholar] [CrossRef] [PubMed]
- Lechuga, L.M. Optical biosensors. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2005; Volume 44, pp. 209–250. ISBN 9780444507150. [Google Scholar]
- Yang, Z.; Albrow-Owen, T.; Cai, W.; Hasan, T. Miniaturization of optical spectrometers. Science 2021, 371. [Google Scholar] [CrossRef]
- Martinez, A.W.; Phillips, S.T.; Butte, M.J.; Whitesides, G.M. Patterned paper as a platform for inexpensive, low-volume, portable bioassays. Angew. Chem. Int. Ed. Engl. 2007, 46, 1318–1320. [Google Scholar] [CrossRef] [Green Version]
- Fu, L.-M.; Wang, Y.-N. Detection methods and applications of microfluidic paper-based analytical devices. TrAC Trends Anal. Chem. 2018, 107, 196–211. [Google Scholar] [CrossRef]
- Carrell, C.; Kava, A.; Nguyen, M.; Menger, R.; Munshi, Z.; Call, Z.; Nussbaum, M.; Henry, C. Beyond the lateral flow assay: A review of paper-based microfluidics. Microelectron. Eng. 2019, 206, 45–54. [Google Scholar] [CrossRef]
- Choi, J.R.; Hu, J.; Tang, R.; Gong, Y.; Feng, S.; Ren, H.; Wen, T.; Li, X.; Wan Abas, W.A.B.; Pingguan-Murphy, B.; et al. An integrated paper-based sample-to-answer biosensor for nucleic acid testing at the point of care. Lab Chip 2016, 16, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Ross, G.M.S.; Bremer, M.G.E.G.; Wichers, J.H.; Van Amerongen, A.; Nielen, M.W.F. Rapid antibody selection using surface plasmon resonance for high-speed and sensitive hazelnut lateral flow prototypes. Biosensors 2018, 8, 130. [Google Scholar] [CrossRef] [Green Version]
- Tsagkaris, A.S.; Uttl, L.; Pulkrabova, J.; Hajslova, J. Screening of carbamate and organophosphate pesticides in food matrices using an affordable and simple spectrophotometric acetylcholinesterase assay. Appl. Sci. 2020, 10, 565. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Wang, H.; Zhang, P.; Sun, C.; Wang, X.; Wang, X.; Yang, R.; Wang, C.; Zhou, L. Rapid multiplex detection of 10 foodborne pathogens with an up-converting phosphor technology-based 10-channel lateral flow assay. Sci. Rep. 2016, 6, 21342. [Google Scholar] [CrossRef] [PubMed]
- Tsagkaris, A.S.; Pulkrabova, J.; Hajslova, J.; Filippini, D. A Hybrid lab-on-a-chip injector system for autonomous carbofuran screening. Sensors 2019, 19, 5579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelis, J.L.D.; Tsagkaris, A.S.; Dillon, M.J.; Hajslova, J.; Elliott, C.T. Smartphone-Based Optical Assays in the Food Safety Field. TrAC Trends Anal. Chem. 2020, 129, 115934. [Google Scholar] [CrossRef]
- Liu, W.; Guo, Y.; Luo, J.; Kou, J.; Zheng, H.; Li, B.; Zhang, Z. A molecularly imprinted polymer based a lab-on-paper chemiluminescence device for the detection of dichlorvos. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 141, 51–57. [Google Scholar] [CrossRef]
- Tseng, S.-Y.; Li, S.-Y.; Yi, S.-Y.; Sun, A.Y.; Gao, D.-Y.; Wan, D. Food quality monitor: Paper-based plasmonic sensors prepared through reversal nanoimprinting for rapid detection of biogenic amine odorants. ACS Appl. Mater. Interfaces 2017, 9, 17306–17316. [Google Scholar] [CrossRef]
- Picó, Y. Chemical Analysis of Food: Techniques and Applications, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Stroock, A.D. Microfluidics. In Optical Biosensors; Elsevier: Amsterdam, The Netherlands, 2008; pp. 659–681. ISBN 9780444531254. [Google Scholar]
- Connelly, J.T.; Kondapalli, S.; Skoupi, M.; Parker, J.S.L.; Kirby, B.J.; Baeumner, A.J. Micro-total analysis system for virus detection: Microfluidic pre-concentration coupled to liposome-based detection. Anal. Bioanal. Chem. 2012, 402, 315–323. [Google Scholar] [CrossRef]
- Soares, R.R.G.; Novo, P.; Azevedo, A.M.; Fernandes, P.; Aires-Barros, M.R.; Chu, V.; Conde, J.P. On-chip sample preparation and analyte quantification using a microfluidic aqueous two-phase extraction coupled with an immunoassay. Lab Chip 2014, 14, 4284–4294. [Google Scholar] [CrossRef]
- Hung, T.Q.; Chin, W.H.; Sun, Y.; Wolff, A.; Bang, D.D. A novel lab-on-chip platform with integrated solid phase pcr and supercritical angle fluorescence (saf) microlens array for highly sensitive and multiplexed pathogen detection. Biosens. Bioelectron. 2017, 90, 217–223. [Google Scholar] [CrossRef]
- Choi, G.; Jung, J.H.; Park, B.H.; Oh, S.J.; Seo, J.H.; Choi, J.S.; Kim, D.H.; Seo, T.S. A centrifugal direct recombinase polymerase amplification (direct-rpa) microdevice for multiplex and real-time identification of food poisoning bacteria. Lab Chip 2016, 16, 2309–2316. [Google Scholar] [CrossRef]
- Bhardwaj, H.; Sumana, G.; Marquette, C.A. A label-free ultrasensitive microfluidic surface plasmon resonance biosensor for aflatoxin b1 detection using nanoparticles integrated gold chip. Food Chem. 2020, 307, 125530. [Google Scholar] [CrossRef]
- Joshi, S.; Annida, R.M.; Zuilhof, H.; van Beek, T.A.; Nielen, M.W.F. Analysis of mycotoxins in beer using a portable nanostructured imaging surface plasmon resonance biosensor. J. Agric. Food Chem. 2016, 64, 8263–8271. [Google Scholar] [CrossRef]
- Sauceda-Friebe, J.C.; Karsunke, X.Y.Z.; Vazac, S.; Biselli, S.; Niessner, R.; Knopp, D. Regenerable immuno-biochip for screening ochratoxin a in green coffee extract using an automated microarray chip reader with chemiluminescence detection. Anal. Chim. Acta 2011, 689, 234–242. [Google Scholar] [CrossRef]
- Wang, Y.; Gan, N.; Zhou, Y.; Li, T.; Hu, F.; Cao, Y.; Chen, Y. Novel label-free and high-throughput microchip electrophoresis platform for multiplex antibiotic residues detection based on aptamer probes and target catalyzed hairpin assembly for signal amplification. Biosens. Bioelectron. 2017, 97, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Weng, X.; Neethirajan, S. Paper-based microfluidic aptasensor for food safety. J. Food Saf. 2018, 38. [Google Scholar] [CrossRef]
- Fernández, F.; Hegnerová, K.; Piliarik, M.; Sanchez-Baeza, F.; Homola, J.; Marco, M.P. A label-free and portable multichannel surface plasmon resonance immunosensor for on site analysis of antibiotics in milk samples. Biosens. Bioelectron. 2010, 26, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Chalyan, T.; Potrich, C.; Schreuder, E.; Falke, F.; Pasquardini, L.; Pederzolli, C.; Heideman, R.; Pavesi, L. AFM1 detection in milk by fab’ functionalized si3n4 asymmetric mach–zehnder interferometric biosensors. Toxins 2019, 11, 409. [Google Scholar] [CrossRef] [Green Version]
- Nelis, J.L.D.; Zhao, Y.; Bura, L.; Rafferty, K.; Elliott, C.T.; Campbell, K. A randomized combined channel approach for the quantification of color- and intensity-based assays with smartphones. Anal. Chem. 2020, 92, 7852–7860. [Google Scholar] [CrossRef]
- Li, Z.; Li, Z.; Zhao, D.; Wen, F.; Jiang, J.; Xu, D. Smartphone-based visualized microarray detection for multiplexed harmful substances in milk. Biosens. Bioelectron. 2017, 87, 874–880. [Google Scholar] [CrossRef]
- Ye, Y.; Wu, T.; Jiang, X.; Cao, J.; Ling, X.; Mei, Q.; Chen, H.; Han, D.; Xu, J.J.; Shen, Y. Portable smartphone-based QDs for the visual onsite monitoring of fluoroquinolone antibiotics in actual food and environmental samples. ACS Appl. Mater. Interfaces 2020, 12, 14552–14562. [Google Scholar] [CrossRef]
- Zhong, L.; Sun, J.; Gan, Y.; Zhou, S.; Wan, Z.; Zou, Q.; Su, K.; Wang, P. Portable smartphone-based colorimetric analyzer with enhanced gold nanoparticles for on-site tests of seafood safety. Anal. Sci. 2019, 35, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-Y.; Liu, B.-W.; Huang, P.; Wu, F.Y. A novel colorimetric aptasensor for detection of chloramphenicol based on lanthanum ion–assisted gold nanoparticle aggregation and smartphone imaging. Anal. Bioanal. Chem. 2019, 411, 7511–7518. [Google Scholar] [CrossRef] [PubMed]
- Hosu, O.; Lettieri, M.; Papara, N.; Ravalli, A.; Sandulescu, R.; Cristea, C.; Marrazza, G. Colorimetric multienzymatic smart sensors for hydrogen peroxide, glucose and catechol screening analysis. Talanta 2019, 204, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wong, J.X.H.; Cui, C.; Li, X.; Yu, H.Z. A smartphone-readable barcode assay for the detection and quantitation of pesticide residues. Analyst 2015, 140, 5518–5525. [Google Scholar] [CrossRef]
- Machado, J.M.D.; Soares, R.R.G.; Chu, V.; Conde, J.P. Multiplexed capillary microfluidic immunoassay with smartphone data acquisition for parallel mycotoxin detection. Biosens. Bioelectron. 2018, 99, 40–46. [Google Scholar] [CrossRef]
- Cheng, N.; Song, Y.; Fu, Q.; Du, D.; Luo, Y.; Wang, Y.; Xu, W.; Lin, Y. Aptasensor based on fluorophore-quencher nano-pair and smartphone spectrum reader for on-site quantification of multi-pesticides. Biosens. Bioelectron. 2018, 117, 75–83. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, L.; Cai, G.; Liu, N.; Liao, M.; Li, Y.; Zhang, X.; Lin, J. A microfluidic biosensor for online and sensitive detection of salmonella typhimurium using fluorescence labeling and smartphone video processing. Biosens. Bioelectron. 2019, 140, 111333. [Google Scholar] [CrossRef]
- Zeinhom, M.M.A.; Wang, Y.; Song, Y.; Zhu, M.-J.; Lin, Y.; Du, D. A portable smart-phone device for rapid and sensitive detection of E. Coli O157:H7 in yoghurt and egg. Biosens. Bioelectron. 2018, 99, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, Y.; Xu, S.; Zhang, H.; Tan, Y.; Ma, C.; Song, R.; Jiang, L.; Yi, C. A 3D Printed smartphone optosensing platform for point-of-need food safety inspection. Anal. Chim. Acta 2017, 966, 81–89. [Google Scholar] [CrossRef]
- Li, X.; Yang, F.; Wong, J.X.H.; Yu, H.-Z. Integrated smartphone-app-chip system for on-site parts-per-billion-level colorimetric quantitation of aflatoxins. Anal. Chem. 2017, 89, 8908–8916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, G.M.S.; Salentijn, G.I.; Nielen, M.W.F. A critical comparison between flow-through and lateral flow immunoassay formats for visual and smartphone-based multiplex allergen detection. Biosensors 2019, 9, 143. [Google Scholar] [CrossRef] [Green Version]
- Valand, R.; Tanna, S.; Lawson, G.; Bengtström, L. A review of Fourier Transform Infrared (FTIR) spectroscopy used in food adulteration and authenticity investigations. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2020, 37, 19–38. [Google Scholar] [CrossRef]
- Danezis, G.P.; Tsagkaris, A.S.; Camin, F.; Brusic, V.; Georgiou, C.A. Food authentication: Techniques, trends & emerging approaches. TrAC Trends Anal. Chem. 2016, 85, 123–132. [Google Scholar] [CrossRef] [Green Version]
- Crocombe, R.A. Portable spectroscopy. Appl. Spectrosc. 2018, 72, 1701–1751. [Google Scholar] [CrossRef]
- SCiO—The World’s Only Pocket-Sized NIR Micro Spectrometer. Available online: https://www.consumerphysics.com/ (accessed on 22 March 2021).
- Lin, Z.; He, L. Recent advance in SERS Techniques for food safety and quality analysis: A brief review. Curr. Opin. Food Sci. 2019, 28, 82–87. [Google Scholar] [CrossRef]
- Hoppmann, E.P.; Yu, W.W.; White, I.M. Highly sensitive and flexible inkjet printed SERS sensors on paper. Methods 2013, 63, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Wang, C.; Pu, S.; Wang, C.; Cheng, F.; Wang, Y.; Fan, M. Rapid and direct detection of illicit dyes on tainted fruit peel using a pva hydrogel surface enhanced raman scattering substrate. Anal. Methods 2016, 8, 4816–4820. [Google Scholar] [CrossRef]
- Shi, Q.; Huang, J.; Sun, Y.; Deng, R.; Teng, M.; Li, Q.; Yang, Y.; Hu, X.; Zhang, Z.; Zhang, G. A SERS-based multiple immuno-nanoprobe for ultrasensitive detection of neomycin and quinolone antibiotics via a lateral flow assay. Microchim. Acta 2018, 185, 3–10. [Google Scholar] [CrossRef]
- Fales, A.M.; Vo-Dinh, T. Silver embedded nanostars for SERS with internal reference (SENSIR). J. Mater. Chem. C 2015, 3, 7319–7324. [Google Scholar] [CrossRef]
- Li, X.; Zhang, S.; Yu, Z.; Yang, T. Surface-enhanced raman spectroscopic analysis of phorate and fenthion pesticide in apple skin using silver nanoparticles. Appl. Spectrosc. 2014, 68, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Lin, X.; Liu, Y.; Zhao, H.; Hasi, W.; Wang, L. Self-assembly of Au@Ag core–shell nanocubes embedded with an internal standard for reliable quantitative SERS measurements. Anal. Methods 2018, 10, 4201–4208. [Google Scholar] [CrossRef]
- Xie, J.; Li, L.; Khan, I.M.; Wang, Z.; Ma, X. Flexible Paper-based SERS substrate strategy for rapid detection of methyl parathion on the surface of fruit. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 231, 118104. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, M.; Kong, L.; Lin, M. Jellylike flexible nanocellulose SERS substrate for rapid in-situ non-invasive pesticide detection in fruits/vegetables. Carbohydr. Polym. 2019, 205, 596–600. [Google Scholar] [CrossRef]
- Moreno, V.; Adnane, A.; Salghi, R.; Zougagh, M.; Ríos, Á. Nanostructured hybrid surface enhancement raman scattering substrate for the rapid determination of sulfapyridine in milk samples. Talanta 2019, 194, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.T.; Flores-Rojas, K.; Guerrero, J.E.; Garrido-Varo, A.; Pérez-Marín, D. Measurement of pesticide residues in peppers by near-infrared reflectance spectroscopy. Pest Manag. Sci. 2010, 66, 580–586. [Google Scholar] [CrossRef] [PubMed]
- de Girolamo, A.; Cervellieri, S.; Visconti, A.; Pascale, M. Rapid analysis of deoxynivalenol in durum wheat by FT-NIR spectroscopy. Toxins 2014, 6, 3129–3143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lasch, P.; Stämmler, M.; Zhang, M.; Baranska, M.; Bosch, A.; Majzner, K. FT-IR hyperspectral imaging and artificial neural network analysis for identification of pathogenic bacteria. Anal. Chem. 2018, 90, 8896–8904. [Google Scholar] [CrossRef] [PubMed]
- Skolik, P.; Morais, C.L.M.; Martin, F.L.; McAinsh, M.R. Attenuated total reflection fourier-transform infrared spectroscopy coupled with chemometrics directly detects pre- and post-symptomatic changes in tomato plants infected with botrytis cinerea. Vib. Spectrosc. 2020, 111, 103171. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; He, C.; Xie, M.; Luo, H.; Wang, Y.; Zhang, J. Rapid screen of aflatoxin-contaminated peanut oil using fourier transform infrared spectroscopy combined with multivariate decision tree. Int. J. Food Sci. Technol. 2018, 53, 2386–2393. [Google Scholar] [CrossRef]
- Meza-Márquez, O.G.; Gallardo-Velázquez, T.; Osorio-Revilla, G.; Dorantes-Álvarez, L. Detection of clenbuterol in beef meat, liver and kidney by mid-infrared spectroscopy (FT-Mid IR) and multivariate analysis. Int. J. Food Sci. Technol. 2012, 47, 2342–2351. [Google Scholar] [CrossRef]
- Kim, J.; Kumar, R.; Bandodkar, A.J.; Wang, J. Advanced materials for printed wearable electrochemical devices: A review. Adv. Electron. Mater. 2017, 3, 1600260. [Google Scholar] [CrossRef]
- Gao, M.; Li, L.; Song, Y. Inkjet printing wearable electronic devices. APL Mater. 2017, 2971–2993. [Google Scholar] [CrossRef]
- Dobbelaere, T.; Vereecken, P.M.; Detavernier, C. HardwareX A USB-controlled potentiostat/galvanostat for thin-film battery characterization. HardwareX 2017, 2, 34–49. [Google Scholar] [CrossRef]
- Dryden, M.D.M.; Wheeler, A.R. DStat: A versatile, open-source potentiostat for electroanalysis and integration. PLoS ONE 2015, e140349. [Google Scholar] [CrossRef] [Green Version]
- Ainla, A.; Mousavi, M.P.S.; Tsaloglou, M.; Redston, J.; Bell, G.; Ferna, M.T. Open-source potentiostat for wireless electrochemical detection with smartphones. Anal. Chem. 2018. [Google Scholar] [CrossRef] [Green Version]
- Ronkainen, N.J.; Brian, H.; Heineman, W.R. Electrochemical biosensors. Chem. Soc. Rev. 2010, 1747–1763. [Google Scholar] [CrossRef]
- Zhao, Y.; Choi, S.Y.; Lou-Franco, J.; Nelis, J.L.D.; Zhou, H.; Cao, C.; Campbell, K.; Elliott, C.; Rafferty, K. Smartphone modulated colorimetric reader with color Subtraction. In Proceedings of the IEEE Sensors, Montreal, QC, Canada, 27–30 October 2019; Volume 2019. [Google Scholar]
- Nelis, J.L.D.; Migliorelli, D.; Jafari, S.; Generelli, S.; Lou-Franco, J.; Salvador, J.P.; Marco, M.P.; Cao, C.; Elliott, C.T.; Campbell, K. The benefits of carbon black, gold and magnetic nanomaterials for point-of-harvest electrochemical quantification of domoic acid. Microchim. Acta 2020, 187, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Nelis, J.L.D.; Migliorelli, D.; Mühlebach, L.; Generelli, S.; Stewart, L.; Elliott, C.T.; Campbell, K. Highly sensitive electrochemical detection of the marine toxins okadaic acid and domoic acid with carbon black modified screen printed electrodes. Talanta 2021, 228, 122215. [Google Scholar] [CrossRef] [PubMed]
- Sierra, T.; Crevillen, A.G.; Escarpa, A. Electrochemical detection based on nanomaterials in ce and microfluidic systems. Electrophoresis 2019, 40, 113–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Xu, D.; Chen, J.; Liu, J.; Li, Y.; Song, J.; Ma, X.; Guo, J. Smartphone-based analytical biosensors. Analyst 2018, 143, 5339–5351. [Google Scholar] [CrossRef]
- Nelis, J.L.D.; Tsagkaris, A.S.; Zhao, Y.; Lou-Franco, J.; Nolan, P.; Zhou, H.; Cao, C.; Rafferty, K.; Hajslova, J.; Elliott, C.T.; et al. The end user sensor tree: An end-user friendly sensor database. Biosens. Bioelectron. 2019, 130, 245–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dungchai, W.; Chailapakul, O.; Henry, C.S. Electrochemical detection for paper-based microfluidics. Anal. Chem. 2009, 81, 5821–5826. [Google Scholar] [CrossRef]
- Nie, Z.; Nijhuis, C.A.; Gong, J.; Chen, X.; Kumachev, A.; Martinez, A.W.; Narovlyansky, M.; Whitesides, G.M. Electrochemical sensing in paper-based microfluidic devices. Lab Chip 2010, 10, 477–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ataide, V.N.; Mendes, L.F.; Gama, L.I.L.M.; de Araujo, W.R.; Paixão, T.R.L.C. Electrochemical paper-based analytical devices: Ten years of development. Anal. Methods 2020, 12, 1030–1054. [Google Scholar] [CrossRef]
- Noviana, E.; McCord, C.P.; Clark, K.M.; Jang, I.; Henry, C.S. Electrochemical paper-based devices: Sensing approaches and progress toward practical applications. Lab Chip 2020, 20, 9–34. [Google Scholar] [CrossRef]
- de Araujo, W.R.; Frasson, C.M.R.; Ameku, W.A.; Silva, J.R.; Angnes, L.; Paixão, T.R.L.C. Single-step reagentless laser scribing fabrication of electrochemical paper-based analytical devices. Angew. Chemie Int. Ed. 2017, 56, 15113–15117. [Google Scholar] [CrossRef]
- Sun, G.; Wang, P.; Ge, S.; Ge, L.; Yu, J.; Yan, M. Photoelectrochemical sensor for pentachlorophenol on microfluidic paper-based analytical device based on the molecular imprinting technique. Biosens. Bioelectron. 2014, 56, 97–103. [Google Scholar] [CrossRef]
- Marín-Barroso, E.; Messina, G.A.; Bertolino, F.A.; Raba, J.; Pereira, S. V Electrochemical immunosensor modified with carbon nanofibers coupled to a paper platform for the determination of gliadins in food samples. Anal. Methods 2019, 11, 2170–2178. [Google Scholar] [CrossRef]
- Vojdani, A. Cross-reaction between gliadin and different food and tissue antigens. Food Nutr. Sci. 2013, 4, 20–32. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Ping, J.; Ye, Z.; Wu, J.; Ying, Y. Impedimetric immunosensor based on gold nanoparticles modified graphene paper for label-free detection of escherichia Coli O157:H. Biosens. Bioelectron. 2013, 49, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Rattanarat, P.; Dungchai, W.; Cate, D.; Volckens, J.; Chailapakul, O.; Henry, C.S. Multilayer paper-based device for colorimetric and electrochemical quantification of metals. Anal. Chem. 2014, 86, 3555–3562. [Google Scholar] [CrossRef]
- Bhardwaj, J.; Devarakonda, S.; Kumar, S.; Jang, J. Development of a paper-based electrochemical immunosensor using an antibody-single walled carbon nanotubes bio-conjugate modified electrode for label-free detection of foodborne pathogens. Sens. Actuators B Chem. 2017, 253, 115–123. [Google Scholar] [CrossRef]
- Ge, S.; Liu, W.; Ge, L.; Yan, M.; Yan, J.; Huang, J.; Yu, J. In situ assembly of porous au-paper electrode and functionalization of magnetic silica nanoparticles with HRP via click chemistry for microcystin-LR immunoassay. Biosens. Bioelectron. 2013, 49, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Arduini, F.; Cinti, S.; Caratelli, V.; Amendola, L.; Palleschi, G.; Moscone, D. Origami multiple paper-based electrochemical biosensors for pesticide detection. Biosens. Bioelectron. 2019, 126, 346–354. [Google Scholar] [CrossRef]
- Cinti, S.; Basso, M.; Moscone, D.; Arduini, F. A paper-based nanomodified electrochemical biosensor for ethanol detection in beers. Anal. Chim. Acta 2017, 960, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Cinti, S.; Moscone, D.; Arduini, F. Preparation of paper-based devices for reagentless electrochemical (bio)sensor strips. Nat. Protoc. 2019, 14, 2437–2451. [Google Scholar] [CrossRef]
- Kant, K.; Shahbazi, M.A.; Dave, V.P.; Ngo, T.A.; Chidambara, V.A.; Than, L.Q.; Bang, D.D.; Wolff, A. Microfluidic devices for sample preparation and rapid detection of foodborne pathogens. Biotechnol. Adv. 2018, 36, 1003–1024. [Google Scholar] [CrossRef] [Green Version]
- Puiu, M.; Bala, C. Microfluidics-integrated biosensing platforms as emergency tools for on-site field detection of foodborne pathogens. TrAC Trends Anal. Chem. 2020, 125, 115831. [Google Scholar] [CrossRef]
- Rackus, D.G.; Shamsi, M.H.; Wheeler, A.R. Electrochemistry, biosensors and microfluidics: A convergence of fields. Chem. Soc. Rev. 2015, 44, 5320–5340. [Google Scholar] [CrossRef] [PubMed]
- Lafleur, J.P.; Jönsson, A.; Senkbeil, S.; Kutter, J.P. Recent advances in lab-on-a-chip for biosensing applications. Biosens. Bioelectron. 2016, 76, 213–233. [Google Scholar] [CrossRef]
- Park, Y.M.; Lim, S.Y.; Shin, S.J.; Kim, C.H.; Jeong, S.W.; Shin, S.Y.; Bae, N.H.; Lee, S.J.; Na, J.; Jung, G.Y.; et al. A film-based integrated chip for gene amplification and electrochemical detection of pathogens causing foodborne illnesses. Anal. Chim. Acta 2018, 1027, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Drechsel, L.; Schulz, M.; Von Stetten, F.; Moldovan, C.; Zengerle, R.; Paust, N. Electrochemical pesticide detection with autodip—A portable platform for automation of crude sample analyses. Lab Chip 2015, 15, 704–710. [Google Scholar] [CrossRef]
- EFSA. The 2010 European Union Report on Pesticide Residues in Food. EFSA J. 2013, 11. [Google Scholar] [CrossRef]
- Uludag, Y.; Esen, E.; Kokturk, G.; Ozer, H.; Muhammad, T.; Olcer, Z.; Basegmez, H.I.O.; Simsek, S.; Barut, S.; Gok, M.Y.; et al. Lab-on-a-chip based biosensor for the real-time detection of aflatoxin. Talanta 2016, 160, 381–388. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Regulation (EC) No 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs; European Commission: Brussels, Belgium, 2006. [Google Scholar]
- Medina-Sánchez, M.; Mayorga-Martinez, C.C.; Watanabe, T.; Ivandini, T.A.; Honda, Y.; Pino, F.; Nakata, A.; Fujishima, A.; Einaga, Y.; Merkoçi, A. Microfluidic platform for environmental contaminants sensing and degradation based on boron-doped diamond electrodes. Biosens. Bioelectron. 2016, 75, 365–374. [Google Scholar] [CrossRef]
- Panini, N.V.; Salinas, E.; Messina, G.A.; Raba, J. Modified paramagnetic beads in a microfluidic system for the determination of zearalenone in feedstuffs samples. Food Chem. 2011, 125, 791–796. [Google Scholar] [CrossRef]
- Lin, Y.; Zhou, Q.; Tang, D.; Niessner, R.; Knopp, D. Signal-on photoelectrochemical immunoassay for aflatoxin b1 based on enzymatic product-etching MnO2 nanosheets for dissociation of carbon dots. Anal. Chem. 2017, 89, 5637–5645. [Google Scholar] [CrossRef]
- Hervás, M.; López, M.A.; Escarpa, A. Integrated electrokinetic magnetic bead-based electrochemical immunoassay on microfluidic chips for reliable control of permitted levels of zearalenone in infant foods. Analyst 2011, 136, 2131–2138. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, D.; Cai, G.; Xiong, Y.; Li, Y.; Wang, M.; Huo, H.; Lin, J. Fast and sensitive detection of foodborne pathogen using electrochemical impedance analysis, urease catalysis and microfluidics. Biosens. Bioelectron. 2016, 86, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Olcer, Z.; Esen, E.; Muhammad, T.; Ersoy, A.; Budak, S.; Uludag, Y. Fast and sensitive detection of mycotoxins in wheat using microfluidics based real-time electrochemical profiling. Biosens. Bioelectron. 2014, 62, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Freitas, T.A.; Proença, C.A.; Baldo, T.A.; Materón, E.M.; Wong, A.; Magnani, R.F.; Faria, R.C. Ultrasensitive immunoassay for detection of citrus tristeza virus in citrus sample using disposable microfluidic electrochemical device. Talanta 2019, 205, 120110. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, T.R.; Martucci, D.H.; Faria, R.C. Simple disposable microfluidic device for salmonella typhimurium detection by magneto-immunoassay. Sens. Actuators B Chem. 2018, 255, 684–691. [Google Scholar] [CrossRef]
- Lu, L.; Gunasekaran, S. Dual-Channel ITO-microfluidic electrochemical immunosensor for simultaneous detection of two mycotoxins. Talanta 2019, 194, 709–716. [Google Scholar] [CrossRef]
- Chiriacò, M.S.; De Feo, F.; Primiceri, E.; Monteduro, A.G.; De Benedetto, G.E.; Pennetta, A.; Rinaldi, R.; Maruccio, G. Portable gliadin-immunochip for contamination control on the food production chain. Talanta 2015, 142, 57–63. [Google Scholar] [CrossRef]
- Crew, A.; Lonsdale, D.; Byrd, N.; Pittson, R.; Hart, J.P. A screen-printed, amperometric biosensor array incorporated into a novel automated system for the simultaneous determination of organophosphate pesticides. Biosens. Bioelectron. 2011, 26, 2847–2851. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, Y.; Wang, D.; He, F.; Rotello, V.M.; Carter, K.R.; Watkins, J.J.; Nugen, S.R. UV-nanoimprint lithography as a tool to develop flexible microfluidic devices for electrochemical detection. Lab Chip 2015, 15, 3086–3094. [Google Scholar] [CrossRef]
- Lillehoj, P.B.; Wei, F.; Ho, C.M. A Self-pumping lab-on-a-chip for rapid detection of botulinum toxin. Lab Chip 2010, 10, 2265–2270. [Google Scholar] [CrossRef]
- Singh, C.; Ali, M.A.; Kumar, V.; Ahmad, R.; Sumana, G. Functionalized MoS2 nanosheets assembled microfluidic immunosensor for highly sensitive detection of food pathogen. Sens. Actuators B Chem. 2018, 259, 1090–1098. [Google Scholar] [CrossRef]
- Ramalingam, S.; Chand, R.; Singh, C.B.; Singh, A. Phosphorene-gold nanocomposite based microfluidic aptasensor for the detection of okadaic acid. Biosens. Bioelectron. 2019, 135, 14–21. [Google Scholar] [CrossRef]
- Li, S.; Li, J.; Luo, J.; Xu, Z.; Ma, X. A Microfluidic chip containing a molecularly imprinted polymer and a DNA aptamer for voltammetric determination of carbofuran. Microchim. Acta 2018, 185, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.; Leung, P.H.M.; Liu, Z.B.; Zhang, Y.; Xiao, L.; Ye, W.; Zhang, X.; Yi, L.; Yang, M. A PDMS Microfluidic Impedance Immunosensor for E. Coli O157:H7 and staphylococcus aureus detection via antibody-immobilized nanoporous membrane. Sens. Actuators B Chem. 2011, 159, 328–335. [Google Scholar] [CrossRef]
- Mondal, K.; Ali, A.; Srivastava, S.; Malhotra, B.D.; Sharma, A. Sensors and Actuators B: Chemical electrospun functional micro/nanochannels embedded in porous carbon electrodes for microfluidic biosensing. Sens. Actuators B Chem. 2016, 229, 82–91. [Google Scholar] [CrossRef]
- Tian, F.; Lyu, J.; Shi, J.; Tan, F.; Yang, M. A polymeric microfluidic device integrated with nanoporous alumina membranes for simultaneous detection of multiple foodborne pathogens. Sens. Actuators B Chem. 2016, 225, 312–318. [Google Scholar] [CrossRef]
- Thiha, A.; Ibrahim, F.; Muniandy, S.; Julian, I.; Jyan, S.; Lin, K.; Fen, B.; Madou, M. All-carbon suspended nanowire sensors as a rapid highly-sensitive label-free chemiresistive biosensing platform. Biosens. Bioelectron. 2018, 107, 145–152. [Google Scholar] [CrossRef]
- Rezazadeh, M.; Seidi, S.; Lid, M.; Pedersen-bjergaard, S. Trends in analytical chemistry the modern role of smartphones in analytical chemistry. Trends Anal. Chem. 2019, 118, 548–555. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, J.; Wang, Y.; Luo, J.; Xu, H.; Xu, S.; Cai, X. A wireless point-of-care testing system for the detection of neuron-specific enolase with microfluidic paper-based analytical devices. Biosens. Bioelectron. 2017, 95, 60–66. [Google Scholar] [CrossRef]
- Xu, K.; Chen, Q.; Zhao, Y.; Ge, C.; Lin, S.; Liao, J. Cost-effective, wireless, and portable smartphone-based electrochemical system for on-site monitoring and spatial mapping of the nitrite contamination in water. Sens. Actuators B Chem. 2020, 319. [Google Scholar] [CrossRef]
- Liao, J.; Chang, F.; Han, X.; Ge, C.; Lin, S. Wireless water quality monitoring and spatial mapping with disposable whole-copper electrochemical sensors and a smartphone. Sens. Actuators B Chem. 2020, 306, 127557. [Google Scholar] [CrossRef]
- Guan, T.; Huang, W.; Xu, N.; Xu, Z.; Jiang, L.; Li, M.; Wei, X.; Liu, Y.; Shen, X.; Li, X.; et al. Point-of-need detection of microcystin-LR using a smartphone-controlled electrochemical analyzer. Sens. Actuators B Chem. 2019, 294, 132–140. [Google Scholar] [CrossRef]
- Ji, D.; Liu, L.; Li, S.; Chen, C.; Lu, Y.; Wu, J.; Liu, Q. Smartphone-based cyclic voltammetry system with graphene modified screen printed electrodes for glucose detection. Biosens. Bioelectron. 2017, 98, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-Y.; Huang, C.-H.; Park, J.; Pathania, D.; Castro, C.M.; Fasano, A.; Weissleder, R.; Lee, H. Integrated magneto-chemical sensor for on-site food allergen detection. ACS Nano 2017, 11, 10062–10069. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.K.; Hubble, L.J.; Martín, A.; Kumar, R.; Barfidokht, A.; Kim, J.; Musameh, M.M.; Kyratzis, I.L.; Wang, J. Wearable flexible and stretchable glove biosensor for on-site detection of organophosphorus chemical threats. ACS Sens. 2017, 2, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Barfidokht, A.; Mishra, R.K.; Seenivasan, R.; Liu, S.; Hubble, L.J.; Wang, J.; Hall, D.A. Wearable electrochemical glove-based sensor for rapid and on-site detection of fentanyl. Sens. Actuators B Chem. 2019, 296, 126422. [Google Scholar] [CrossRef] [PubMed]
- Hipple, J.A., Jr. Portable mass spectrometer. Nature 1942, 150, 111–112. [Google Scholar] [CrossRef]
- Yang, M.; Kim, T.-Y.; Hwang, H.-C.; Yi, S.-K.; Kim, D.-H. Developmen of a palm portable mass spectrometer. J. Am. Soc. Mass Spectrom. 2008, 19, 1442–1448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouyang, Z.; Cooks, R.G. Miniature mass spectrometers. Annu. Rev. Anal. Chem. 2009, 2, 187–214. [Google Scholar] [CrossRef]
- Mielczarek, P.; Silberring, J.; Smoluch, M. Miniaturization in mass spectrometry. Mass Spectrom. Rev. 2019. [Google Scholar] [CrossRef]
- Snyder, D.T.; Pulliam, C.J.; Ouyang, Z.; Cooks, R.G. Miniature and fieldable mass spectrometers: Recent advances. Anal. Chem. 2016, 88, 2–29. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, L.C.; Pereira, I.; De Carvalho, T.C.; Allochio Filho, J.F.; Romão, W.; Vaz, B.G. Paper Spray ionization and portable mass spectrometers: A review. Anal. Methods 2019, 11, 999–1013. [Google Scholar] [CrossRef]
- Diaz, J.A.; Giese, C.F.; Gentry, W.R. Portable double-focusing mass-spectrometer system for field gas monitoring. Field Anal. Chem. Technol. 2001, 5, 156–167. [Google Scholar] [CrossRef]
- Bryden, W.A.; Benson, R.C.; Eeelberger, S.A.; Phillips, T.E.; Cotter, R.J.; Fenselau, C. The Tiny-TOF mass spectrometer for chemical and biological Sensing. Johns Hopkins APL Tech. Dig. 1995, 16, 296–310. [Google Scholar] [CrossRef]
- Black, C.; Chevallier, O.P.; Elliott, C.T. The current and potential applications of ambient mass spectrometry in detecting food fraud. TrAC Trends Anal. Chem. 2016, 82, 268–278. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.-Y.; Huang, X.-M.; Zhai, J.-F.; Bai, H.; Li, X.-X.; Ma, X.-X.; Ma, Q. Research advances in ambient ionization and miniature mass spectrometry. Chin. J. Anal. Chem. 2019, 47, 335–346. [Google Scholar] [CrossRef]
- Huang, G.; Xu, W.; Visbal-Onufrak, M.A.; Ouyang, Z.; Cooks, R.G. Direct analysis of melamine in complex matrices using a handheld mass spectrometer. Analyst 2010, 135, 705–711. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) No 594/2012 of 5 July 2012 Amending Regulation (EC) 1881/2006 as regards the maximum levels of the contaminants Ochratoxin A, non dioxin-like PCBs and melamine in foodstuffs. Off. J. Eur. Union 2012, 176, 43–45. [Google Scholar]
- Soparawalla, S.; Tadjimukhamedov, F.K.; Wiley, J.S.; Ouyang, Z.; Cooks, R.G. In situ analysis of agrochemical residues on fruit using ambient ionization on a handheld mass spectrometer. Analyst 2011, 136, 4392–4396. [Google Scholar] [CrossRef]
- Wiley, J.S.; Shelley, J.T.; Cooks, R.G. Handheld low-temperature plasma probe for portable “point-and- shoot” ambient ionization mass spectrometry. Anal. Chem. 2013, 85, 6545–6552. [Google Scholar] [CrossRef]
- Janfelt, C.; Græsbøll, R.; Lauritsen, F.R. Portable electrospray ionization mass spectrometry (ESI-MS) for analysis of contaminants in the Field. Int. J. Environ. Anal. Chem. 2012, 92, 397–404. [Google Scholar] [CrossRef]
- Gerbig, S.; Neese, S.; Penner, A.; Spengler, B.; Schulz, S. Real-time food authentication using a miniature mass spectrometer. Anal. Chem. 2017, 89, 10717–10725. [Google Scholar] [CrossRef]
- Li, L.; Chen, T.C.; Ren, Y.; Hendricks, P.I.; Cooks, R.G.; Ouyang, Z. Mini 12, miniature mass spectrometer for clinical and other applications—Introduction and characterization. Anal. Chem. 2014, 86, 2909–2916. [Google Scholar] [CrossRef] [PubMed]
- Pulliam, C.J.; Bain, R.M.; Wiley, J.S.; Ouyang, Z.; Cooks, R.G. Mass spectrometry in the home and garden. J. Am. Soc. Mass Spectrom. 2015, 26, 224–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Q.; Bai, H.; Li, W.; Wang, C.; Li, X.; Cooks, R.G.; Ouyang, Z. Direct identification of prohibited substances in cosmetics and foodstuffs using ambient ionization on a miniature mass spectrometry system. Anal. Chim. Acta 2016, 912, 65–73. [Google Scholar] [CrossRef] [Green Version]
- Mini β Miniature Mass Spectrometer. Available online: http://www.purspec.us/params (accessed on 16 June 2021).
- Kang, M.; Lian, R.; Zhang, X.; Li, Y.; Zhang, Y.; Zhang, Y.; Zhang, W.; Ouyang, Z. Rapid and on-site detection of multiple fentanyl compounds by dual-ion trap miniature mass spectrometry system. Talanta 2020, 217, 121057. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Yu, K.; Li, N.; Liu, Y.; Liu, X.; Zhang, H.; Yang, B.; Wu, W.; Gao, J.; et al. Rapid monitoring approach for microplastics using portable pyrolysis-mass spectrometry. Anal. Chem. 2020, 92, 4656–4662. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Rios, G.A.; Vasiljevic, T.; Gionfriddo, E.; Yu, M.; Pawliszyn, J. Towards on-site analysis of complex matrices by solid-phase microextraction-transmission mode coupled to a portable mass spectrometer via direct analysis in real time. Analyst 2017, 142, 2928–2935. [Google Scholar] [CrossRef] [PubMed]
- Tomkins, B.A.; Ilgner, R.H. Determination of atrazine and four organophosphorus pesticides in ground water using Solid Phase Microextraction (SPME) followed by gas chromatography with selected-ion monitoring. J. Chromatogr. A 2002, 972, 183–194. [Google Scholar] [CrossRef]
- Hrbek, V.; Vaclavik, L.; Elich, O.; Hajslova, J. Authentication of milk and milk-based foods by Direct Analysis in Real Time Ionization–High Resolution Mass Spectrometry (DART–HRMS) Technique: A critical assessment. Food Control 2014, 36, 138–145. [Google Scholar] [CrossRef]
- Blokland, M.H.; Gerssen, A.; Zoontjes, P.W.; Pawliszyn, J.; Nielen, M.W.F. Potential of recent ambient ionization techniques for future food contaminant analysis using (trans)portable mass spectrometry. Food Anal. Methods 2019, 13, 706–717. [Google Scholar] [CrossRef] [Green Version]
- Wright, S.; Malcolm, A.; Wright, C.; O’Prey, S.; Crichton, E.; Dash, N.; Moseley, R.W.; Zaczek, W.; Edwards, P.; Fussell, R.J.; et al. A Microelectromechanical systems-enabled, miniature triple quadrupole mass spectrometer. Anal. Chem. 2015, 87, 3115–3122. [Google Scholar] [CrossRef] [PubMed]
- Jager, J.; Gerssen, A.; Pawliszyn, J.; Sterk, S.S.; Nielen, M.W.F.; Blokland, M.H. USB-powered coated blade spray ion source for on-site testing using transportable mass spectrometry. J. Am. Soc. Mass Spectrom. 2020, 31, 2243–2249. [Google Scholar] [CrossRef] [PubMed]
- Gallo, M.; Ferrara, L.; Calogero, A.; Montesano, D.; Naviglio, D. Relationships between Food and Diseases: What to Know to Ensure Food Safety. Food Res. Int. 2020, 137. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EC) No 149/2008 of 29 January 2008 Amending Regulation (EC) No 396/2005 of the European Parliament and of the Council by Establishing Annexes II, III and IV Setting Maximum Residue Levels for Products Covered by Annex I Thereto. Off. J. Eur. Union 2008, 58, 1–398. [Google Scholar]
- Geballa-Koukoula, A.; Gerssen, A.; Nielen, M.W.F. From smartphone lateral flow immunoassay screening to direct MS Analysis: Development and validation of a semi-quantitative Direct Analysis in Real-Time Mass Spectrometric (DART-MS) approach to the analysis of deoxynivalenol. Sensors 2021, 21, 1861. [Google Scholar] [CrossRef] [PubMed]
- Geballa-Koukoula, A.; Gerssen, A.; Nielen, M.W.F. Direct analysis of lateral flow immunoassays for deoxynivalenol using electrospray ionization mass spectrometry. Anal. Bioanal. Chem. 2020. [Google Scholar] [CrossRef]







 ).
).
 ).
).| Criteria | Affordable | Sensitive | Specific | User-Friendly | Rapid and Robust | Equipment-Free | Deliverable to End-user | Overall Score |
|---|---|---|---|---|---|---|---|---|
| Paper-based colorimetric | 5 | 1 | 4 | 5 | 5 | 5 | 5 |  4.3 4.3 |
| Smartphone-based optical | 4 | 2 | 4 | 4 | 2 | 4 | 4 |  3.4 3.4 |
| Smartphone-based electrochemical | 2 | 5 | 4 | 3 | 3 | 2 | 3 |  3.1 3.1 |
| Microfluidic chip-based electrochemical | 2 | 5 | 4 | 2 | 3 | 1 | 2 |  2.7 2.7 |
| Microfluidic chip-based optical | 2 | 4 | 4 | 2 | 3 | 1 | 2 |  2.6 2.6 |
| Paper-based electrochemical | 3 | 2 | 4 | 3 | 3 | 2 | 2 |  2.6 2.6 |
| Raman/IR -based | 2 | 1 | 5 | 3 | 4 | 2 | 2 |  2.6 2.6 |
| Portable MS as screening | 1 | 5 | 5 | 1 | 3 | 1 | 1 |  2.4 2.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jafari, S.; Guercetti, J.; Geballa-Koukoula, A.; Tsagkaris, A.S.; Nelis, J.L.D.; Marco, M.-P.; Salvador, J.-P.; Gerssen, A.; Hajslova, J.; Elliott, C.; et al. ASSURED Point-of-Need Food Safety Screening: A Critical Assessment of Portable Food Analyzers. Foods 2021, 10, 1399. https://doi.org/10.3390/foods10061399
Jafari S, Guercetti J, Geballa-Koukoula A, Tsagkaris AS, Nelis JLD, Marco M-P, Salvador J-P, Gerssen A, Hajslova J, Elliott C, et al. ASSURED Point-of-Need Food Safety Screening: A Critical Assessment of Portable Food Analyzers. Foods. 2021; 10(6):1399. https://doi.org/10.3390/foods10061399
Chicago/Turabian StyleJafari, Safiye, Julian Guercetti, Ariadni Geballa-Koukoula, Aristeidis S. Tsagkaris, Joost L. D. Nelis, M.-Pilar Marco, J.-Pablo Salvador, Arjen Gerssen, Jana Hajslova, Chris Elliott, and et al. 2021. "ASSURED Point-of-Need Food Safety Screening: A Critical Assessment of Portable Food Analyzers" Foods 10, no. 6: 1399. https://doi.org/10.3390/foods10061399