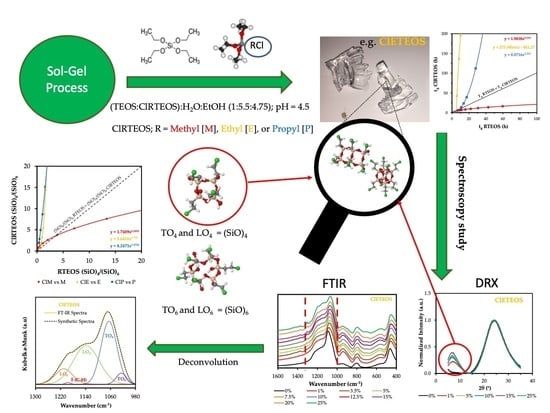
Hybrid Xerogels: Study of the Sol-Gel Process and Local Structure by Vibrational Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of the Xerogels
2.3. Characterization
3. Results and Discussion
3.1. Influence of Organochlorine Substituents on Gelation Time
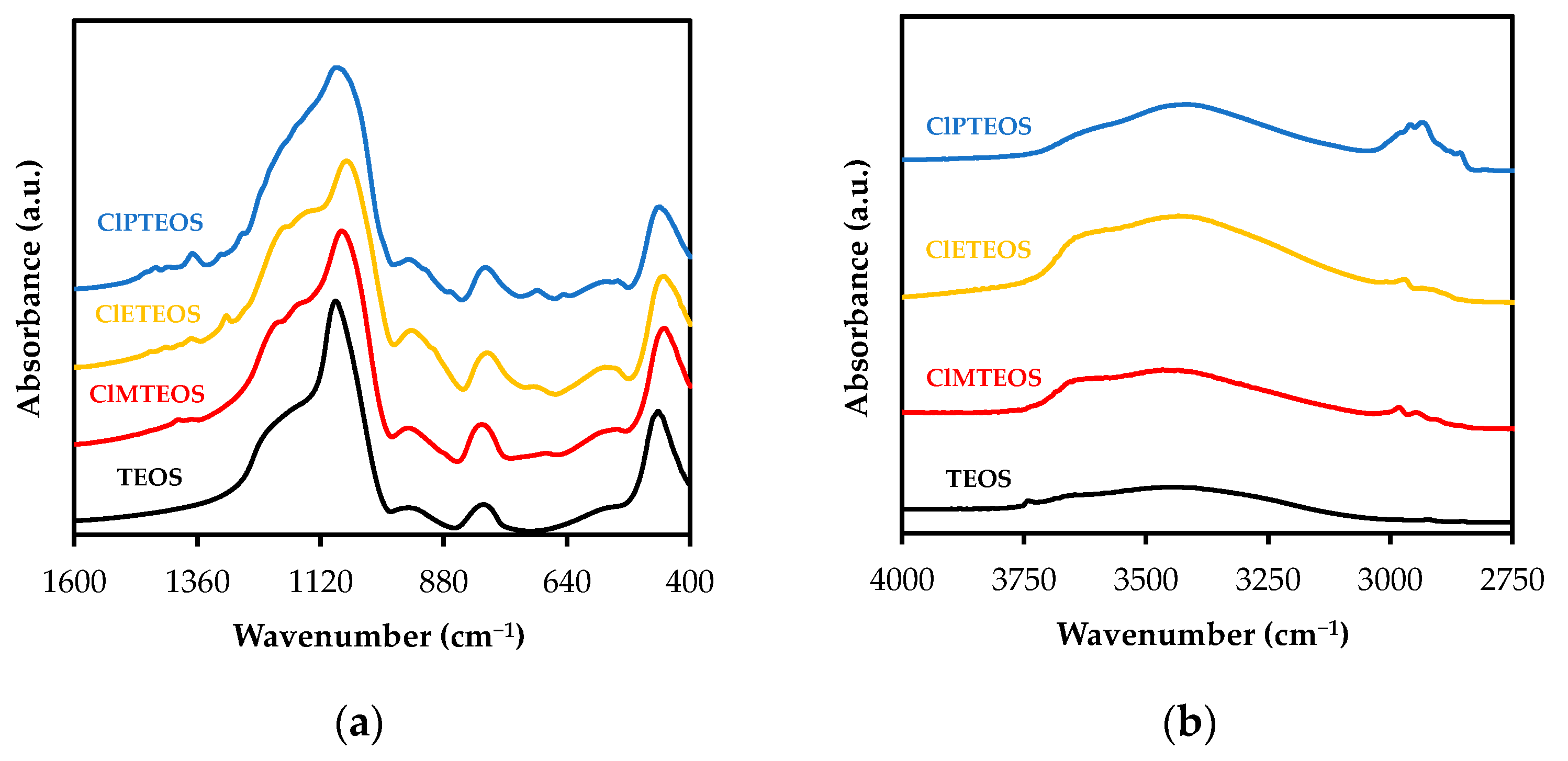
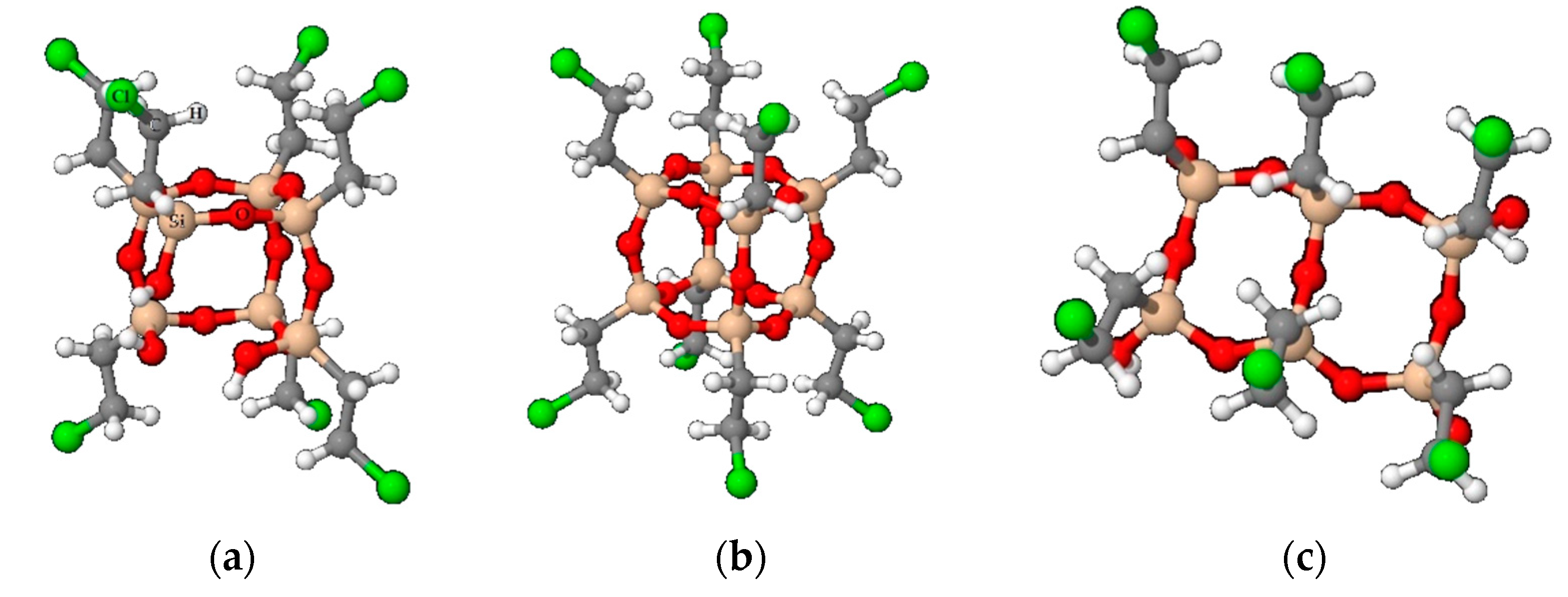
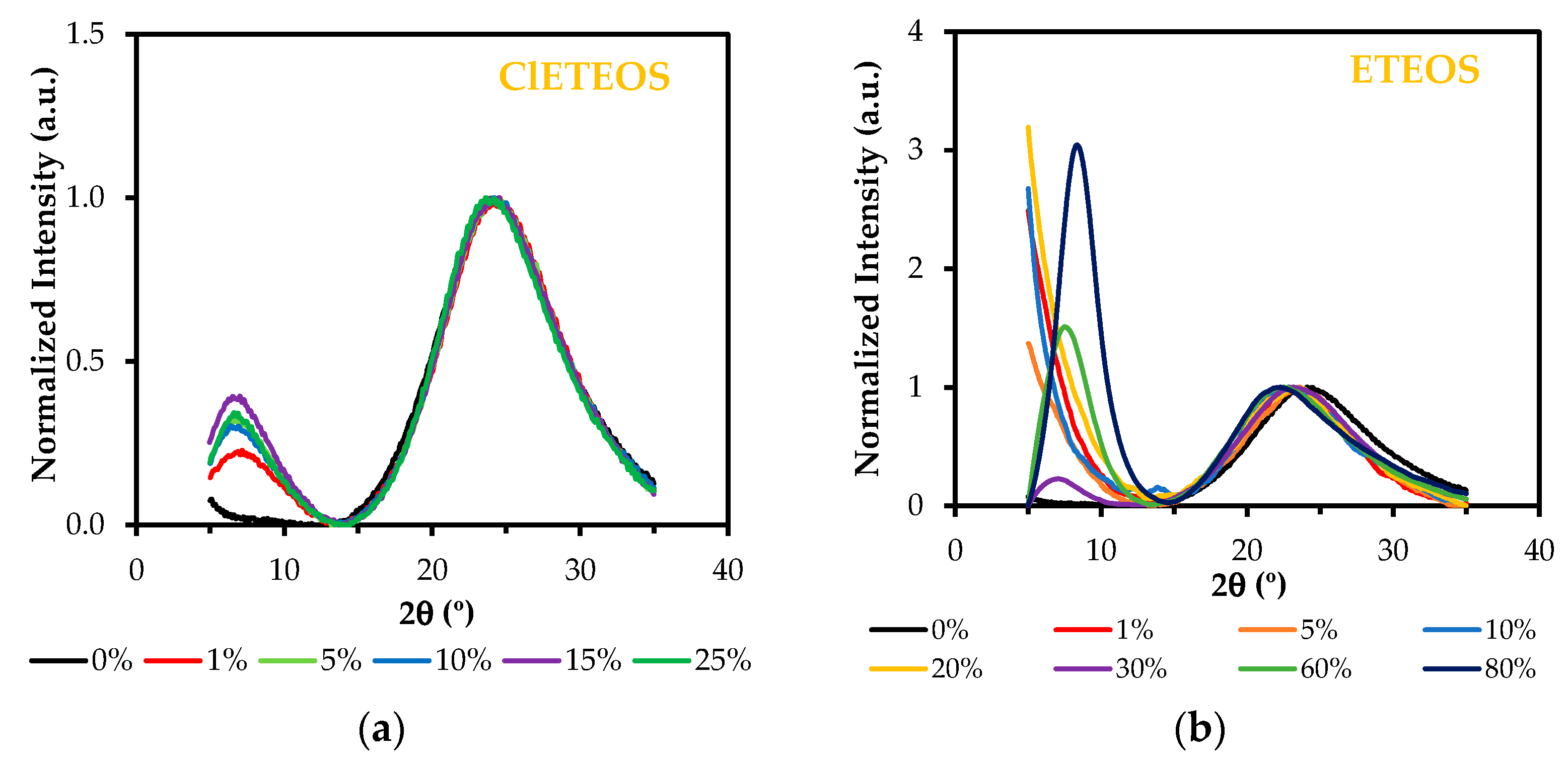
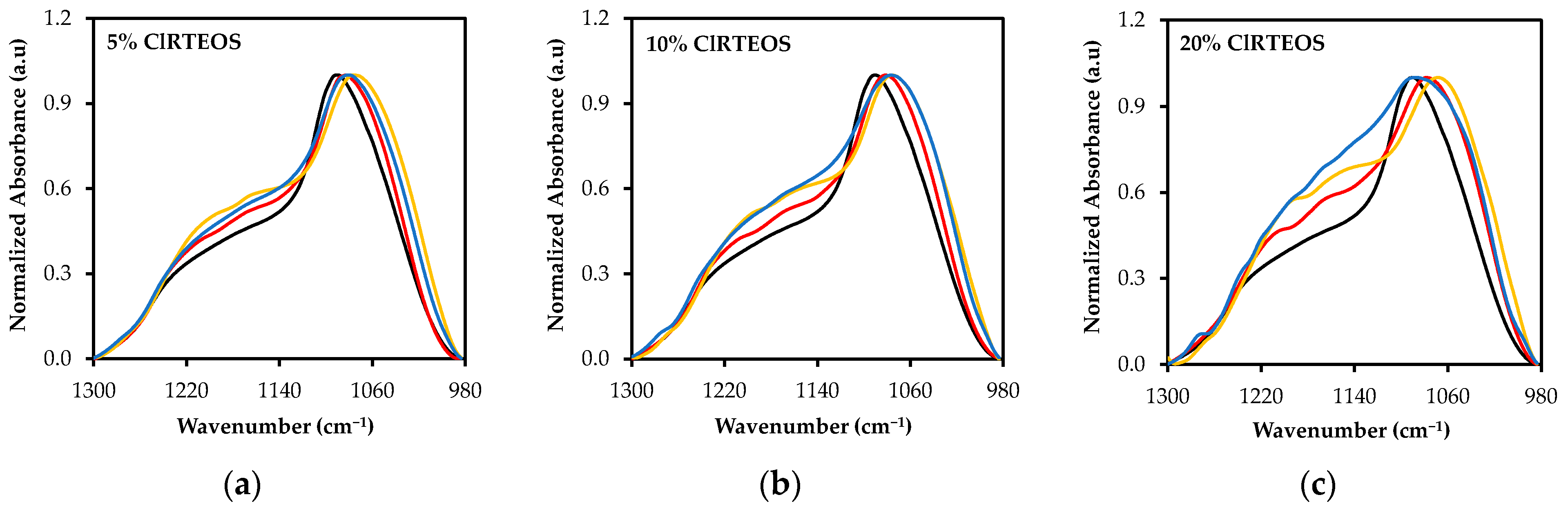
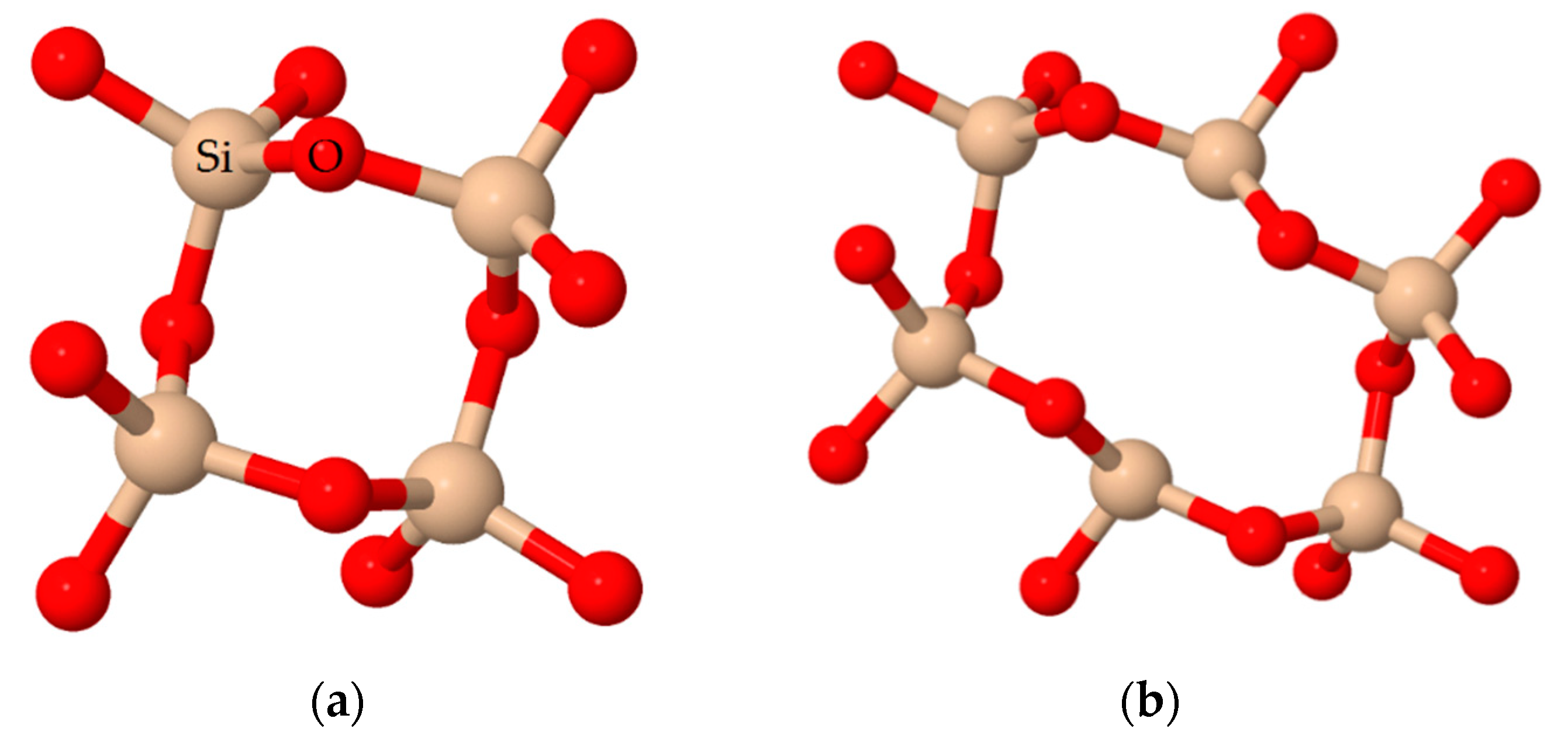
3.2. Study of the Local Structure of Hybrid Xerogels Using FTIR and XRD
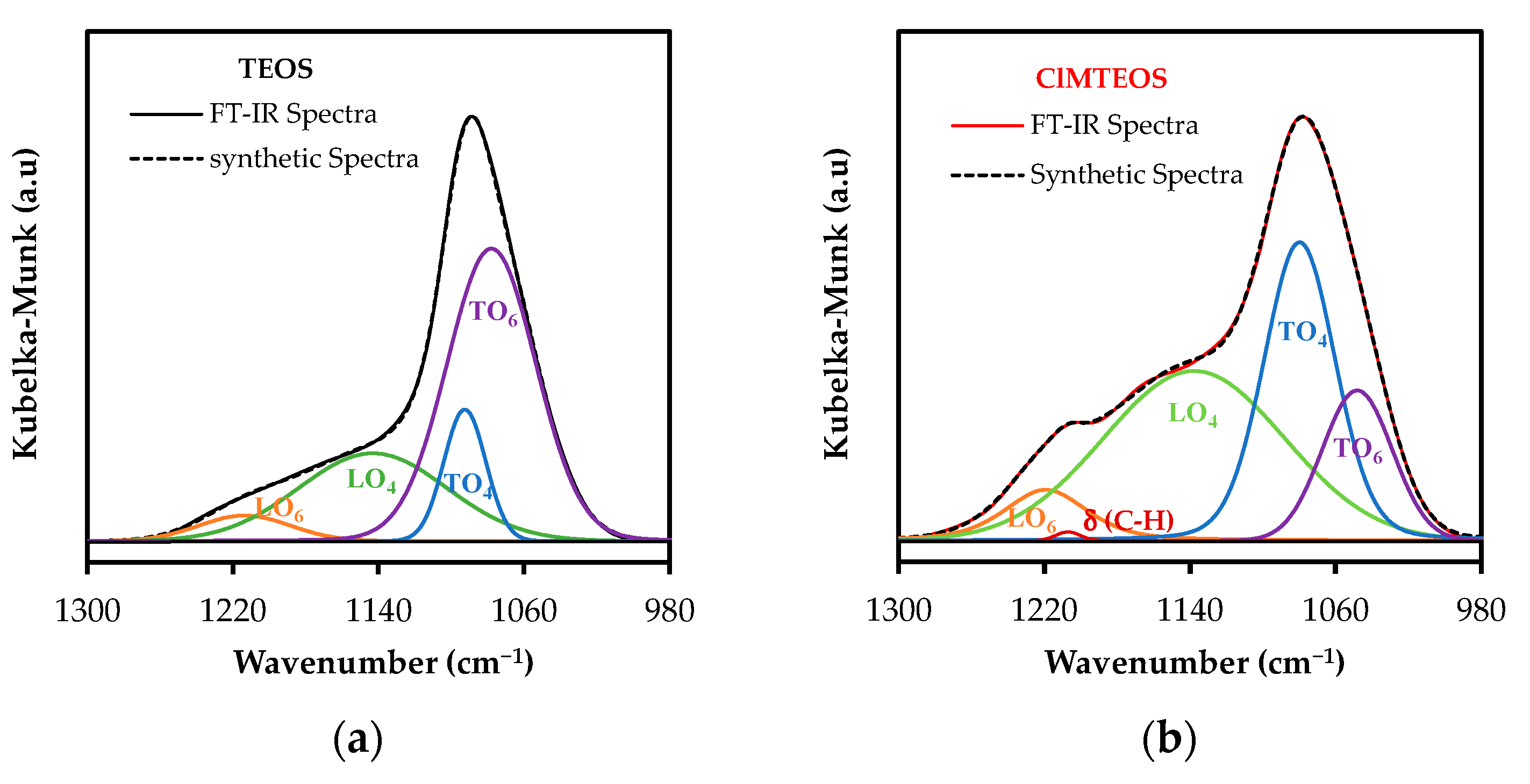
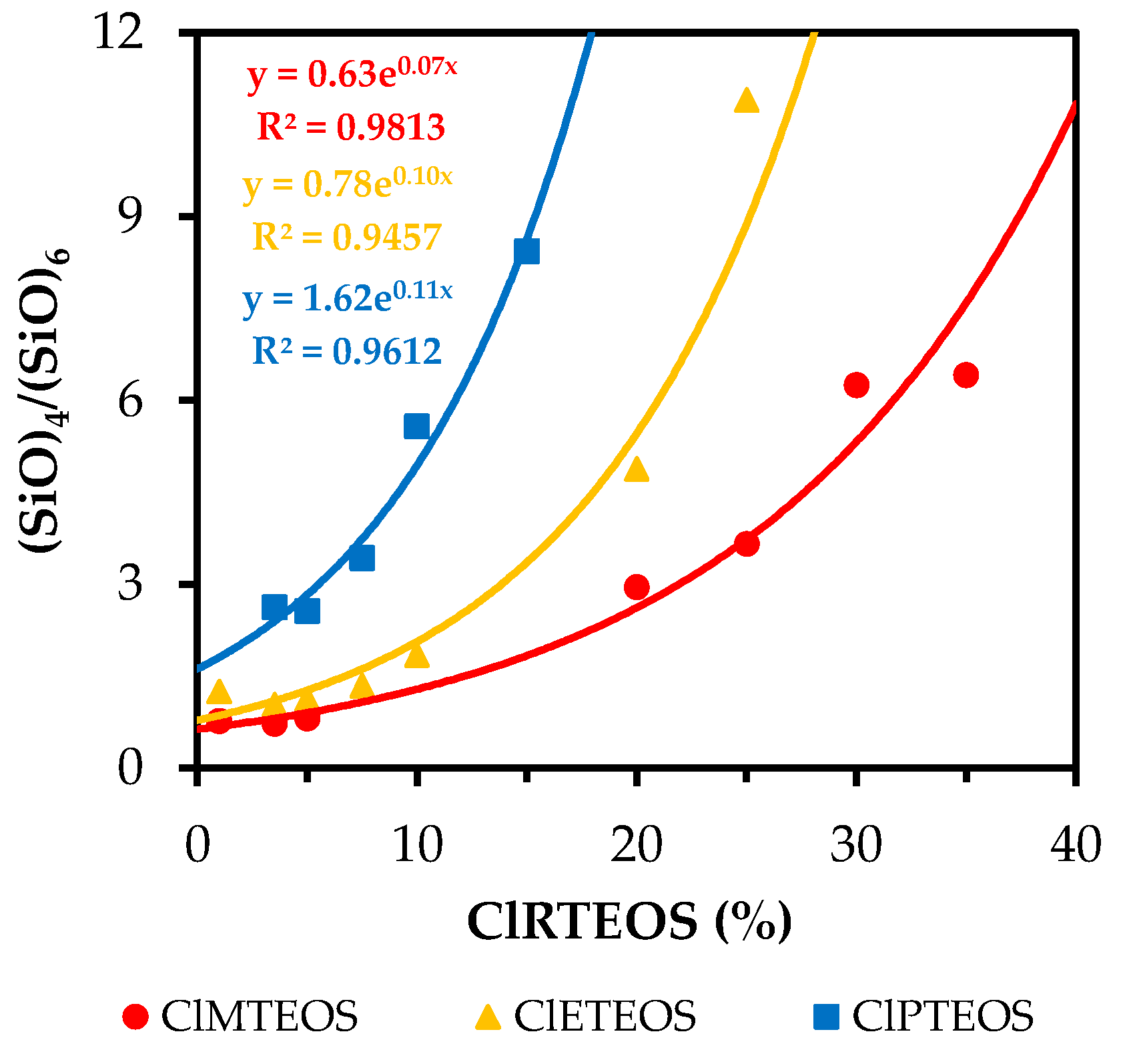
3.3. Spectral Deconvolution of the 1300–700 cm−1 Region
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pastore, A.; Badocco, D.; Pastore, P. Influence of surfactant chain length, counterion and OrMoSil precursors on reversibility and working interval of pH colorimetric sensors. Talanta 2020, 212, 120739. [Google Scholar] [CrossRef]
- Gillanders, R.N.; Campbell, I.A.; Glackin, J.M.E.; Samuel, I.D.W.; Turnbull, G.A. Ormosil-coated conjugated polymers for the detection of explosives in aqueous environments. Talanta 2018, 179, 426–429. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Suslick, K.S. Ultrasonic Preparation of Porous Silica-Dye Microspheres: Sensors for Quantification of Urinary Trimethylamine N-Oxide. ACS Appl. Mater. Interfaces 2018, 10, 15820–15828. [Google Scholar] [CrossRef]
- Shamir, D.; Elias, I.; Albo, Y.; Meyerstein, D.; Burg, A. ORMOSIL-entrapped copper complex as electrocatalyst for the heterogeneous de-chlorination of alkyl halides. Inorg. Chim. Acta 2020, 500, 119225. [Google Scholar] [CrossRef]
- Ponamoreva, O.N.; Afonina, E.L.; Kamanina, O.A.; Lavrova, D.G.; Arlyapov, V.A.; Alferov, V.A.; Boronin, A.M. Yeast Debaryomyces hansenii within ORMOSIL Shells as a Heterogeneous Biocatalyst. Appl. Biochem. Microbiol. 2018, 54, 736–742. [Google Scholar] [CrossRef]
- Lin, W.; Zhang, X.; Cai, Q.; Yang, W.; Chen, H. Dehydrogenation-driven assembly of transparent and durable superhydrophobic ORMOSIL coatings on cellulose-based substrates. Cellulose 2020, 27, 7805–7821. [Google Scholar] [CrossRef]
- Liu, Z.; Tian, S.; Li, Q.; Wang, J.; Pu, J.; Wang, G.; Zhao, W.; Feng, F.; Qin, J.; Ren, L. Integrated Dual-Functional ORMOSIL Coatings with AgNPs@rGO Nanocomposite for Corrosion Resistance and Antifouling Applications. ACS Sustain. Chem. Eng. 2020, 8, 6786–6797. [Google Scholar] [CrossRef]
- Scotland, K.M.; Shetranjiwalla, S.; Vreugdenhil, A.J. Curable hybrid materials for corrosion protection of steel: Development and application of UV-cured 3-methacryloxypropyltrimethoxysilane-derived coating. J. Coat. Technol. Res. 2020, 17, 977–989. [Google Scholar] [CrossRef]
- Bouvet-Marchand, A.; Graillot, A.; Abel, M.; Koudia, M.; Boutevin, G.; Loubat, C.; Grosso, D. Distribution of fluoroalkylsilanes in hydrophobic hybrid sol-gel coatings obtained by co-condensation. J. Mater. Chem. A 2018, 6, 24899–24910. [Google Scholar] [CrossRef]
- Yue, D.; Feng, Q.; Huang, X.; Zhang, X.; Chen, H. In situ fabrication of a superhydrophobic ORMOSIL coating on wood by an ammonia-HMDS vapor treatment. Coatings 2019, 9, 556. [Google Scholar] [CrossRef] [Green Version]
- Malek, S.K.; Nodeh, H.R.; Akbari-Adergani, B. Silica-based magnetic hybrid nanocomposite for the extraction and preconcentration of some organophosphorus pesticides before gas chromatography. J. Sep. Sci. 2018, 41, 2934–2941. [Google Scholar] [CrossRef] [PubMed]
- Moriones, P.; Ríos, X.; Echeverría, J.C.; Garrido, J.J.; Pires, J.; Pinto, M. Hybrid organic-inorganic phenyl stationary phases for the gas separation of organic binary mixtures. Colloids Surf. A Physicochem. Eng. Asp. 2011, 389, 69–75. [Google Scholar] [CrossRef]
- Tran, H.N.; Nghiem, T.H.L.; Vu, D.T.T.; Pham, M.T.; Nguyen, T.V.; Tran, T.T.; Chu, V.H.; Tong, K.T.; Tran, T.T.; Le, X.T.T.; et al. Dye-doped silica-based nanoparticles for bioapplications. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 043001. [Google Scholar] [CrossRef]
- Judeinstein, P.; Sanchez, C. Hybrid organic-inorganic materials: A land of multidisciplinarity. J. Mater. Chem. 1996, 6, 511–525. [Google Scholar] [CrossRef]
- Alemán, J.; Chadwick, A.V.; He, J.; Hess, M.; Horie, K.; Jones, R.G.; Kratochvíl, P.; Meisel, I.; Mita, I.; Moad, G.; et al. Definitions of terms relating to the structure and processing of sols, gels, networks, and inorganic-organic hybrid materials (IUPAC recommendations 2007). Pure Appl. Chem. 2007, 79, 1801–1829. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science; Academic Press: New York, NY, USA, 1990; ISBN 978-0-08-057103-4. [Google Scholar]
- Fidalgo, A.; Ilharco, L.M. The defect structure of sol-gel-derived silica/polytetrahydrofuran hybrid films by FTIR. J. Non Cryst. Solids 2001, 283, 144–154. [Google Scholar] [CrossRef]
- Chemtob, A.; Ni, L.; Croutxé-Barghorn, C.; Boury, B. Ordered hybrids from template-free organosilane self-assembly. Chem. A Eur. J. 2014, 20, 1790–1806. [Google Scholar] [CrossRef] [PubMed]
- Issa, A.A.; Luyt, A.S. Kinetics of alkoxysilanes and organoalkoxysilanes polymerization: A review. Polymers 2019, 11, 537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, X.; Chen, D.; Liu, Y. Mechanisms of silicon alkoxide hydrolysis-oligomerization reactions: A DFT investigation. ChemPhysChem 2012, 13, 2392–2404. [Google Scholar] [CrossRef]
- Issa, A.A.; El-Azazy, M.; Luyt, A.S. Kinetics of alkoxysilanes hydrolysis: An empirical approach. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef]
- Echeverría, J.C.; Moriones, P.; Arzamendi, G.; Garrido, J.J.; Gil, M.J.; Cornejo, A.; Martínez-Merino, V. Kinetics of the acid-catalyzed hydrolysis of tetraethoxysilane (TEOS) by 29Si NMR spectroscopy and mathematical modeling. J. Sol-Gel Sci. Technol. 2018, 86, 316–328. [Google Scholar] [CrossRef]
- Moriones, P.; Arzamendi, G.; Cornejo, A.; Garrido, J.J.; Echeverria, J.C. Comprehensive Kinetics of Hydrolysis of Organotriethoxysilanes by 29Si NMR. J. Phys. Chem. A 2019, 123, 10364–10371. [Google Scholar] [CrossRef]
- Colin, B.; Lavastre, O.; Fouquay, S.; Michaud, G.; Simon, F.; Laferte, O.; Brusson, J.-M. Contactless Raman Spectroscopy-Based Monitoring of Physical States of Silyl-Modified Polymers during Cross-Linking. Green Sustain. Chem. 2016, 06, 151–166. [Google Scholar] [CrossRef] [Green Version]
- Innocenzi, P.; Falcaro, P.; Grosso, D.; Babonneau, F. Microstructural evolution and order-disorder transitions in mesoporous silica films studied by FTIR spectroscopy. Mater. Res. Soc. Symp. Proc. 2002, 726, 271–281. [Google Scholar] [CrossRef]
- Ponton, S.; Dhainaut, F.; Vergnes, H.; Samelor, D.; Sadowski, D.; Rouessac, V.; Lecoq, H.; Sauvage, T.; Caussat, B.; Vahlas, C. Investigation of the densification mechanisms and corrosion resistance of amorphous silica films. J. Non Cryst. Solids 2019, 515, 34–41. [Google Scholar] [CrossRef] [Green Version]
- Fidalgo, A.; Ciriminna, R.; Ilharco, L.M.; Pagliaro, M. Role of the alkyl-alkoxide precursor on the structure and catalytic properties of hybrid sol-gel catalysts. Chem. Mater. 2005, 17, 6686–6694. [Google Scholar] [CrossRef]
- Saputra, R.E.; Astuti, Y.; Darmawan, A. Hydrophobicity of silica thin films: The deconvolution and interpretation by Fourier-transform infrared spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 199, 12–20. [Google Scholar] [CrossRef]
- Stocker, M.K.; Sanson, M.L.; Bernardes, A.A.; Netto, A.M.; Brambilla, R. Acid–base sensor based on sol–gel encapsulation of bromothymol blue in silica: Application for milk spoilage detection. J. Sol-Gel Sci. Technol. 2021, 98, 568–579. [Google Scholar] [CrossRef]
- Fidalgo, A.; Ilharco, L.M. Correlation between physical properties and structure of silica xerogels. J. Non Cryst. Solids 2004, 347, 128–137. [Google Scholar] [CrossRef]
- Izaak, T.I.; Martynova, D.O.; Stonkus, O.A.; Slavinskaya, E.M.; Boronin, A.I. Deposition of silver nanoparticles into porous system of sol-gel silica monoliths and properties of silver/porous silica composites. J. Sol-Gel Sci. Technol. 2013, 68, 471–478. [Google Scholar] [CrossRef]
- Capeletti, L.B.; Zimnoch, J.H. Fourier Transform Infrared and Raman Characterization of Silica-Based Materials. In Applications of Molecular Spectroscopy to Current Research in the Chemical and Biological Sciences, 1st ed.; Stauffer, M., Ed.; Intechopen: Kansas City, KS, USA, 2016; pp. 3–22. ISBN 978-953-51-2681-2. [Google Scholar]
- Echeverría, J.C.; Faustini, M.; Garrido, J.J. Effects of the porous texture and surface chemistry of silica xerogels on the sensitivity of fiber-optic sensors toward VOCs. Sens. Actuators B Chem. 2016, 222, 1166–1174. [Google Scholar] [CrossRef]
- Echeverría, J.C.; Calleja, I.; Moriones, P.; Garrido, J.J. Fiber optic sensors based on hybrid phenyl-silica xerogel films to detect n-hexane: Determination of the isosteric enthalpy of adsorption. Beilstein J. Nanotechnol. 2017, 8, 475–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz-Quesada, G.; Espinal-Viguri, M.; Garrido, J. Novel Organochlorinated Xerogels: From Microporous Materials to Ordered Domains. Polymers 2021, 13, 1415. [Google Scholar] [CrossRef]
- Rios, X.; Moriones, P.; Echeverría, J.C.; Luquín, A.; Laguna, M.; Garrido, J.J. Characterisation of hybrid xerogels synthesised in acid media using methyltriethoxysilane (MTEOS) and tetraethoxysilane (TEOS) as precursors. Adsorption 2011, 17, 583–593. [Google Scholar] [CrossRef]
- Rios, X.; Moriones, P.; Echeverría, J.C.; Luquin, A.; Laguna, M.; Garrido, J.J. Ethyl group as matrix modifier and inducer of ordered domains in hybrid xerogels synthesised in acidic media using ethyltriethoxysilane (ETEOS) and tetraethoxysilane (TEOS) as precursors. Mater. Chem. Phys. 2013, 141, 166–174. [Google Scholar] [CrossRef]
- Moriones, P. Sintesis y Caracterización de Xerogeles Silíceos Híbridos (RTEOS/TEOS.; R = P, Ph). Ph.D. Thesis, Universidad Publica de Navarra, Pamplona, Spain, 2015. Available online: https://academica-e.unavarra.es/handle/2454/20351 (accessed on 14 November 2020).
- Torres-Luna, J.A.; Carriazo, J.G. Porous aluminosilicic solids obtained by thermal-acid modification of a commercial kaolinite-type natural clay. Solid State Sci. 2019, 88, 29–35. [Google Scholar] [CrossRef]
- Sakka, S. Handbook of Sol-Gel Science and technology processing characterization and applications. In Characterization of Sol-Gel materials and Products; Kluwer Academic Publishers: Amsterdam, The Netherlands, 2005; Volume II, ISBN 1-4020-7967-2. [Google Scholar]
- Berrier, E.; Courtheoux, L.; Bouazaoui, M.; Capoen, B.; Turrell, S. Correlation between gelation time, structure and texture of low-doped silica gels. Phys. Chem. Chem. Phys. 2010, 12, 14477–14484. [Google Scholar] [CrossRef]
- Salon, B.M.C.; Belgacem, M.N. Competition between hydrolysis and condensation reactions of trialkoxysilanes, as a function of the amount of water and the nature of the organic group. Colloids Surf. A Physicochem. Eng. Asp. 2010, 366, 147–154. [Google Scholar] [CrossRef]
- Pierre, A.C. Introduction to Sol-Gel Processing; Springer: Berlin/Heidelberg, Germany, 2020; ISBN 9780792381211. [Google Scholar]
- Benbow, J.J. Ultrastructure Processing of Advance Materials.; Uhlmann, D.R., Ulrich, D.R., Eds.; John Wiley & Sons, INC.: New York, NY, USA, 1989; Volume 44, ISBN 0471529869. [Google Scholar]
- Depla, A.; Verheyen, E.; Veyfeyken, A.; Van Houteghem, M.; Houthoofd, K.; Van Speybroeck, V.; Waroquier, M.; Kirschhock, C.E.A.; Martens, J.A. UV-Raman and 29Si NMR spectroscopy investigation of the nature of silicate oligomers formed by acid catalyzed hydrolysis and polycondensation of tetramethylorthosilicate. J. Phys. Chem. C 2011, 115, 11077–11088. [Google Scholar] [CrossRef]
- Depla, A.; Lesthaeghe, D.; Van Erp, T.S.; Aerts, A.; Houthoofd, K.; Fan, F.; Li, C.; Van Speybroeck, V.; Waroquier, M.; Kirschhock, C.E.A.; et al. 29Si NMR and UV-raman investigation of initial oligomerization reaction pathways in acid-catalyzed silica Sol-Gel chemistry. J. Phys. Chem. C 2011, 115, 3562–3571. [Google Scholar] [CrossRef]
- Fidalgo, A.; Ilharco, L.M. Chemical Tailoring of Porous Silica Xerogels: Local Structure by Vibrational Spectroscopy. Chem. A Eur. J. 2004, 10, 392–398. [Google Scholar] [CrossRef]
- Hayami, R.; Ideno, Y.; Sato, Y.; Tsukagoshi, H.; Yamamoto, K.; Gunji, T. Soluble ethane-bridged silsesquioxane polymer by hydrolysis–condensation of bis(trimethoxysilyl)ethane: Characterization and mixing in organic polymers. J. Polym. Res. 2020, 27, 1–10. [Google Scholar] [CrossRef]
- Ramezani, M.; Vaezi, M.R.; Kazemzadeh, A. The influence of the hydrophobic agent, catalyst, solvent and water content on the wetting properties of the silica films prepared by one-step sol-gel method. Appl. Surf. Sci. 2015, 326, 99–106. [Google Scholar] [CrossRef]
- Innocenzi, P. Infrared spectroscopy of sol–gel derived silica-based films: A spectra-microstructure overview. J. Non Cryst. Solids 2003, 316, 309–319. [Google Scholar] [CrossRef]
- Handke, M.; Kowalewska, A. Siloxane and silsesquioxane molecules—Precursors for silicate materials. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2011, 79, 749–757. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry, 3rd ed.; Meyers, R.A., Ed.; Wiley & Sons, Ltd.: New York, NY, USA, 2006; pp. 1–23. ISBN 978-0-471-97670-7. [Google Scholar]
- Launer, P.J.; Arkles, B. Infrared Analysis of Orgaonsilicon Compounds. In Silicon Compounds: Silanes and Silicones, 3rd ed.; Gelest Inc.: Morrisville, PA, USA, 2013; pp. 175–178. [Google Scholar]
- Chen, G.; Zhou, Y.; Wang, X.; Li, J.; Xue, S.; Liu, Y.; Wang, Q.; Wang, J. Construction of porous cationic frameworks by crosslinking polyhedral oligomeric silsesquioxane units with N-heterocyclic linkers. Sci. Rep. 2015, 5, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Park, E.S.; Ro, H.W.; Nguyen, C.V.; Jaffe, R.L.; Yoon, D.Y. Infrared spectroscopy study of microstructures of poly(silsesquioxane)s. Chem. Mater. 2008, 20, 1548–1554. [Google Scholar] [CrossRef]
- Kamiya, K.; Dohkai, T.; Wada, M.; Hashimoto, T.; Matsuoka, J.; Nasu, H. X-ray diffraction of silica gels made by sol-gel method under different conditions. J. Non Cryst. Solids 1998, 240, 202–211. [Google Scholar] [CrossRef]
- García-Cerda, L.A.; Mendoza-González, O.; Pérez-Robles, J.F.; González-Hernández, J. Structural characterization and properties of colloidal silica coatings on copper substrates. Mater. Lett. 2002, 56, 450–453. [Google Scholar] [CrossRef]
- Hagiwara, Y.; Shimojima, A.; Kuroda, K. Alkoxysilylated-derivatives of double-four-ring silicate as novel building blocks of silica-based materials. Chem. Mater. 2008, 20, 1147–1153. [Google Scholar] [CrossRef]
- Nowacka, M.; Kowalewska, A.; Makowski, T. Structural studies on ladder phenylsilsesquioxane oligomers formed by polycondensation of cyclotetrasiloxanetetraols. Polymer 2016, 87, 81–89. [Google Scholar] [CrossRef]
- Ospino, I.; Luquin, A.; Jiménez-Ruiz, M.; Pérez-Landazábal, J.I.; Recarte, V.; Echeverría, J.C.; Laguna, M.; Urtasun, A.A.; Garrido, J.J. Computational Modeling and Inelastic Neutron Scattering Contributions to the Study of Methyl-silica Xerogels: A Combined Theoretical and Experimental Analysis. J. Phys. Chem. C 2017, 121, 22836–22845. [Google Scholar] [CrossRef]
- Handke, M.; Jastrzȩbski, W. Vibrational spectroscopy of the ring structures in silicates and siloxanes. J. Mol. Struct. 2004, 704, 63–69. [Google Scholar] [CrossRef]
- Shi, Y.; Neuefeind, J.; Ma, D.; Page, K.; Lamberson, L.A.; Smith, N.J.; Tandia, A.; Song, A.P. Ring size distribution in silicate glasses revealed by neutron scattering first sharp diffraction peak analysis. J. Non Cryst. Solids 2019, 516, 71–81. [Google Scholar] [CrossRef]
- Tan, C.Z.; Arndt, J. Interaction of longitudinal and transverse optic modes in silica glass. J. Chem. Phys. 2000, 112, 5970–5974. [Google Scholar] [CrossRef]
- Caresani, J.R.; Lattuada, R.M.; Radtke, C.; Dos Santos, J.H.Z. Attempts made to heterogenize MAO via encapsulation within silica through a non-hydrolytic sol-gel process. Powder Technol. 2014, 252, 56–64. [Google Scholar] [CrossRef]
- Choi, S.S.; Lee, A.S.; Lee, H.S.; Baek, K.Y.; Choi, D.H.; Hwang, S.S. Synthesis and characterization of ladder-like structured polysilsesquioxane with carbazole group. Macromol. Res. 2011, 19, 261–265. [Google Scholar] [CrossRef]
- Gallardo, J.; Durán, A.; Di Martino, D.; Almeida, R.M. Structure of inorganic and hybrid SiO2 sol-gel coatings studied by variable incidence infrared spectroscopy. J. Non Cryst. Solids 2002, 298, 219–225. [Google Scholar] [CrossRef]



| Precursor | R | X | δSi | δR | δOEt |
|---|---|---|---|---|---|
| TEOS | OC2H5 | 2.32 | 0.32 | - | −0.08 |
| MTEOS | CH3 | 2.29 | 0.31 | 0.20 | −0.17 |
| ETEOS | C2H5 | 2.29 | 0.31 | 0.28 | −0.20 |
| PTEOS | C3H7 | 2.28 | 0.30 | 0.35 | −0.22 |
| ClMTEOS | CH2Cl | 2.32 | 0.32 | −0.09 | −0.08 |
| ClETEOS | C2H4Cl | 2.31 | 0.32 | 0.02 | −0.11 |
| ClPTEOS | C3H6Cl | 2.30 | 0.31 | 0.11 | −0.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz-Quesada, G.; Espinal-Viguri, M.; López-Ramón, M.V.; Garrido, J.J. Hybrid Xerogels: Study of the Sol-Gel Process and Local Structure by Vibrational Spectroscopy. Polymers 2021, 13, 2082. https://doi.org/10.3390/polym13132082
Cruz-Quesada G, Espinal-Viguri M, López-Ramón MV, Garrido JJ. Hybrid Xerogels: Study of the Sol-Gel Process and Local Structure by Vibrational Spectroscopy. Polymers. 2021; 13(13):2082. https://doi.org/10.3390/polym13132082
Chicago/Turabian StyleCruz-Quesada, Guillermo, Maialen Espinal-Viguri, María Victoria López-Ramón, and Julián J. Garrido. 2021. "Hybrid Xerogels: Study of the Sol-Gel Process and Local Structure by Vibrational Spectroscopy" Polymers 13, no. 13: 2082. https://doi.org/10.3390/polym13132082