Co-Adjuvant Therapy Efficacy of Catechin and Procyanidin B2 with Docetaxel on Hormone-Related Cancers In Vitro
Abstract
:1. Introduction
2. Results
2.1. Cytotoxic Effects on Health Cells
2.2. Antitumor Effects
2.2.1. Anti-Proliferative Activity
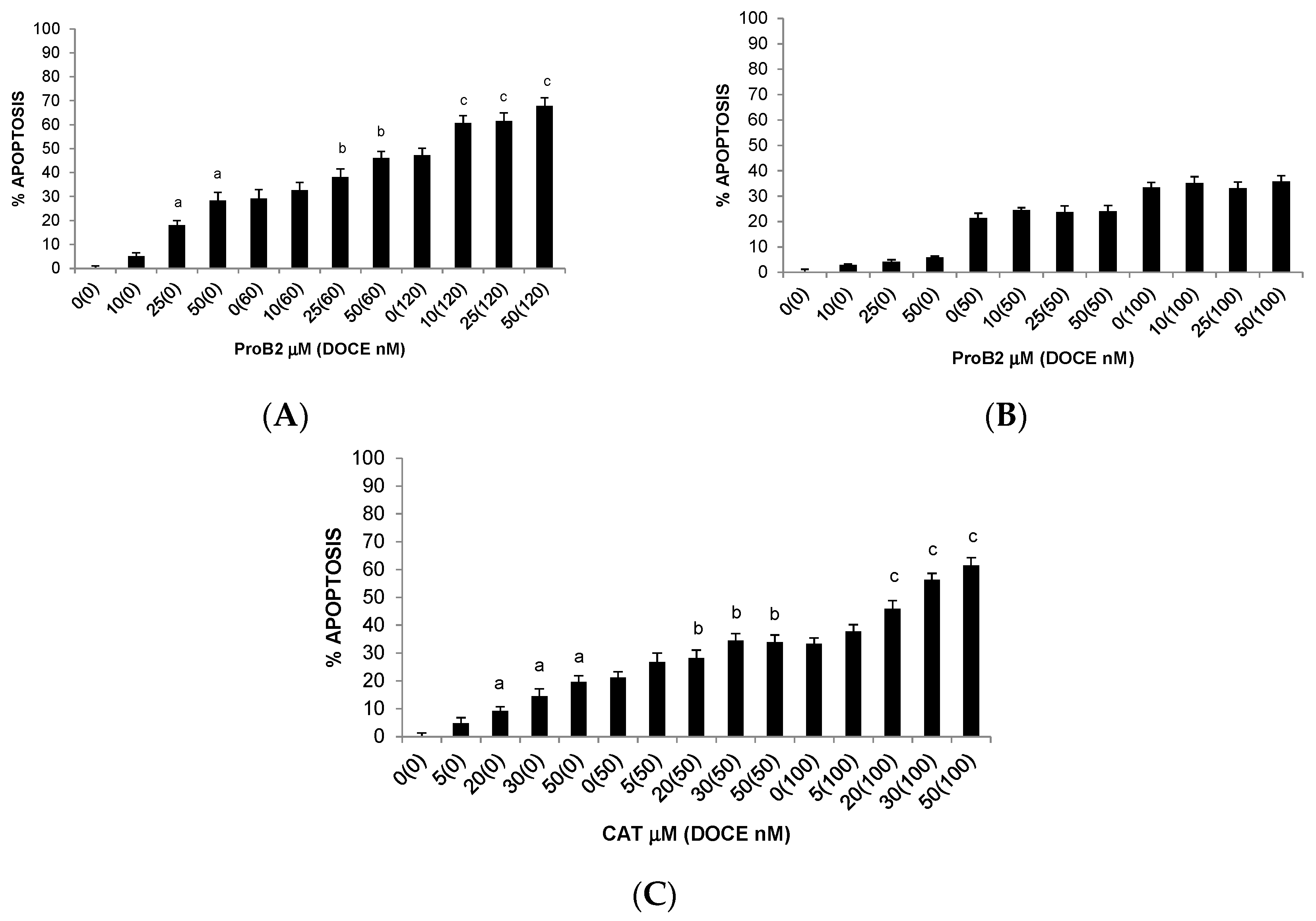
2.2.2. Pro-Apoptotic Activity
2.2.3. Sensitization of Cancer Cells to DOCE Growth-Suppressive Effect
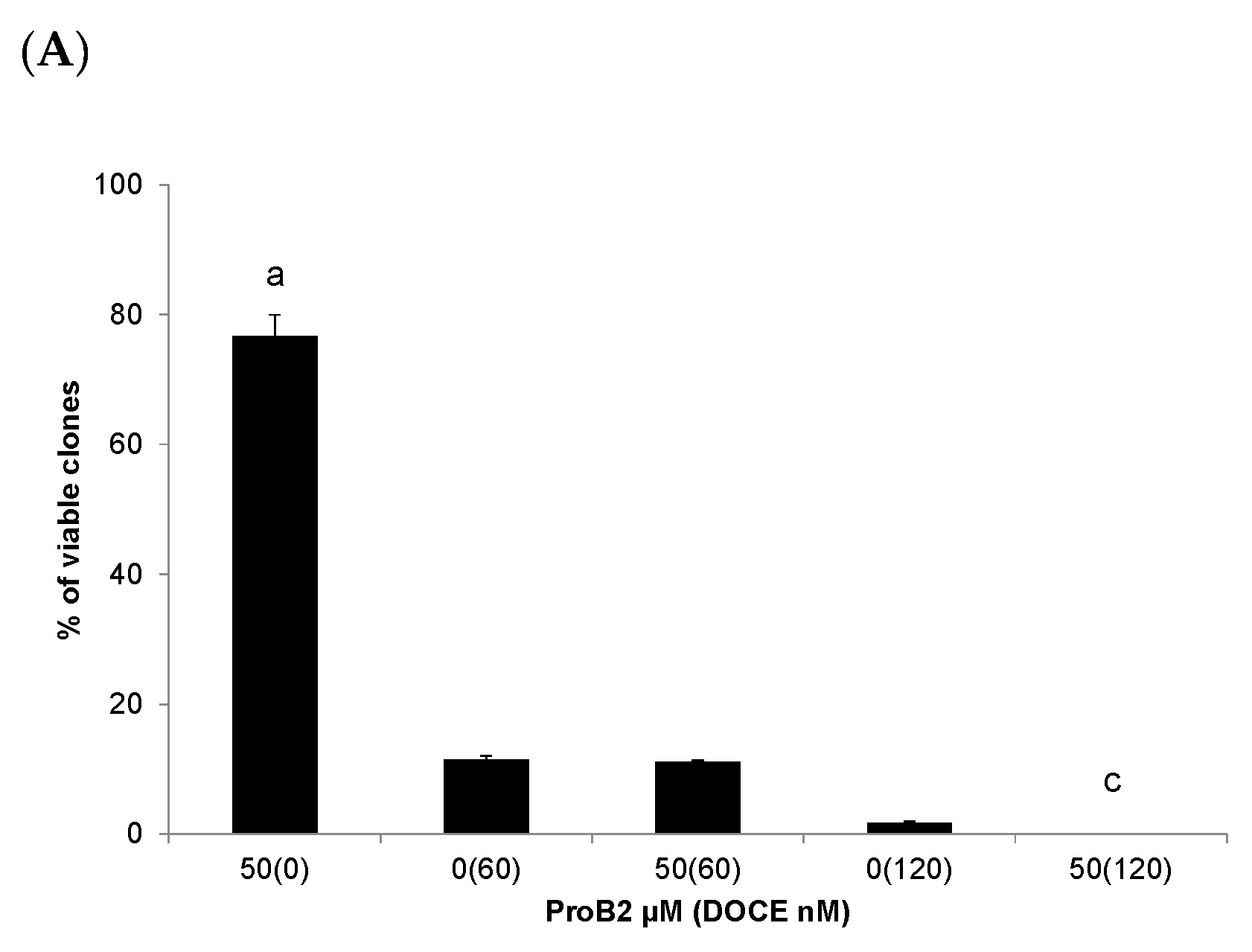
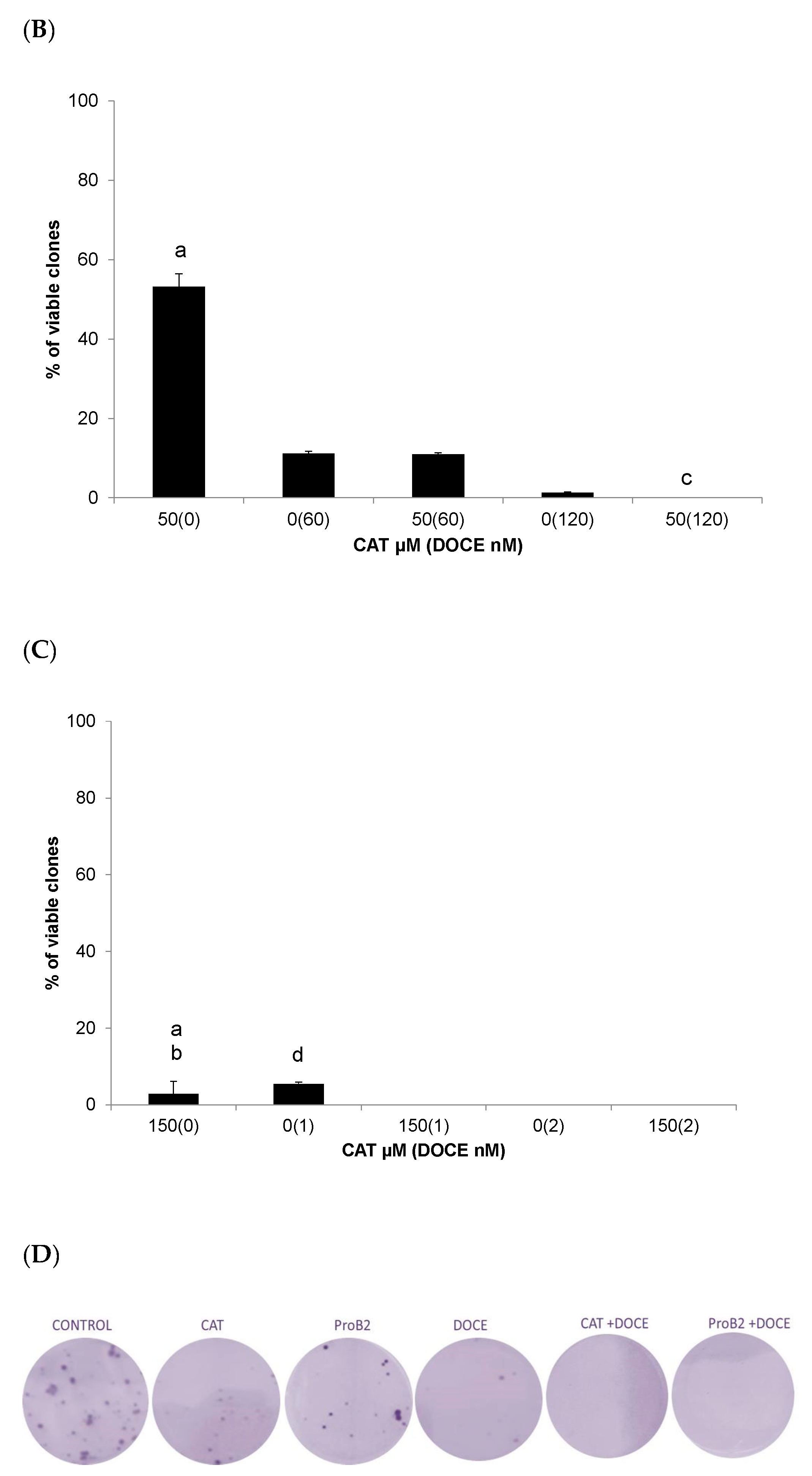
2.2.4. Colony Formation Test
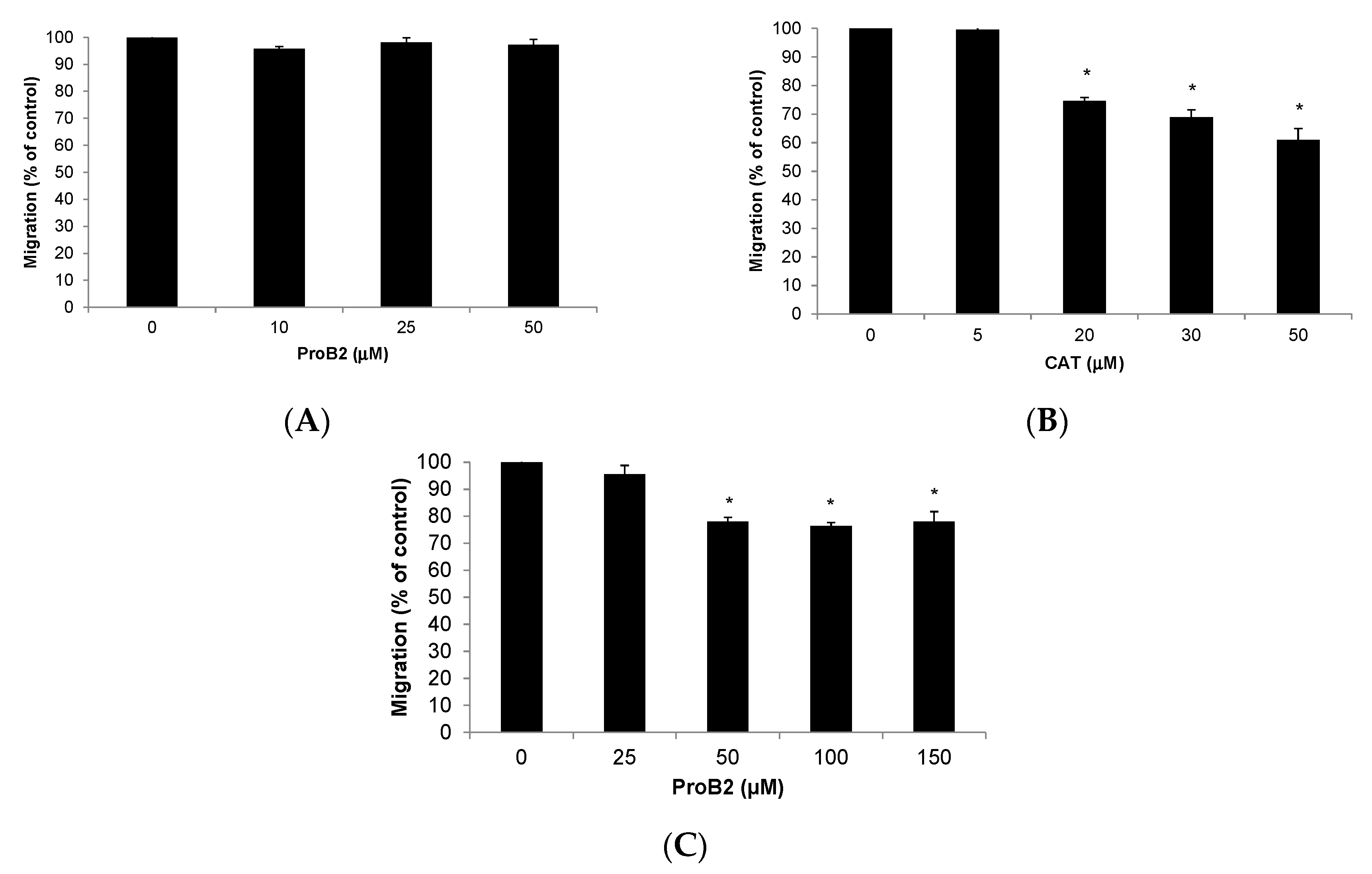
2.2.5. Cell Migration Assay
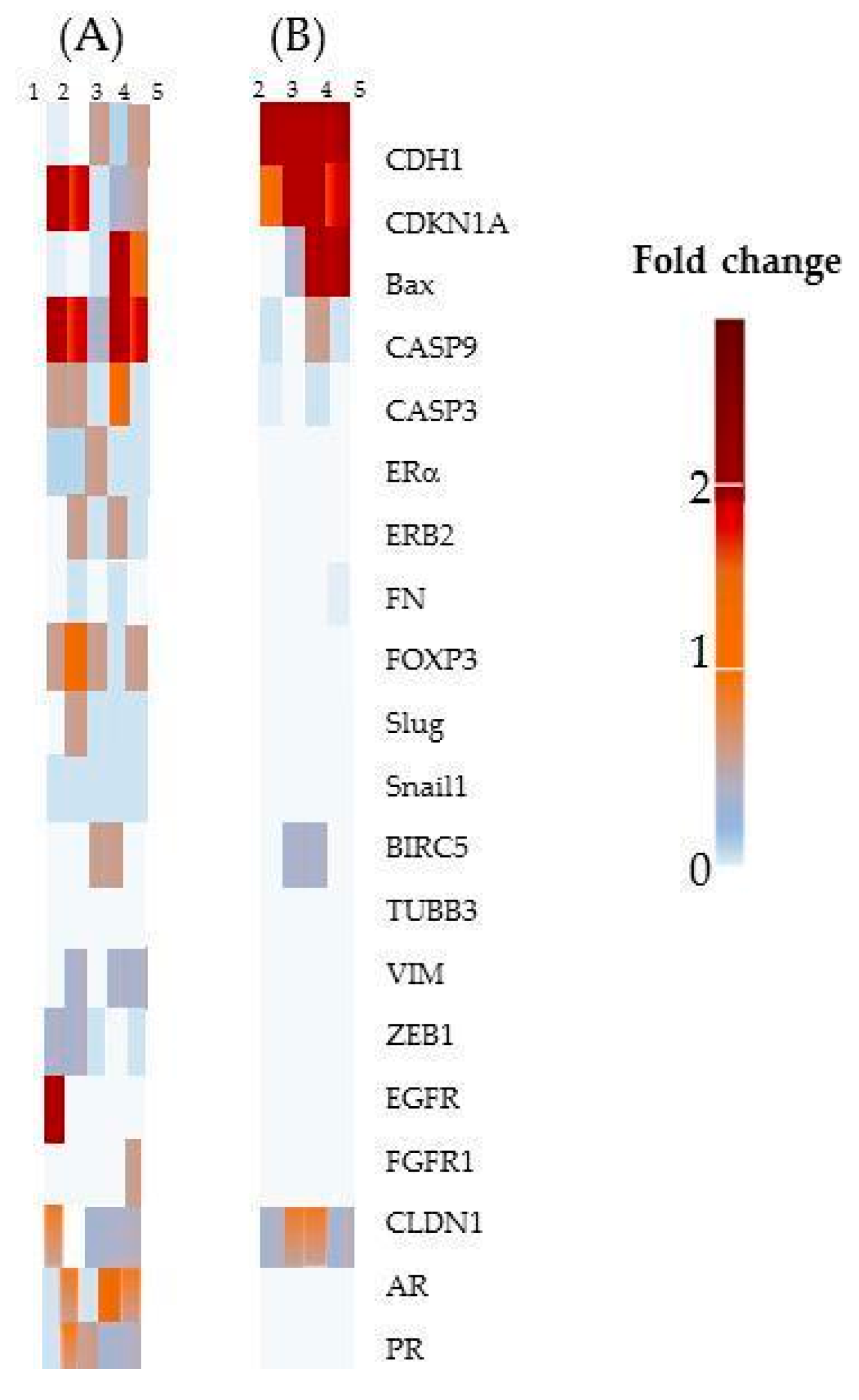
2.2.6. Gene expression
- Sensitization of cancer cells to DOCE growth-suppressive effect
- CAT and ProB2-induced anti-migratory effect
3. Discussion
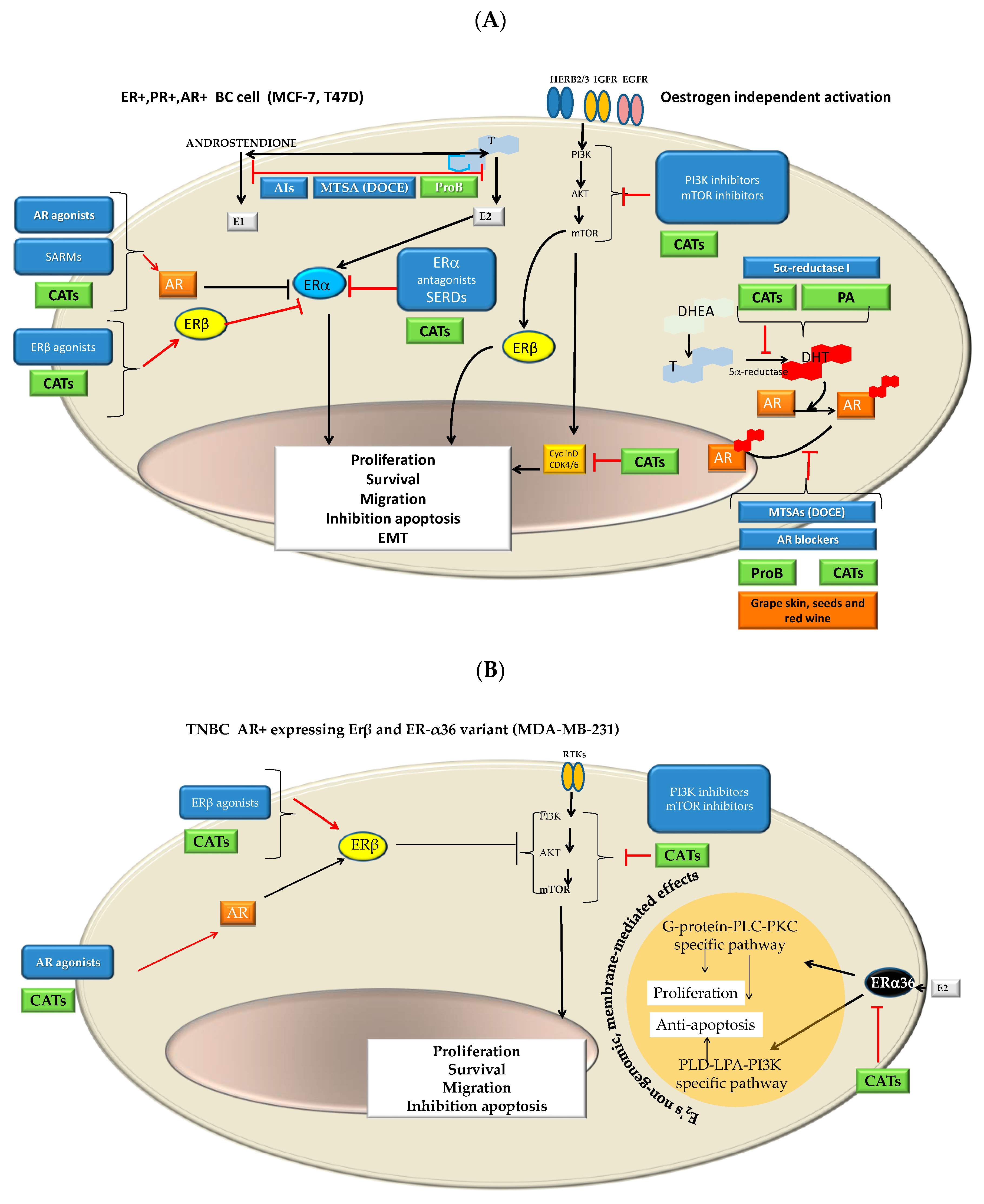
- It is considered that the binding affinity of CATs to ERβ and ERα is likely conditioned by the structure and the dose as well as the cell type [66,68,69,72]. CAT it is a flavan-3-ol, whose family is considered structurally similar to isoflavones that exhibit some structural similarities with 17-b-estradiol as well as with other steroid hormones and steroid hormone antagonists. Thus, CAT could act as an ER agonist or antagonist whose action is both ER concentration- and ER isomorph-dependent or flavane type-dependent. The number of hydroxyl groups, mainly those in the flavonoid B-ring, appeared to be of importance, while changes in the A- or B-hydroxylation rings are given minor importance [82,83].
- Different CATs can antagonize androgen, resulting in a decrease in AR-mediated transcriptional activation [74]. Furthermore, Kampa et al. [75] suggested that flavanol dimers B1-B4 (oligomeric Pro) and in particular, oleylated B2 could be considered a therapeutic agent for advanced PC since it had a powerful agonist effect on membrane AR in androgen-independent DU145 PC cells (ProB2 > ProB3 = ProB4 > ProB1).
- In the present study, we observed that MCF-7 and DU145 cells were most sensitive to ProB2 and/or CAT than the remaining BC and PC cells, respectively. It is known that MCF-7 cells and T47D cells both are ER+, PR+ AR+ cells, but MCF-7 cells differ from T47D cells in that they continually express ER while T47D cells lose ER and PR expression under estrogen withdrawal [85] as well as in ER and PR expression levels [29,86]. Additionally, AR activity is necessary for ER+/AR+ MCF-7 and T47D cell line growth [87]. MCF-7 and T47D both express AR, but two different regulatory pathways may be involved in the androgen-induced stimulation of proliferation, resulting in different repercussions on proliferative activity. MCF-7 cells show AR-mediated and AR-independent MCF-7 mechanisms, whereas T47D exhibits an AR-mediated one. In addition, MCF-7 cells express a wild-type AR characterized by a shortened CAG repeat, while T47D presents a CAG repeat length considered in the normal range [88]. This shortening represents a more active receptor [89]. In addition, these cell lines differ in AR-FL (full-length AR) and splice variants from the AR gene (ARVs) expression. The latter are constitutively active androgen-independent transcription factors that are implicated in resistance to treatment in PC and in BC. ARVs vary widely among the BC and PC cell lines tested. As an example, MDA-MB-231 cells exhibits the highest ARV to AR-FL ratio, followed by T47D and MCF-7 cells. Additionally, MDA-MB-231 cells exhibit low AR-FL and high AR-V3 expression. AR45, AR-V1, AR-V7 and AR-V9 splice variants rather than T47D and MCF-7 cells [90]. On the other hand, DU145 and PC3 cells do not express AR and Erβ, while PC3 express ERα [91].
- Additionally, it should be remembered that MCF7 and T47D are PR+. Regarding PC cells lines used in the present study, PC3 and DU145 differ in the expression of PR isoforms (isoforms A and B). While PC3 presents the two promotors methylated and inactivated, DU145 presents them unmethylated and activated. It should be noted that two promoters control the expression of PR isoforms and hypermethylation of cytosine-rich areas in promoters, which is considered a functional inactivation [92]. The overexpression of the isoform A is involved in cyclin D1 activity; proliferative promoters increase (TGFβ1) as well as pro-invasive and pro-migratory changes (basal membrane disruption, etc.). Interestingly, those effects can be counteracted by antiestrogens [93].
- Grape seed extracts and red wine, and specifically ProB2 dimers (from grape seeds as well as red wine), could suppress aromatase activity [76,77,78,79], inducing hormonal changes attributed to AI [79]. DU145 and PC3 cells used in the present study have constitutive aromatase activity, but with different levels [94]. Regarding BC cell lines, there are contradictory data on aromatase expression, with the presence [95,96] or absence of this enzyme [97]. In the present study, we found that PC and BC cell lines show different degrees of susceptibility to the effects of CAT and ProB2.
- 5α-reductase 1 is present in both PC cell lines used in this study, while 5α-reductase 2 is present in DU145 PC cells [98]. With respect to BC cell lines, MCF-7 and T47D BC cells used in this study both express 5α-reductase 1 [99]. It was suggested that some CATs from green tea seed extract exert 5α-reductase inhibitory characteristics. On the other hand, it seems that natural compounds that act as inhibitors possessing a catechol group have selectivity for the type 1 isozyme [80]. Additionally, polymeric anthocyanins seem to be type 2 isozyme inhibitors [81]. Thereby, it was suggested that they are useful for the prevention as well as the treatment of androgen-dependent disorders [80].
- Combined therapy with receptor modulators and chemotherapy drugs such as DOCE has long since been given improving clinical outcomes (CHAARTED trial, STAMPEDE trial, PROXIMA trial, etc.) [59,100,101,102,103]. Moreover, it has recently been found that SERMs can act as modulators of microtubules at taxane sites [104]. As it is shown in Figure 12A–C, ProB2 and/or CAT could exert a pleiotropic effect on hormone receptors and hormone synthesis, in particular in ER+PR+, AR+ BC cells such as MCF-7 cells (already discussed above). Additionally, as it is shown in Figure 12A, CATs and DOCE have some similar mechanisms of action such as AIs or AR modulators. These aspects may have contributed to the sensitizing effect of DOCE in the combined treatment of ProB2 plus DOCE and CAT plus DOCE shown in this study.
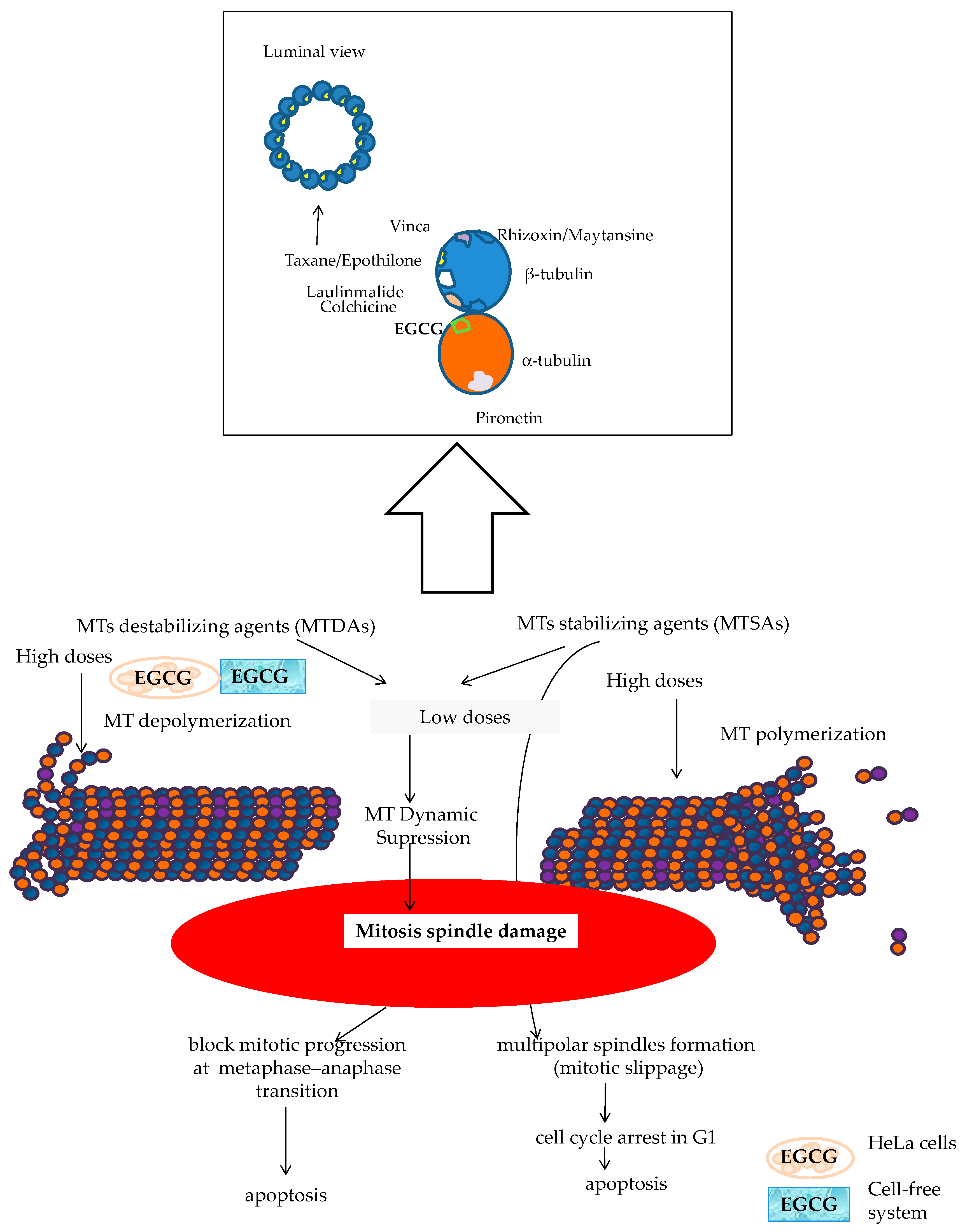
- The dual targeting of the microtubule structure could sensitize cancer cells to chemotherapy treatment (Figure 13). Microtubule-targeting agents (MTAs) such as DOCE are an effective treatment for solid tumors such as BC and PC. This drug is the standard treatment for BC and PC, both metastatic and resistant to other treatments [59,60,101,102,103]. A large number of naturally sourced MTAs are antimitotic agents by acting on tubulin protein and its polymers microtubules [105,106]. These drugs can be classified according to their effect in microtubule-destabilizing agents ((MTDAs) vinca alcaloids, cryptophycins, etc.) and microtubule-stabilizing agents (MTSAs) that include Paclitaxel and its semisynthetic analogue DOCE [105], the chemotherapy drug tested in the present study. DOCE binds to the β-tubulin subunits of microtubules at the taxane-binding side, inducing the suppression of MT dynamics (low doses) or the stabilization of microtubules, and thereafter, cell-cycle arrest [105].
- The molecules tested in our study could act as MTAs, and their administration with DOCE could contribute to the sensitization to DOCE by CAT and ProB2 observed in our study (Figure 13). Recent reports suggest that natural compounds such as CATs can exert anti-proliferative activity against parasites [107] and lung, cervix [108] and hepatoma cancer [109] cell lines could be mediated by union to β-tubulin chain [103], α-tubulin protein and α-β heterodimer [109]. In silico modeling has provided additional insights into potential mechanisms of some types of CATs by binding to α-tubulin molecules at the interface, between α-and β-heterodimers, which could be responsible for the depolymerization of MTs in cervix cancer cells. Additionally, a cell-free system study showed that EGCG causes the inhibition of microtubule polymerization [108]. In our future research, we will analyze the effect of ProB2 and CAT, with and without DOCE, on α-and β-tubulin as well as microtubules.
- Among the cells used in this study, the MCF-7 cell line has been shown to be the most sensitive to treatment. Overall, our results suggest that CDKN1A, BAX, CASP9 and E-cadherin up-regulated expression contributing to the ProB2- and CAT-induced sensitization to DOCE in MCF-7 cells, since:
- CDK 1 is of particular importance as it is essential to ensure cell cycle progression and apoptosis [110,111,112]. CDK inhibitors had been postulated as a promising therapeutic strategy for advanced cancer [113]. CDKN1A suppresses tumor growth and apoptosis [41,42,111,113]. It is postulated that preventing the up-regulation of the CDK1 axis improves the sensitivity of cancer cells to chemotherapeutic agents, increasing their effectiveness. Combined treatment with CDK inhibitors and chemotherapeutic agents significantly increases the effectiveness of the chemotherapeutic agent [108,109,110,111,112,113,114,115], including docetaxel [114]. In addition, inactivating CDK1 agents can induce long-term cytotoxicity (clonogenic assay) [116]. In the present study, we observed that MCF-7 cells treated with CAT plus DOCE and ProB2 plus DOCE caused a high increase in CDK1 inhibitor. Additionally, in this study, the combined treatment of PRoB2 plus DOCE as well as CAT plus DOCE inhibited the formation of colonies drastically in relation to DOCE alone.
- BAX and CASP9 promote apoptosis [43,44,45,46,47] and both CASP3 and CASP9 seem to be involved in taxane-induced cell death [46]. Interestingly, we found that both BAX (≈ eight-fold) and (≈ two-fold) increased in CAT plus DOCE-treated cells and ProB2 plus DOCE-treated cells, respectively, in relation to DOCE.
- E-cadherine, an epithelial phenotype maintaining gene, can inhibit mitogenic signaling [48] and its suppression is associated to DOCE chemoresistance in hormonal tumors [49,50,53]. As far as we observed, with a great increase in E-cadherine under combined treatment compared to DOCE single treatment, a highly sensitizing effect could be expected.
- Sensitization to DOCE induced by the compounds tested on MCF-7 cells does not appear to be influenced by the expression of beta tubulin III (TUBB3), baculoviral inhibitor of apoptosis repeat-containing 5 (BIRC5) and forkhead box protein P3 (FOXOP3). It is known that FOXOP3 is involved in apoptosis [117] and that BIRC5 is a bi-functional protein that acts not only as a mitotic regulator but also inhibits caspase activation [118].
4. Material and Methods
4.1. Cell Culture
4.2. Treatments
4.3. Viability Assay
4.4. Sensitization of Cancer Cells to Growth Suppression by DOCE
4.5. Apoptosis Assay
4.6. Colony Formation Assay
4.7. Cell Migration Assay
4.8. Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR)
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Risbridger, G.P.; Davis, I.D.; Birrell, S.N.; Tilley, W. Breast and prostate cancer: More similar than different. Nat. Rev. Cancer 2010, 10, 205–212. [Google Scholar] [CrossRef]
- Misawa, A.; Inoue, S. Estrogen-Related Receptors in Breast Cancer and Prostate Cancer. Front. Endocrinol. 2015, 6, 83. [Google Scholar] [CrossRef] [Green Version]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Lamy, P.-J.; Trétarre, B.; Rébillard, X.; Sanchez, M.; Cénée, S.; Ménégaux, F. Family history of breast cancer increases the risk of prostate cancer: Results from the EPICAP study. Oncotarget 2018, 9, 23661–23669. [Google Scholar] [CrossRef] [Green Version]
- Frank, C.; Sundquist, J.; Hemminki, A.; Hemminki, K. Familial Associations between Prostate Cancer and Other Cancers. Eur. Urol. 2017, 71, 162–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Primers. 2019, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Zardavas, D.; Irrthum, A.; Swanton, C.; Piccart, M. Clinical management of breast cancer heterogeneity. Nat. Rev. Clin. Oncol. 2015, 12, 381–394. [Google Scholar] [CrossRef]
- Bertucci, F.; Finetti, P.; Goncalves, A.; Birnbaum, D. The therapeutic response of ER+/HER2− breast cancers differs accord-ing to the molecular Basal or Luminal subtype. NPJ Breast Cancer 2020, 6, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toss, A.; Cristofanilli, M. Molecular characterization and targeted therapeutic approaches in breast cancer. Breast Cancer Res. 2015, 17, 60. [Google Scholar] [CrossRef] [PubMed]
- Venema, C.M.; Bense, R.D.; Steenbruggen, T.G.; Nienhuis, H.H.; Qiu, S.Q.; van Kruchten, M.; Brown, M.; Tamimi, R.M.; Hospers, G.A.; Schröder, C.P.; et al. Consideration of breast cancer subtype in targeting the androgen receptor. Pharmacol. Ther. 2019, 200, 135–147. [Google Scholar] [CrossRef]
- Karantanos, T.; Corn, P.G.; Thompson, T.C. Prostate cancer progression after androgen deprivation therapy: Mechanisms of castrate resistance and novel therapeutic approaches. Oncogene 2013, 32, 5501–55011. [Google Scholar] [CrossRef] [PubMed]
- Tolkach, Y.; Kristiansen, G. The Heterogeneity of Prostate Cancer: A Practical Approach. Pathobiology 2018, 85, 108–116. [Google Scholar] [CrossRef]
- Grindstad, T.; Richardsen, E.; Andersen, S.; Skjefstad, K.; Khanehkenari, M.R.; Donnem, T.; Ness, N.; Nordby, Y.; Bremnes, R.M.; Al-Saad, S.; et al. Progesterone Receptors in Prostate Cancer: Progesterone receptor B is the isoform associated with disease progression. Sci. Rep. 2018, 8, 11358. [Google Scholar] [CrossRef] [PubMed]
- Di Zazzo, E.; Galasso, G.; Giovannelli, P.; Di Donato, M.; Castoria, G. Estrogens and Their Receptors in Prostate Cancer: Therapeutic Implications. Front. Oncol. 2018, 8, 2. [Google Scholar] [CrossRef] [Green Version]
- Hwang, C. Overcoming docetaxel resistance in prostate cancer: A perspective review. Ther. Adv. Med Oncol. 2012, 4, 329–340. [Google Scholar] [CrossRef] [Green Version]
- Murray, S.; Briasoulis, E.; Linardou, H.; Bafaloukos, D.; Papadimitriou, C. Taxane resistance in breast cancer: Mechanisms, predictive biomarkers and circumvention strategies. Cancer Treat. Rev. 2012, 38, 890–903. [Google Scholar] [CrossRef] [PubMed]
- Nurgali, K.; Jagoe, R.T.; Abalo, R. Editorial: Adverse Effects of Cancer Chemotherapy: Anything New to Improve Tolerance and Reduce Sequelae? Front. Pharmacol. 2018, 9, 245. [Google Scholar] [CrossRef]
- Lin, S.R.; Chang, C.H.; Hsu, C.F.; Tsai, M.J.; Cheng, H.; Leong, M.K.; Sung, P.J.; Chen, J.C.; Weng, C.F. Natural compounds as potential adju-vants to cancer therapy: Preclinical evidence. Br. J. Pharmacol. 2020, 177, 1409–1423. [Google Scholar] [CrossRef] [Green Version]
- de la Iglesia, R.; Milagro, F.I.; Campión, J.; Boqué, N.; Martíne, J.A. Healthy properties of proanthocyanidins. Biofactors 2010, 36, 159–168. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Haq, I.-U.; Patel, S.; Pan, X.; Naz, S.; Silva, A.S.; Saeed, F.; Suleria, H.A.R. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.B.; Pow-Sang, J.; Egan, K.M.; Spiess, P.E.; Dickinson, S.; Salup, R.; Helal, M.; McLarty, J.; Williams, C.R.; Schreiber, F.; et al. Randomized, Placebo-Controlled Trial of Green Tea Catechins for Prostate Cancer Prevention. Cancer Prev. Res. 2015, 8, 879–887. [Google Scholar] [CrossRef] [Green Version]
- Cui, K.; Li, X.; Du, Y.; Tang, X.; Arai, S.; Geng, Y.; Xi, Y.; Xu, H.; Zhou, Y.; Ma, W.; et al. Chemoprevention of prostate cancer in men with high-grade prostatic intraepithelial neoplasia (HGPIN): A systematic review and adjusted indirect treatment comparison. Oncotarget 2017, 8, 36674–36684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawada, N. Risk and preventive factors for prostate cancer in Japan: The Japan Public Health Center-based prospective (JPHC) study. J. Epidemiol. 2017, 27, 2–7. [Google Scholar] [CrossRef] [Green Version]
- Gu, L.; Kelm, M.A.; Hammerstone, J.F.; Beecher, G.; Holden, J.; Haytowitz, D.; Gebhardt, S.; Prior, R.L. Concentrations of Proanthocyanidins in Common Foods and Estimations of Normal Consumption. J. Nutr. 2004, 134, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Kelm, M.A.; Hammerstone, J.F.; Beecher, G.; Holden, J.; Haytowitz, D.; Prior, R.L. Screening of foods containing proantho-cyanidins and their structural characterization using LC-MS/MS and thiolytic degradation. J. Agric. Food Chem. 2003, 51, 7513–7521. [Google Scholar] [CrossRef]
- Araujo, F.; Nitzke, J.A.; Blauth, C.; Vogt, E. Chocolate and red wine–A comparison between flavonoids content. Food Chem. 2010, 120, 109–112. [Google Scholar]
- He, Z.; Zhang, J.; Yuan, X.; Xi, J.; Liu, Z.; Zhang, Y. Stratification of Breast Cancer by Integrating Gene Expression Data and Clinical Variables. Molecules 2019, 24, 631. [Google Scholar] [CrossRef] [Green Version]
- Goodspeed, A.; Heiser, L.M.; Gray, J.; Costello, J.C. Tumor-Derived Cell Lines as Molecular Models of Cancer Phar-macogenomics. Mol. Cancer Res. 2016, 14, 3–13. [Google Scholar] [CrossRef] [Green Version]
- The Broad Institute Cancer Cell Line Encyclopedia (CCLE). The Broad Institute of MIT&Harvad. 2021. Available online: ttp://www.broadinstitute.org/ccle/home (accessed on 3 June 2021).
- Welcome—IARC TP53 Database. Available online: https://p53.iarc.fr/ (accessed on 27 November 2020).
- Moran, D.M.; Mak, C.G. Nutlin-3a induces cytoskeletal rearrangement and inhibits the migration and invasion capacity of p53 wild-type cancer cells. Mol. Cancer Ther. 2010, 9, 895–905. [Google Scholar] [CrossRef] [Green Version]
- Concin, N.; Zeillinger, C.; Tong, D.; Stimpfl, M.; König, M.; Print, D.; Concin, N.; Zeillinger, C.; Tong, D.; Stimpfl, M.; et al. Comparison of p53 mutational status with mRNA and protein expression in a panel of 24 human breast carcinoma cell lines. Breast Cancer Res. Treat. 2003, 79, 37–46. [Google Scholar] [CrossRef]
- Dias, K.; Dvorkin-Gheva, A.; Hallett, R.M.; Wu, Y.; Hassell, J.; Pond, G.R.; Levine, M.; Whelan, T.; Bane, A.L. Claudin-Low Breast Cancer; Clinical & Patho-logical Characteristics. PLoS ONE 2017, 12, e0168669. [Google Scholar]
- Jiang, G.; Zhang, S.; Yazdanparast, A.; Li, M.; Pawar, A.V.; Liu, Y.; Inavolu, S.M.; Cheng, L. Comprehensive comparison of molecular portraits between cell lines and tumors in breast cancer. BMC Genom. 2016, 17, 525. [Google Scholar] [CrossRef] [Green Version]
- Barretina, J.; Caponigro, G.; Stransky, N.; Venkatesan, K.; Margolin, A.A.; Kim, S.; Wilson, C.J.; Lehár, J.; Kryukov, G.V.; Sonkin, D.; et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 2012, 48, 603–607, Erratum in Nature 2012, 492, 290. Erratum in Nature 2019, 565, E5–E6. [Google Scholar] [CrossRef] [PubMed]
- Saunus, J.M.; Smart, C.E.; Kutasovic, J.R.; Johnston, R.; Croft, P.K.-D.; Miranda, M.; Rozali, E.N.; Vargas, A.C.; Reid, L.E.; Lorsy, E.; et al. Multidimensional phenotyping of breast cancer cell lines to guide preclinical research. Breast Cancer Res. Treat. 2017, 167, 289–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neve, R.M.; Chin, K.; Fridlyand, J.; Yeh, J.; Baehner, F.L.; Fevr, T.; Clark, L.; Bayani, N.; Coppe, J.-P.; Tong, F.; et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell 2006, 10, 515–527. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.Y.; Oh, S.H.; Suh, Y.A.; Baek, J.H.; Papadimitrakopoulou, V.; Huang, S.; Hong, W.K. Response of non-small cell lung cancer cells to the inhibitors of phosphatidylinositol 3-kinase/Akt- and MAPK kinase 4/c-Jun NH2-terminal kinase pathways: An ef-fective therapeutic strategy for lung cancer. Clin. Cancer Res. 2005, 11, 6065–6074. [Google Scholar] [CrossRef] [Green Version]
- Franken, N.A.P.; Rodermond, H.M.; Stap, J.; Haveman, J.; Van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef] [PubMed]
- Gandalovičová, A.; Rosel, D.; Fernandes, M.; Veselý, P.; Heneberg, P.; Čermák, V.; Petruzelka, L.; Kumar, S.; Sanz-Moreno, V.; Brábek, J. Migrastatics—Anti-metastatic and Anti-invasion Drugs: Promises and Challenges. Trends Cancer 2017, 3, 391–406. [Google Scholar] [CrossRef] [Green Version]
- Abbas, T.; Dutta, A. p21 in cancer: Intricate networks and multiple activities. Nat. Rev. Cancer 2009, 9, 400–414. [Google Scholar] [CrossRef]
- Liao, X.-H.; Lu, D.-L.; Wang, N.; Liu, L.-Y.; Wang, Y.; Li, Y.-Q.; Yan, T.-B.; Sun, X.-G.; Hu, P.; Zhang, T.-C. Estrogen receptor α mediates proliferation of breast cancer MCF-7 cells via a p21/PCNA/E2F1-dependent pathway. FEBS J. 2014, 281, 927–942. [Google Scholar] [CrossRef] [Green Version]
- Westphal, D.; Kluck, R.; Dewson, G. Building blocks of the apoptotic pore: How Bax andBak are activated and oligomerize during apoptosis. Cell Death Differ. 2014, 21, 196–205. [Google Scholar] [CrossRef] [Green Version]
- Kalkavan, H.; Green, D. MOMP, cell suicide as a BCL-2 family business. Cell Death Differ. 2018, 25, 46–55. [Google Scholar] [CrossRef]
- Delbridge, A.R.D.; Strasser, A. The BCL-2 protein family, BH3-mimetics and cancer therapy. Cell Death Differ. 2015, 22, 1071–1080. [Google Scholar] [CrossRef] [Green Version]
- Jelínek, M.; Balušíková, K.; Schmiedlová, M.; Němcová-Fürstová, V.; Šrámek, J.; Stančíková, J.; Zanardi, I.; Ojima, I.; Kovář, J. The role of individual caspases in cell death induction by taxanes in breast cancer cells. Cancer Cell Int. 2015, 15, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; He, M.; Li, L.; Liang, Z.; Zou, Z.; Tao, A. Cell-in-Cell Death Is Not Restricted by Caspase-3 Deficiency in MCF-7 Cells. J. Breast Cancer 2016, 19, 231–241. [Google Scholar] [CrossRef]
- Jeanes, A.; Gottardi, C.; Yap, A. Cadherins and cancer: How does cadherin dysfunction promote tumor progression? On-Cogene 2008, 27, 6920–6929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marín-Aguilera, M.; Codony-Servat, J.; Reig, Ò.; Lozano, J.J.; Fernández, P.L.; Pereira, M.V.; Jiménez, N.; Donovan, M.; Puig, P.; Mengual, L.; et al. Epithelial-to-Mesenchymal Transition Mediates Docetaxel Resistance and High Risk of Relapse in Prostate Cancer. Mol. Cancer Ther. 2014, 13, 1270–1284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Işeri, O.D.; Kars, M.D.; Arpaci, F.; Atalay, C.; Pak, I.; Gündüz, U. Drug resistant MCF-7 cells exhibit epithelial-mesenchymal transition gene expression pattern. Biomed. Pharmacother. 2011, 65, 40–45. [Google Scholar] [CrossRef]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular principles of metastasis: A hallmark of cancer revisit-ed. Sig. Transduct. Target Ther. 2020, 5, 28. [Google Scholar] [CrossRef]
- Olea-Flores, M.; Juárez-Cruz, J.C.; Mendoza-Catalán, M.A.; Padilla-Benavides, T.; Navarro-Tito, N. Signaling Pathways In-duced by Leptin during Epithelial⁻Mesenchymal Transition in Breast Cancer. Int. J. Mol. Sci. 2018, 19, 3493. [Google Scholar] [CrossRef] [Green Version]
- Li, W.-J.; Zhong, S.-L.; Wu, Y.-J.; Xu, W.-D.; Xu, J.-J.; Tang, J.-H.; Zhao, J.-H. Systematic expression analysis of genes related to multidrug-resistance in isogenic docetaxel- and adriamycin-resistant breast cancer cell lines. Mol. Biol. Rep. 2013, 40, 6143–6150. [Google Scholar] [CrossRef] [PubMed]
- GeneCards® Home Page. Available online: http://www.genecards.org/ (accessed on 1 August 2012).
- Gene NCBI. National Center for Biotechnology Information. U.S. National Library of Medicine. 8600 Rockville Pike, Bethesda, MD, USA. Available online: https://www.ncbi.nlm.nih.gov/gene (accessed on 1 June 2021).
- Allison, K.H.; Hammond, M.E.H.; Dowsett, M.; McKernin, S.E.; Carey, L.A.; Fitzgibbons, P.L.; Hayes, D.F.; Lakhani, S.R.; Chavez-MacGregor, M.; Perlmutter, J.; et al. Estrogen and Progester-one Receptor Testing in Breast Cancer: ASCO/CAP Guideline Update. J. Clin. Oncol. 2020, 38, 346–1366. [Google Scholar] [CrossRef]
- Parker, C.; Castro, E.; Fizazi, K.; Heidenreich, A.; Ost, P.; Procopio, G.; Tombal, B.; Gillessen, S. ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1119–1134. [Google Scholar] [CrossRef]
- Jividen, K.; Yang, C.S.; Szlachta, K.; Ratan, A.; Paschal, B.M. Genomic analysis of DNA repair genes and androgen signaling in prostate cancer. BMC Cancer 2018, 18, 960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akaza, H.; Procopio, G.; Pripatnanont, C.; Facchini, G.V.; Fava, S.; Wheatley, D.; Leung, K.C.; Butt, M.; Silva, A.; Castillo, L.; et al. Metastatic Castration-Resistant Pros-tate Cancer Previously Treated With Docetaxel-Based Chemotherapy: Treatment Patterns from the PROXIMA Prospective Registry. J. Glob. Oncol. 2018, 4, 1–12. [Google Scholar]
- Farha, N.G.; Kasi, A. Docetaxel. [Updated 2020 Apr 13]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2020; Available online: https://www.ncbi.nlm.nih.gov/books/NBK537242/ (accessed on 31 May 2021).
- Yiannakopoulou, E.C. Interaction of Green Tea Catechins with Breast Cancer Endocrine Treatment: A Systematic Review. Pharmacol. 2014, 94, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Henning, S.M.; Heber, D.; Vadgama, J.V. Sensitization to docetaxel in prostate cancer cells by green tea and quer-cetin. J. Nutr. Biochem. 2015, 26, 408–415. [Google Scholar] [CrossRef] [Green Version]
- Kilic, U.; Sahin, K.; Tuzcu, M.; Basak, N.; Orhan, C.; Elibol-Can, B.; Kilic, E.; Sahin, F.; Kucuk, O. Enhancement of Cisplatin sensitivity in human cervical cancer: Epigallocatechin-3-gallate. Front Nutr. 2015, 1, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Przystupski, D.; Michel, O.; Rossowska, J.; Kwiatkowski, S.; Saczko, J.; Kulbacka, J. The modulatory effect of green tea catechin on drug resistance in human ovarian cancer cells. Med. Chem. Res. 2019, 28, 657–667. [Google Scholar] [CrossRef] [Green Version]
- Flores-Pérez, A.; Marchat, L.A.; Sánchez, L.L.; Romero-Zamora, D.; Arechaga-Ocampo, E.; Ramírez-Torres, N.; Chávez, J.D.; Carlos-Reyes, Á.; Astudillo-de la Vega, H.; Ruiz-García, E.; et al. Differ-ential proteomic analysis reveals that EGCG inhibits HDGF and activates apoptosis to increase the sensitivity of non-small cells lung cancer to chemotherapy. Proteom. Clin. Appl. 2016, 10, 172–182. [Google Scholar] [CrossRef]
- Damianaki, A.; Bakogeorgou, E.; Kampa, M.; Notas, G.; Hatzoglou, A.; Panagiotou, S.; Gemetzi, C.; Kouroumalis, E.; Martin, P.-M.; Castanas, E. Potent inhibitory action of red wine polyphenols on human breast cancer cells. J. Cell. Biochem. 2000, 78, 429–441. [Google Scholar] [CrossRef]
- Goodin, M.G.; Fertuck, K.C.; Zacharewski, T.R.; Rosengren, R.J.; Goodin, M.G.; Fertuck, K.C.; Zacharewski, T.R.; Rosengren, R.J. Estrogen Receptor-Mediated Actions of Polyphenolic Catechins in Vivo and in Vitro. Toxicol. Sci. 2002, 69, 354–361. [Google Scholar] [CrossRef] [Green Version]
- Kuruto-Niwa, R.; Inoue, S.; Ogawa, S.; Muramatsu, M.; Nozawa, R. Effects of tea catechins on the ERE-regulated estrogenic activity. J. Agric. Food Chem. 2000, 48, 6355–6361. [Google Scholar] [CrossRef] [PubMed]
- Puranik, N.V.; Srivastava, P.; Bhatt, G.; John Mary, D.J.S.; Limaye, A.M.; Sivaraman, J. Determination and analysis of agonist and antagonist potential of naturally occurring flavonoids for estrogen receptor (ERα) by various param-eters and molecular modelling approach. Sci. Rep. 2019, 9, 7450. [Google Scholar] [CrossRef] [PubMed]
- Ratna, W.N.; Bhatt, V.D.; Chaudhary, K.; Ariff, A.B.; Bavadeka, S.A.; Boggeti, R. Selective Estrogen-Receptor Modulators Genistein, Resveratrol, and Catechin fail to stimulate the Hepatic Expression of Estrogen-Responsive Genes Encoding the Avian Apolipoprotein II and Vitellogenin. FASEB J. 2012, 26, 673.7. [Google Scholar]
- Hallman, K.; Aleck, K.; Quigley, M.; Dwyer, B.; Lloyd, V.; Szmyd, M. The regulation of steroid receptors by epigallocate-chin-3-gallate in breast cancer cells. Breast Cancer 2017, 24, 365–373. [Google Scholar]
- De Amicis, F.; Russo, A.; Avena, P.; Santoro, M.; Vivacqua, A.; Bonofiglio, D.; Mauro, L.; Aquila, S.; Tramontano, D.; Fuqua, S.A.; et al. In vitro mechanism for downregulation of ER-α expression by epigallocatechin gallate in ER+/PR+ human breast cancer cells. Mol. Nutr. Food Res. 2013, 57, 840–853. [Google Scholar] [CrossRef] [Green Version]
- Farabegoli, F.; Barbi, C.; Lambertini, E.; Piva, R. (−)-Epigallocatechin-3-gallate downregulates estrogen receptor alpha function in MCF-7 breast carcinoma cells. Cancer Detect. Prev. 2007, 31, 499–504. [Google Scholar] [CrossRef]
- Siddiqui, I.A.; Asim, M.; Hafeez, B.B.; Adhami, V.M.; Tarapore, R.S.; Mukhtar, H. Green tea polyphenol EGCG blunts androgen re-ceptor function in prostate cancer. FASEB J. 2011, 25, 1198–1207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kampa, M.; Theodoropoulou, K.; Mavromati, F.; Pelekanou, V.; Notas, G.; Lagoudaki, E.D.; Nifli, A.P.; Morel-Salmi, C.; Stathopoulos, E.N.; Vercauteren, J.; et al. Novel oligomeric proan-thocyanidin derivatives interact with membrane androgen sites and induce regression of hormone-independent prostate cancer derivatives interact with membrane androgen sites and induce regression of hormone-independent prostate cancer. J. Pharmacol. Exp. Ther. 2011, 337, 24–32. [Google Scholar] [CrossRef] [Green Version]
- Eng, E.T.; Ye, J.; Williams, D.; Phung, S.; Moore, R.; Young, M.K.; Gruntmanis, U.; Braunstein, G.; Chen, S. Suppression of estrogen biosynthesis by procyanidin dimers in red wine and grape seeds. Cancer Res. 2003, 63, 8516–8522. [Google Scholar] [PubMed]
- Eng, E.T.; Williams, D.; Mandava, U.; Kirma, N.; Tekmal, R.R.; Chen, S. Suppression of aromatase (estrogen synthetase) by red wine phytochemicals. Breast Cancer Res. Treat. 2001, 67, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Eng, E.T.; Williams, D.; Mandava, U.; Kirma, N.; Tekmal, R.R.; Chen, S. Anti-aromatase chemicals in red wine. Ann. N. Y. Acad. Sci. 2006, 963, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Shufelt, C.; Merz, C.N.; Yang, Y.; Kirschner, J.; Polk, D.; Stanczyk, F.; Paul-Labrador, M.; Braunstein, G.D. Red versus white wine as a nutritional aromatase inhibitor in premenopausal women: A pilot study. J. Womens Health (Larchmt) 2012, 21, 281–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiipakka, R.A.; Zhang, H.-Z.; Dai, W.; Dai, Q.; Liao, S. Structure–activity relationships for inhibition of human 5α-reductases by polyphenols. Biochem. Pharmacol. 2002, 63, 1165–1176. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Fan, M.; Tang, Y.; Yang, H.P.; Hwang, J.-Y.; Kim, E.-K. In Vivo Effects of Polymerized Anthocyanin from Grape Skin on Benign Prostatic Hyperplasia. Nutrients 2019, 11, 2444. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.Y.; Ha, T.Y.; Ahn, J.Y.; Kim, S.R.; Kang, K.S.; Hwang, I.K.; Kim, S. Estrogenic Activities of Isoflavones and Flavones and their Structure-Activity Relationships. Planta Med. 2007, 74, 25–32. [Google Scholar] [CrossRef]
- Resende, F.A.; de Oliveira, A.P.; de Camargo, M.S.; Vilegas, W.; Varanda, E.A. Evaluation of estrogenic potential of flavo-noids using a recombinant yeast strain and MCF7/BUS cell proliferation assay. PLoS ONE 2013, 8, e74881. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Zhao, B.; Song, Z.; Han, S.; Wang, M. Estrogen receptor-α36 is involved in epigallocatechin-3-gallate induced growth inhibition of ER-negative breast cancer stem/progenitor cells. J. Pharmacol. Sci. 2016, 130, 85–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sweeney, E.E.; McDaniel, R.E.; Maximov, P.Y.; Fan, P.; Jordan, V.C. Models and mechanisms of acquired antihormone resistance in breast cancer: Significant clinical progress despite limitations. Horm. Mol. Biol. Clin. Investig. 2012, 9, 143–163. [Google Scholar] [CrossRef] [Green Version]
- Mota, A.L.; Evangelista, A.F.; Macedo, T.; Oliveira, R.; Scapulatempo-Neto, C.; Vieira, R.A.; Marques, M.M.C. Molecular characterization of breast cancer cell lines by clinical immunohistochemical markers. Oncol. Lett. 2017, 13, 4708–4712. [Google Scholar] [CrossRef] [Green Version]
- D’Amato, N.C.; Gordon, M.A.; Babbs, B.L.; Spoelstra, N.S.; Butterfield, K.T.C.; Torkko, K.C.; Phan, V.T.; Barton, V.N.; Rogers, T.J.; Sartorius, C.A.; et al. Cooperative Dynamics of AR and ER Activity in Breast Cancer. Mol. Cancer Res. 2016, 14, 1054–1067. [Google Scholar] [CrossRef] [Green Version]
- Magklara, A.; Brown, T.J.; Diamandis, E.P. Characterization of androgen receptor and nuclear receptor co-regulator ex-pression in human breast cancer cell lines exhibiting differential regulation of kallikreins 2 and 3. Int. J. Cancer 2002, 100, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, N.L.; Driver, D.; Miesfeld, R.L. The length and location of CAG trinucleotide repeats in the androgen receptor N-terminal domain affect transactivation function. Nucleic Acids Res. 1994, 22, 3181–3186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, D.G.; Hickey, T.; Irvine, C.; Wijayakumara, D.D.; Lu, L.; Tilley, W.D.; Selth, L.; MacKenzie, P.I. Identification of Androgen Receptor Splice Variant Transcripts in Breast Cancer Cell Lines and Human Tissues. Horm. Cancer 2014, 5, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Lafront, C.; Germain, L.; Weidmann, C.; Audet-Walsh, É. A Systematic Study of the Impact of Estrogens and Selective Es-trogen Receptor Modulators on Prostate Cancer Cell Proliferation. Sci. Rep. 2020, 10, 4024. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, M.; Tanaka, Y.; Perinchery, G.; Dharia, A.; Kotcherguina, I.; Fujimoto, S.; Dahiya, R. Methylation and inactivation of estrogen, progesterone, and androgen receptors in prostate cancer. J. Natl. Cancer Inst. 2002, 94, 384–390. [Google Scholar] [CrossRef] [Green Version]
- Sampayo, R.; Recouvreux, S.; Simian, M. The hyperplastic phenotype in PR-A and PR-B transgenic mice: Lessons on the role of estrogen and progesterone receptors in the mouse mammary gland and breast cancer. Vitam. Horm. 2013, 93, 185–201. [Google Scholar]
- Ellem, S.J.; Schmitt, J.F.; Pedersen, J.S.; Frydenberg, M.; Risbridger, G. Local Aromatase Expression in Human Prostate Is Altered in Malignancy. J. Clin. Endocrinol. Metab. 2004, 89, 2434–2441. [Google Scholar] [CrossRef] [Green Version]
- Ryde, C.M.; Nicholls, J.E.; Dowsett, M. Steroid and growth factor modulation of aromatase activity in MCF7 and T47D breast carcinoma cell lines. Cancer Res. 1992, 52, 1411–1415. [Google Scholar]
- Sonne-Hansen, K.; Lykkesfeldt, A. Endogenous aromatization of testosterone results in growth stimulation of the human MCF-7 breast cancer cell line. J. Steroid Biochem. Mol. Biol. 2005, 93, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Aka, J.A.; Lin, S.-X. Correction: Comparison of Functional Proteomic Analyses of Human Breast Cancer Cell Lines T47D and MCF7. PLoS ONE 2012, 7. [Google Scholar] [CrossRef]
- Negri-Cesi, P.; Colciago, A.; Poletti, A.; Motta, M. 5alpha-reductase isozymes and aromatase are differentially expressed and active in the androgen-independent human prostate cancer cell lines DU145 and PC3. Prostate 1999, 41, 224–232. [Google Scholar] [CrossRef]
- Wiebe, J.P.; Lewis, M.J. Activity and expression of progesterone metabolizing 5alpha-reductase, 20alpha-hydroxysteroid oxi-doreductase and 3alpha(beta)-hydroxysteroid oxidoreductases in tumorigenic (MCF-7, MDA-MB-231, T-47D) and nontu-morigenic (MCF-10A) human breast cancer cells. BMC Cancer 2003, 3, 9. [Google Scholar] [CrossRef] [Green Version]
- Rice, M.A.; Malhotra, S.V.; Stoyanova, T. Second-Generation Antiandrogens: From Discovery to Standard of Care in Castra-tion Resistant Prostate Cancer. Front Oncol. 2019, 9, 801. [Google Scholar] [CrossRef]
- Kyriakopoulos, C.E.; Chen, Y.H.; Carducci, M.A.; Liu, G.; Jarrard, D.F.; Hahn, N.M.; Shevrin, D.H.; Dreicer, R.; Hussain, M.; Eisenberger, M.; et al. Chemohormonal Therapy in Meta-static Hormone-Sensitive Prostate Cancer: Long-Term Survival Analysis of the Randomized Phase III E3805 CHAARTED Trial. J. Clin. Oncol. 2018, 36, 1080–1087. [Google Scholar] [CrossRef] [Green Version]
- Lumachi, F.; Santeufemia, D.; Basso, S.M. Current medical treatment of estrogen receptor-positive breast cancer. World J. Biol. Chem. 2015, 6, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Cook, A.; Beesley, S.; O’Sullivan, J.M.; Birtle, A.J.; Thalmann, G.; Graham, J.D.; Spears, M.R.; Brock, S.; Srinivasan, R.; Protheroe, A.; et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): Survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 2016, 387, 1163–1177. [Google Scholar] [CrossRef]
- Lo, Y.-C.; Cormier, O.; Liu, T.; Nettles, K.W.; Katzenellenbogen, J.A.; Stearns, T.; Altman, R.B. Pocket similarity identifies selective estrogen receptor modulators as microtubule modulators at the taxane site. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukhtar, E.; Adhami, V.M.; Mukhtar, H. Targeting Microtubules by Natural Agents for Cancer Therapy. Mol. Cancer Ther. 2014, 13, 275–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, S.; Hwang, D.J.; Li, W.; Miller, D.D. Current Advances of Tubulin Inhibitors in Nanoparticle Drug Delivery and Vas-cular Disruption/Angiogenesis. Molecules 2016, 21, 1468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sumit, H.; Tehseen, D.; Lalit, S. Molecular. Docking studies of Filarial β-Tubulin protein models with anti-filarial phytochem-icals. Biomed. Biotechnol. Res. J. 2019, 3, 162–170. [Google Scholar]
- Chakrabarty, S.; Ganguli, A.; Das, A.; Nag, D.; Chakrabarti, G. Epigallocatechin-3-gallate shows anti-proliferative activity in HeLa cells targeting tubulin-microtubule equilibrium. Chem. Interact. 2015, 242, 380–389. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Y.Y.; Sun, W.J.; Zhang, M.H.; Zheng, Y.F.; Shen, H.M.; Yang, J.; Zhu, X.Q. FBS or BSA Inhibits EGCG Induced Cell Death through Covalent Binding and the Reduction of Intracellular ROS Production. Biomed. Res. Int. 2016, 2016, 5013409. [Google Scholar] [CrossRef] [Green Version]
- Santamaría, D.; Barrière, C.; Cerqueira, A.; Hunt, S.; Tardy, C.; Newton, K.; Caceres, J.; Dubus, P.; Malumbres, M.; Barbacid, M. Cdk1 is sufficient to drive the mammalian cell cycle. Nat. Cell Biol. 2007, 448, 811–815. [Google Scholar] [CrossRef]
- Karimian, A.; Ahmadi, Y.; Yousefi, B. Multiple functions of p21 in cell cycle, apoptosis and transcriptional regulation after DNA damage. DNA Repair 2016, 42, 63–71. [Google Scholar] [CrossRef]
- Cao, J.; Zhu, Z.; Wang, H.; Nichols, T.C.; Lui, G.Y.L.; Deng, S.; Rejto, P.A.; VanArsdale, T.; Hardwick, J.S.; Weinrich, S.L.; et al. Combining CDK4/6 inhibition with taxanes enhances anti-tumor efficacy by sustained impairment of pRB-E2F pathways in squamous cell lung cancer. Oncogene 2019, 38, 4125–4141. [Google Scholar] [CrossRef]
- Ding, L.; Cao, J.; Lin, W.; Chen, H.; Xiong, X.; Ao, H.; Yu, M.; Lin, J.; Cui, Q. The Roles of Cyclin-Dependent Kinases in Cell-Cycle Progression and Therapeutic Strategies in Human Breast Cancer. Int. J. Mol. Sci. 2020, 21, 1960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chohan, T.A.; Qayyum, A.; Rehman, K.; Tariq, M.; Akash, M.S.H. An insight into the emerging role of cyclin-dependent ki-nase inhibitors as potential therapeutic agents for the treatment of advanced cancers. Biomed Pharmacother. 2018, 107, 1326–1341. [Google Scholar] [CrossRef]
- Bai, J.; Li, Y.; Zhang, G. Cell cycle regulation and anticancer drug discovery. Cancer Biol. Med. 2017, 14, 348–362. [Google Scholar]
- Zeng, X.; Xu, W.K.; Lok, T.M.; Ma, H.T.; Poon, R.Y.C. Imbalance of the spindle-assembly checkpoint promotes spindle poi-son-mediated cytotoxicity with distinct kinetics. Cell Death Dis. 2019, 10, 314. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Liu, C.; Chen, D.; Yang, W.H.; Liu, X.; Liu, C.G.; Dugas, C.M.; Tang, F.; Zheng, P.; Liu, Y.; et al. FOXP3 Controls an miR-146/NF-κB Negative Feedback Loop That Inhibits Apoptosis in Breast Cancer Cells. Cancer Res. 2015, 75, 1703–1713. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Hu, C.; Li, H. Survivin as a novel target protein for reducing the proliferation of cancer cells (Review). Biomed. Rep. 2018, 8, 399–406. [Google Scholar] [CrossRef] [Green Version]
- Karamouzis, M.V.; Papavassiliou, K.A.; Adamopoulos, C.; Papavassiliou, A.G. Targeting Androgen/Estrogen Receptors Crosstalk in Cancer. Trends Cancer 2016, 2, 35–48. [Google Scholar] [CrossRef]
- Chaudhri, R.A.; Hadadi, A.; Lobachev, K.S.; Schwartz, Z.; Boyan, B.D. Estrogen receptor-alpha 36 mediates the an-ti-apoptotic effect of estradiol in triple negative breast cancer cells via a membrane-associated mechanism. Biochim. Biophys. Acta 2014, 1843, 2796–2806. [Google Scholar] [CrossRef] [Green Version]
- Papadopoulou, N.; Papakonstanti, E.A.; Kallergi, G.; Alevizopoulos, K.; Stournaras, C. Membrane androgen receptor acti-vation in prostate and breast tumor cells: Molecular signaling and clinical impact. IUBMB Life 2009, 61, 56–61. [Google Scholar] [CrossRef]
- Giovannelli, P.; Di Donato, M.; Auricchio, F.; Castoria, G.; Migliaccio, A. Androgens Induce Invasiveness of Triple Negative Breast Cancer Cells through AR/Src/PI3-K Complex Assembly. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Bai, S.; Zhang, B.Y. Impact of taxanes on androgen receptor signaling. Asian J. Androl. 2019, 21, 249–252. [Google Scholar] [CrossRef] [PubMed]
- McLoughlin, E.C.; O’Boyle, N.M. Colchicine-Binding Site Inhibitors from Chemistry to Clinic: A Review. Pharmaceuticals 2020, 13, 8, Erratum in Pharmaceuticals 2020, 13, 72. [Google Scholar] [CrossRef] [Green Version]
- COSMIC, the Catalogue of Somatic Mutations in Cancer. Cell Lines Project. Available online: http://cancer.sanger.ac.uk/cancergenome/projects/cell_lines/ (accessed on 3 June 2021).
- Núñez-Iglesias, M.J.; Novío, S.; García, C.; Pérez-Muñuzuri, E.; Soengas, P.; Cartea, E.; Velasco, P.; Freire-Garabal, M. Glu-cosinolate-Degradation Products as Co-Adjuvant Therapy on Prostate Cancer in Vitro. Int. J. Mol. Sci. 2019, 20, 4977. [Google Scholar] [CrossRef] [Green Version]
- Xiao, D.; Choi, S.; Johnson, D.E.; Vogel, V.G.; Johnson, C.S.; Trump, D.L.; Lee, Y.J.; Singh, S.V. Diallyl trisulfide-induced apoptosis in human prostate cancer cells involves c-Jun N-terminal kinase and extracellular-signal regulated kinase-mediated phosphorylation of Bcl-2. Oncogene 2004, 23, 5594–5606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, L.; Yang, C.; Wang, J.; Li, W.; Wen, R.; Chen, J.; Zheng, J. SATB1 is overexpressed in metastatic prostate cancer and promotes prostate cancer cell growth and invasion. J. Transl. Med. 2013, 11, 111. [Google Scholar] [CrossRef] [Green Version]
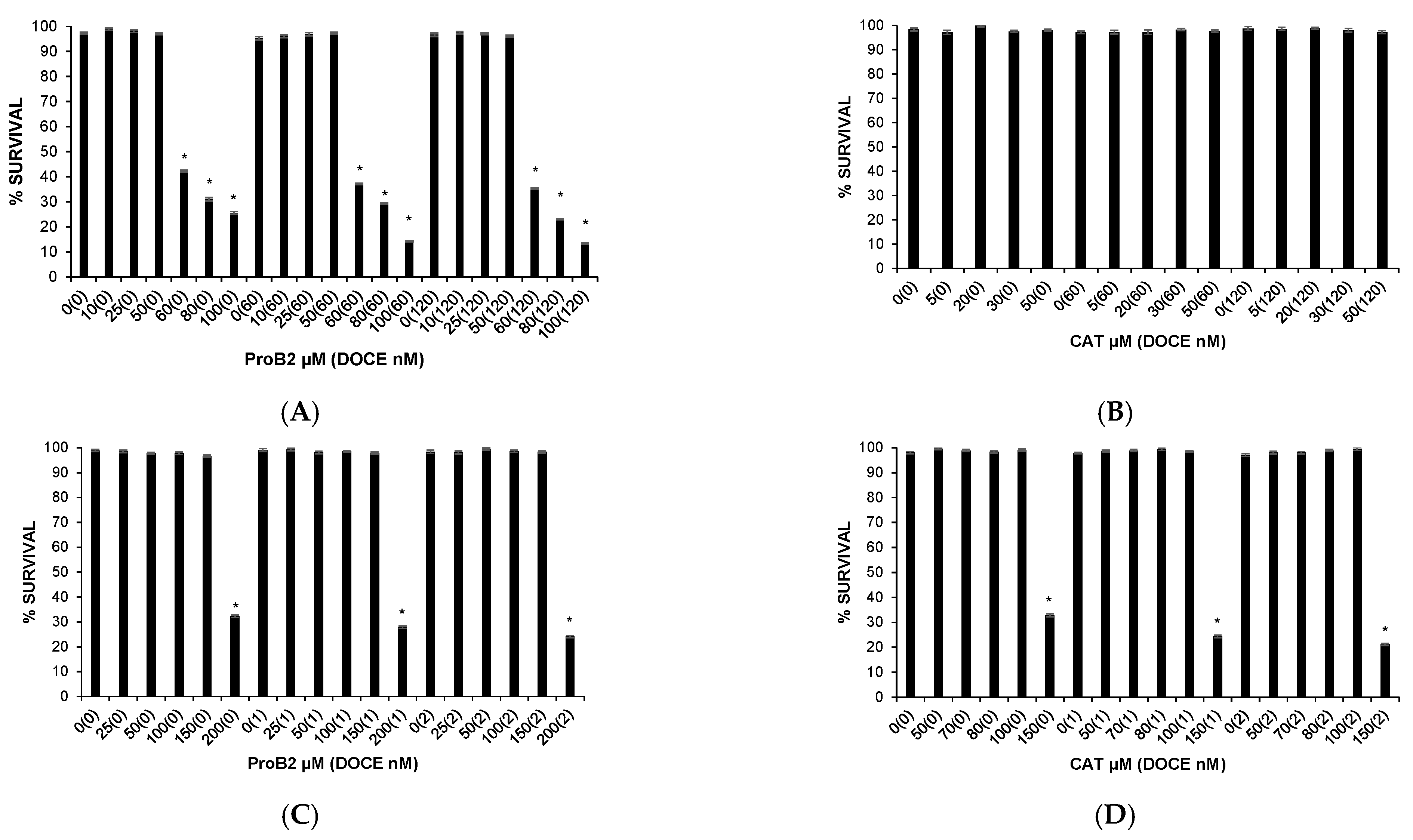

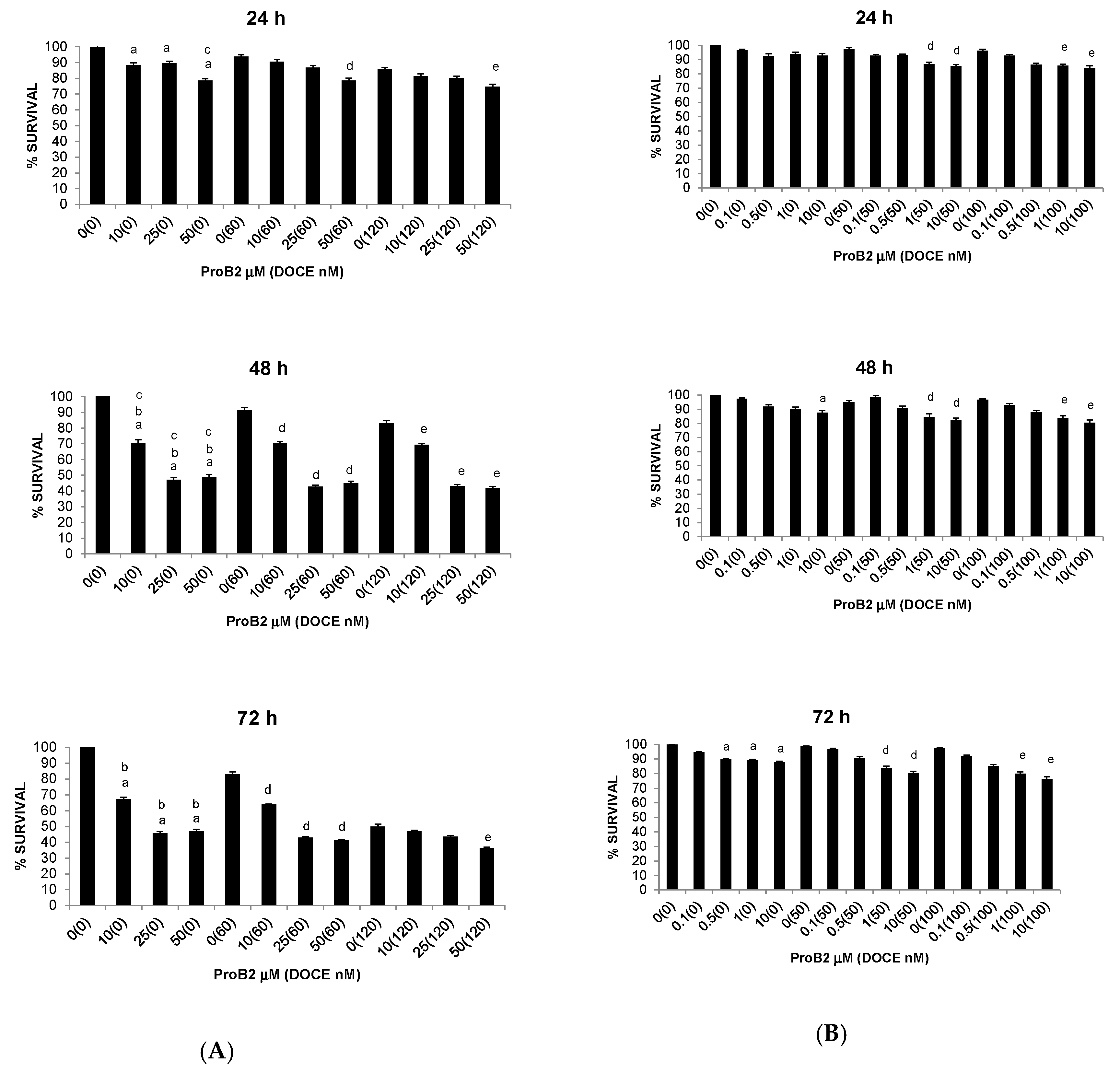
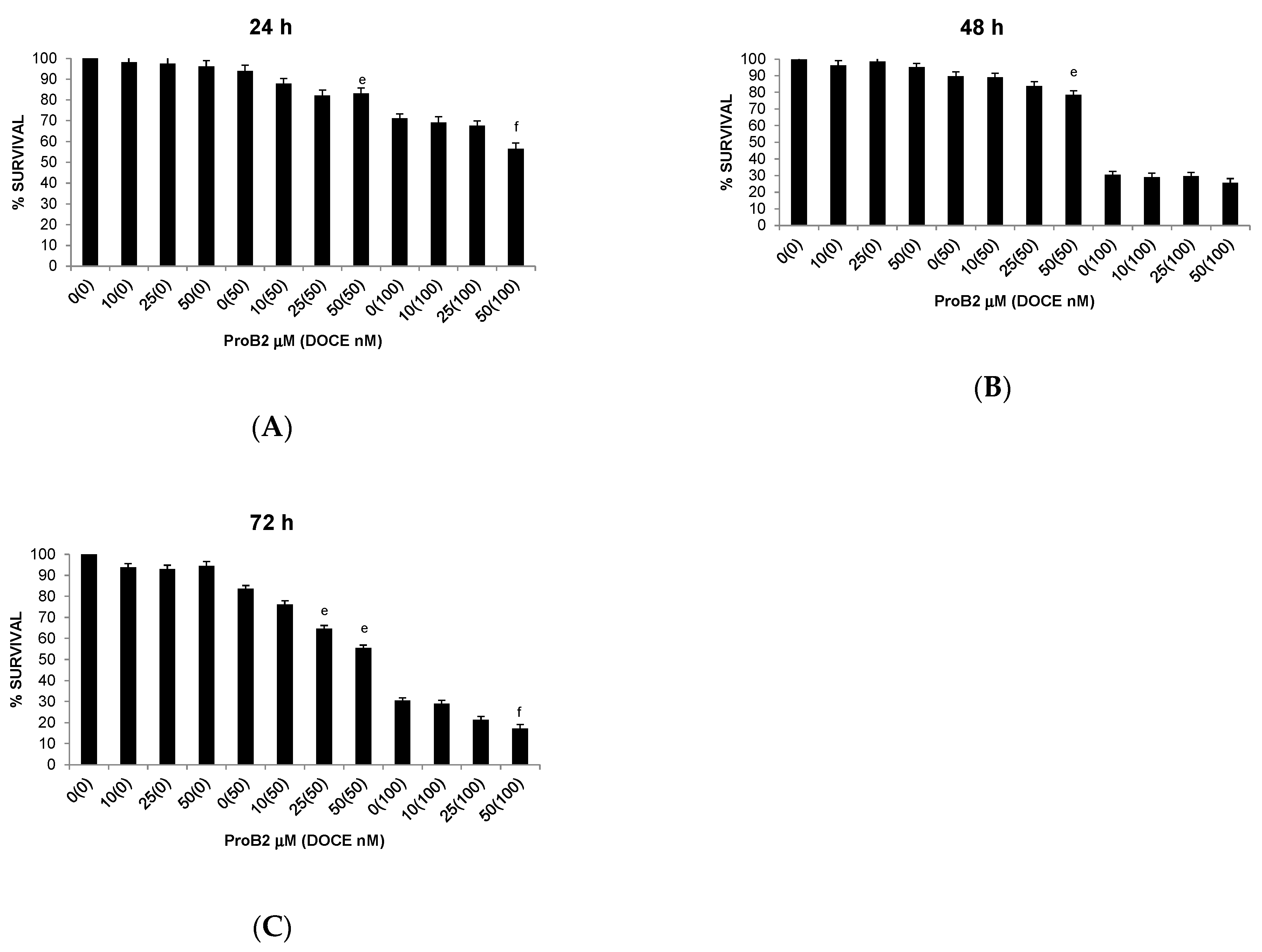
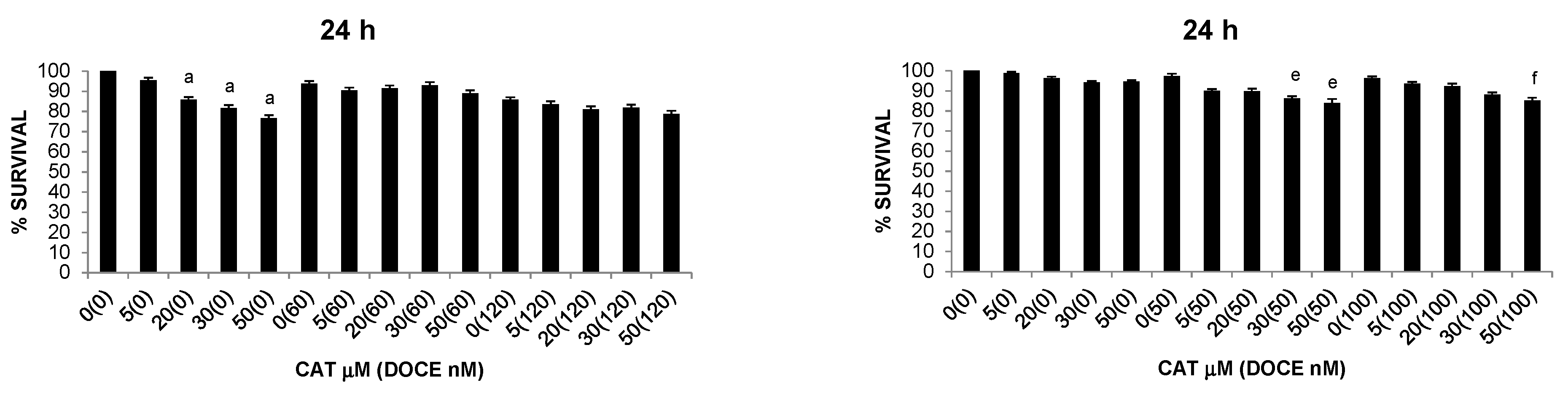




| (A) | |||||||
| DOCE (nM) | ProB2 (µM) | Mean | SD | DOCE (nM) | CAT(µM) | Mean | SD |
| 0 | 0 | 98.84 | 0.87 | 0 | 0 | 98.01 | 0.87 |
| 0 | 25 | 98.51 | 0.92 | 0 | 50 | 99.6 | 0.79 |
| 0 | 50 | 97.83 | 0.65 | 0 | 70 | 98.87 | 0.88 |
| 0 | 100 | 97.75 | 1.03 | 0 | 80 | 98.32 | 0.86 |
| 0 | 150 | 96.62 | 0.78 | 0 | 100 | 99.05 | 0.68 |
| 0 | 200 | 32.22 * | 0.93 | 0 | 150 | 32.8 * | 1.15 |
| 1 | 0 | 99.11 | 1.11 | 1 | 0 | 97.87 | 0.5 |
| 1 | 25 | 99.27 | 1.06 | 1 | 50 | 98.67 | 0.74 |
| 1 | 50 | 98.08 | 0.93 | 1 | 70 | 98.86 | 0.87 |
| 1 | 100 | 98.45 | 0.68 | 1 | 80 | 99.34 | 0.75 |
| 1 | 150 | 97.9 | 0.86 | 1 | 100 | 98.43 | 0.67 |
| 1 | 200 | 27.86 * | 0.92 | 1 | 150 | 24.32 * | 0.87 |
| 2 | 0 | 98.33 | 1.21 | 2 | 0 | 97.08 | 1.21 |
| 2 | 25 | 98.08 | 1.23 | 2 | 50 | 98.06 | 1.13 |
| 2 | 50 | 99.43 | 0.96 | 2 | 70 | 97.97 | 0.94 |
| 2 | 100 | 98.52 | 0.87 | 2 | 80 | 98.87 | 0.89 |
| 2 | 150 | 98.31 | 0.79 | 2 | 100 | 99.56 | 1.27 |
| 2 | 200 | 24.12 * | 0.93 | 2 | 150 | 21.13 * | 0.83 |
| (B) | |||||||
| DOCE (nM) | ProB2 (µM) | Mean | SD | DOCE (nM) | CAT (µM) | Mean | SD |
| 0 | 0 | 97.32 | 0.61 | 0 | 0 | 98.33 | 1.17 |
| 0 | 10 | 98.91 | 0.87 | 0 | 5 | 97.09 | 1.72 |
| 0 | 25 | 98.10 | 1.13 | 0 | 20 | 99.93 | 1.22 |
| 0 | 50 | 97.02 | 0.65 | 0 | 30 | 97.53 | 0.96 |
| 0 | 60 | 42.21 * | 0.83 | 0 | 50 | 98.04 | 0.78 |
| 0 | 80 | 31.01 * | 1.29 | 60 | 0 | 97.13 | 1.04 |
| 0 | 100 | 25.47 * | 1.09 | 60 | 5 | 97.21 | 1.62 |
| 60 | 0 | 95.31 | 1.24 | 60 | 20 | 97.22 | 1.77 |
| 60 | 10 | 96.04 | 1.20 | 60 | 30 | 98.28 | 0.94 |
| 60 | 25 | 96.92 | 1.28 | 60 | 50 | 97.67 | 0.79 |
| 60 | 50 | 97.31 | 0.76 | 120 | 0 | 98.75 | 1.65 |
| 60 | 60 | 37.06 * | 0.56 | 120 | 5 | 98.59 | 1.31 |
| 60 | 80 | 29.23 * | 0.72 | 120 | 20 | 98.81 | 0.84 |
| 60 | 100 | 14.26 * | 0.37 | 120 | 30 | 98.01 | 1.52 |
| 120 | 0 | 96.72 | 1.36 | 120 | 50 | 97.27 | 1.19 |
| 120 | 10 | 97.44 | 1.03 | ||||
| 120 | 25 | 97.03 | 0.74 | ||||
| 120 | 50 | 96.15 | 0.82 | ||||
| 120 | 60 | 35.24 * | 0.59 | ||||
| 120 | 80 | 22.97 * | 0.43 | ||||
| 120 | 100 | 13.28 * | 0.47 | ||||
| Gene Symbol * | Description † | Treatment | Related to Control | Related to DOCE |
|---|---|---|---|---|
| CDH1 | Cadherin 1, type 1, E-cadherin (epithelial) | DOCE | 0.00770 | |
| CAT | 0.51759 | 67.19063 a | ||
| ProB2 | 0.10638 | 13.68735 a | ||
| CAT+DOCE | 0.30540 | 39.51263 a | ||
| ProB2+DOCE | 0.07310 | 9.49801 a | ||
| CDKN1A | Cyclin-dependent kinase inhibitor 1A | DOCE | 5.06 a | |
| CAT | 1.60 a | 0.97 | ||
| ProB2 | 0.22 | 7.39 b | ||
| CAT+DOCE | 0.38 | 8.22 b | ||
| ProB2+DOCE | 0.49 | 2.40 b | ||
| BAX | BCL-2-associated X protein | DOCE | 0.05 | |
| CAT | 0.01 | 0.02 | ||
| ProB2 | 0.15 | 0.37 | ||
| CAT+DOCE | 7.25 a | 8.46 b | ||
| ProB2DOCE | 1.37 | 1.92 b | ||
| CASP9 | Caspase-9 | DOCE | 18.02465 a | |
| CAT | 1.55971 a | 0.08442 | ||
| ProB2 | 0.36939 | 0.02025 | ||
| CAT+DOCE | 8.27195 a | 0.46528 | ||
| ProB2+DOCE | 1.48718 a | 0.08252 |
| Cell Line | T. Type a | T. source | Pan-Can | IHC-ST b | Tx-st | Diffs | TP53 Status c | ER/PR/HERB2/AR d |
|---|---|---|---|---|---|---|---|---|
| MCF-7 | A, NOS | PE | Lum/HER2 | A | Lum | Lum1 | Wt | ER +/PR+/HERB2-/AR+ |
| T47D | Idc, NOS | PE | Lum/HER2 | A | Lum | Lum2 | m | ER+/PR+/HERB2-/AR+ |
| MDA-MB-231 | A, NOS | PE | ------ | C | Claudin low | ------ | m | ER-/PR-/HERB2-/AR+ |
| PC3 | AD, Grade IV | Bom-d | ------ | ------ | ------ | ------ | m | ER/PR/AR- |
| DU145 | AD, NOS | Brm-d | ------ | ------ | ------ | ------ | m | ER/PRAR- |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Núñez-Iglesias, M.J.; Novio, S.; García, C.; Pérez-Muñuzuri, M.E.; Martínez, M.-C.; Santiago, J.-L.; Boso, S.; Gago, P.; Freire-Garabal, M. Co-Adjuvant Therapy Efficacy of Catechin and Procyanidin B2 with Docetaxel on Hormone-Related Cancers In Vitro. Int. J. Mol. Sci. 2021, 22, 7178. https://doi.org/10.3390/ijms22137178
Núñez-Iglesias MJ, Novio S, García C, Pérez-Muñuzuri ME, Martínez M-C, Santiago J-L, Boso S, Gago P, Freire-Garabal M. Co-Adjuvant Therapy Efficacy of Catechin and Procyanidin B2 with Docetaxel on Hormone-Related Cancers In Vitro. International Journal of Molecular Sciences. 2021; 22(13):7178. https://doi.org/10.3390/ijms22137178
Chicago/Turabian StyleNúñez-Iglesias, Mª Jesús, Silvia Novio, Carlota García, Mª Elena Pérez-Muñuzuri, María-Carmen Martínez, José-Luis Santiago, Susana Boso, Pilar Gago, and Manuel Freire-Garabal. 2021. "Co-Adjuvant Therapy Efficacy of Catechin and Procyanidin B2 with Docetaxel on Hormone-Related Cancers In Vitro" International Journal of Molecular Sciences 22, no. 13: 7178. https://doi.org/10.3390/ijms22137178






