Protective Effects of Ethanolic Extract from Rhizome of Polygoni avicularis against Renal Fibrosis and Inflammation in a Diabetic Nephropathy Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of ER-PA
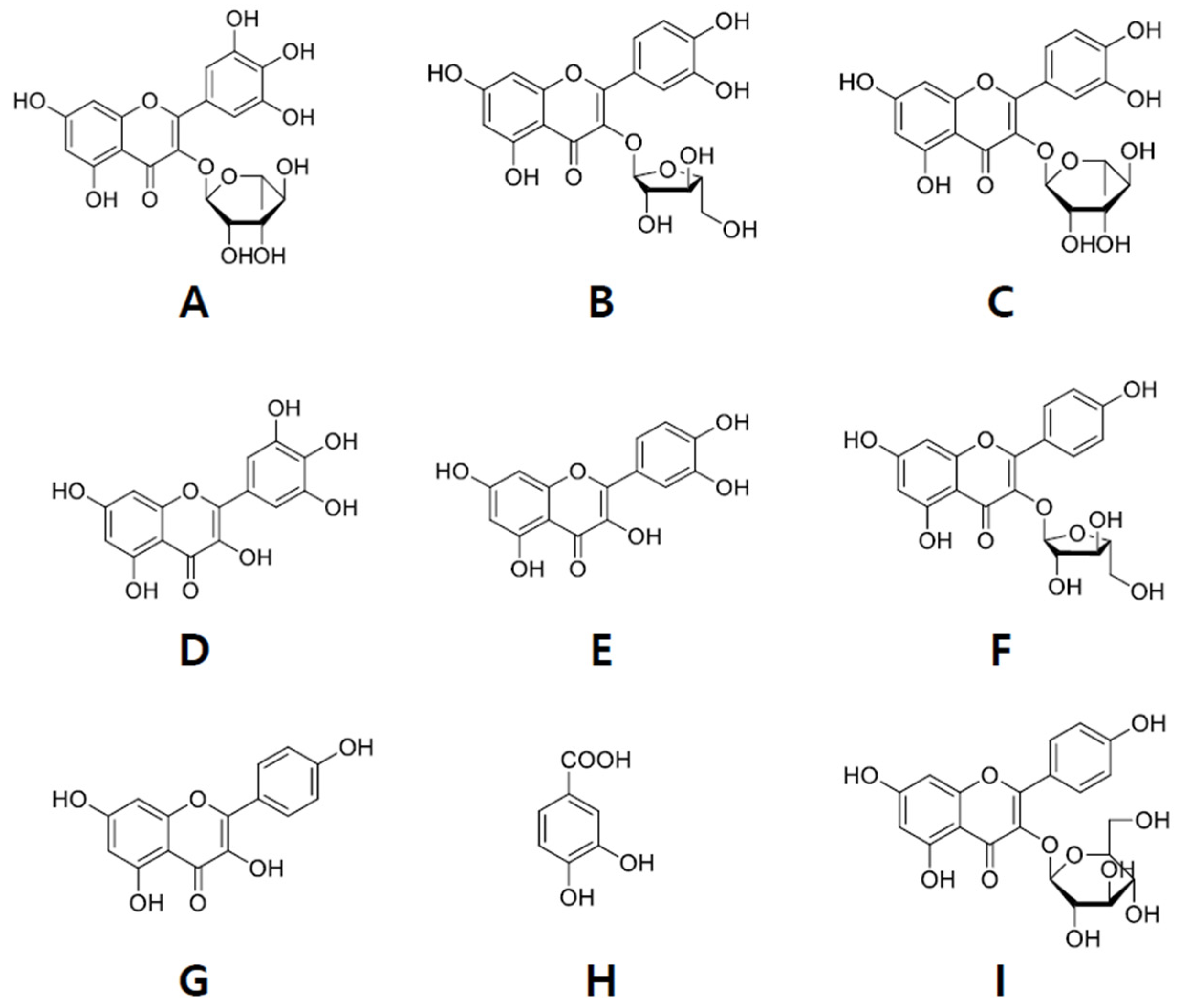
2.2. Isolation of Compounds from ER-PA
2.3. Experimental Animals and Diets
2.4. Cell Cultures
2.5. Estimation of Blood Glucose and the Oral Glucose Tolerance Test (OGTT)
2.6. Analysis of Plasma Biochemical Markers
2.7. Monitoring Renal Function
2.8. Immunohistochemical and Morphological Staining of Kidney Tissue
2.9. Western Blot Analysis of Kidney Samples
2.10. Reverse Transcription-Quantitative (RT-q) PCR
2.11. Statistical Analysis
3. Results
3.1. Effects of the Ethanolic Extract from Rhizome of PA on Fluid Metabolism
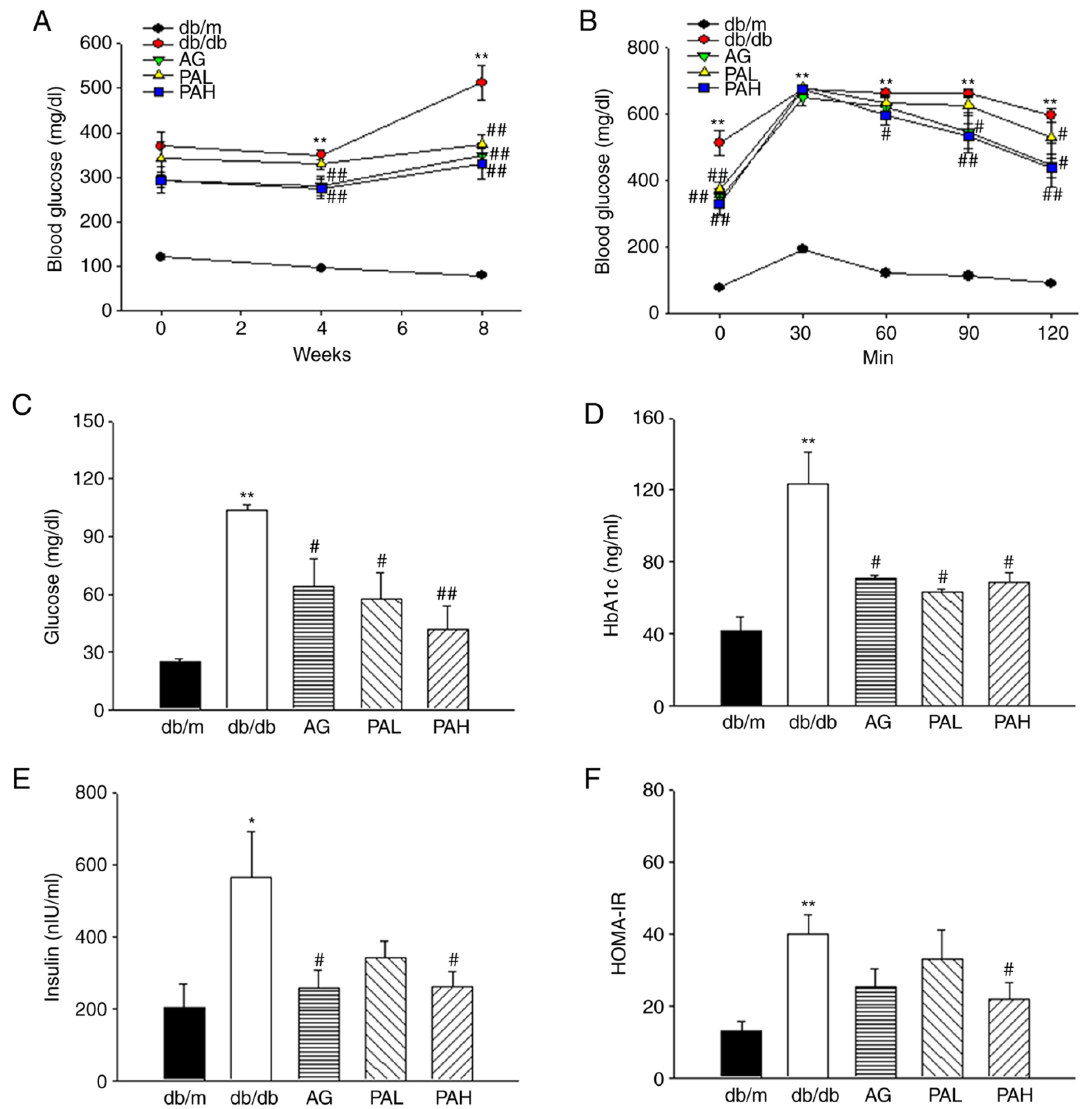
3.2. Effects of the Ethanolic Extract from Rhizome of PA on Glucose Tolerance and Insulin Resistance
3.3. Effects of the Ethanolic Extract from Rhizome of PA on Renal Function and Glomerular Morphological Changes
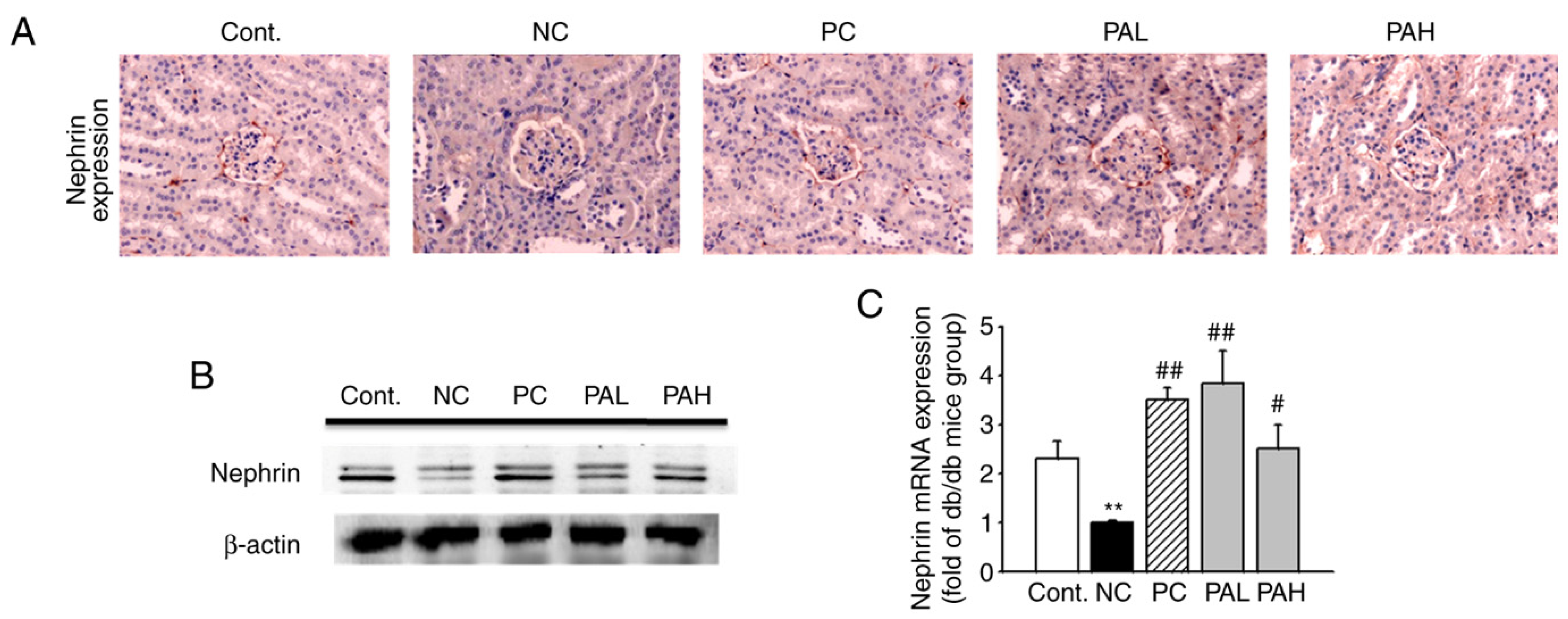
3.4. Effects of the Ethanolic Extract from Rhizome of PA on Nephrin Levels
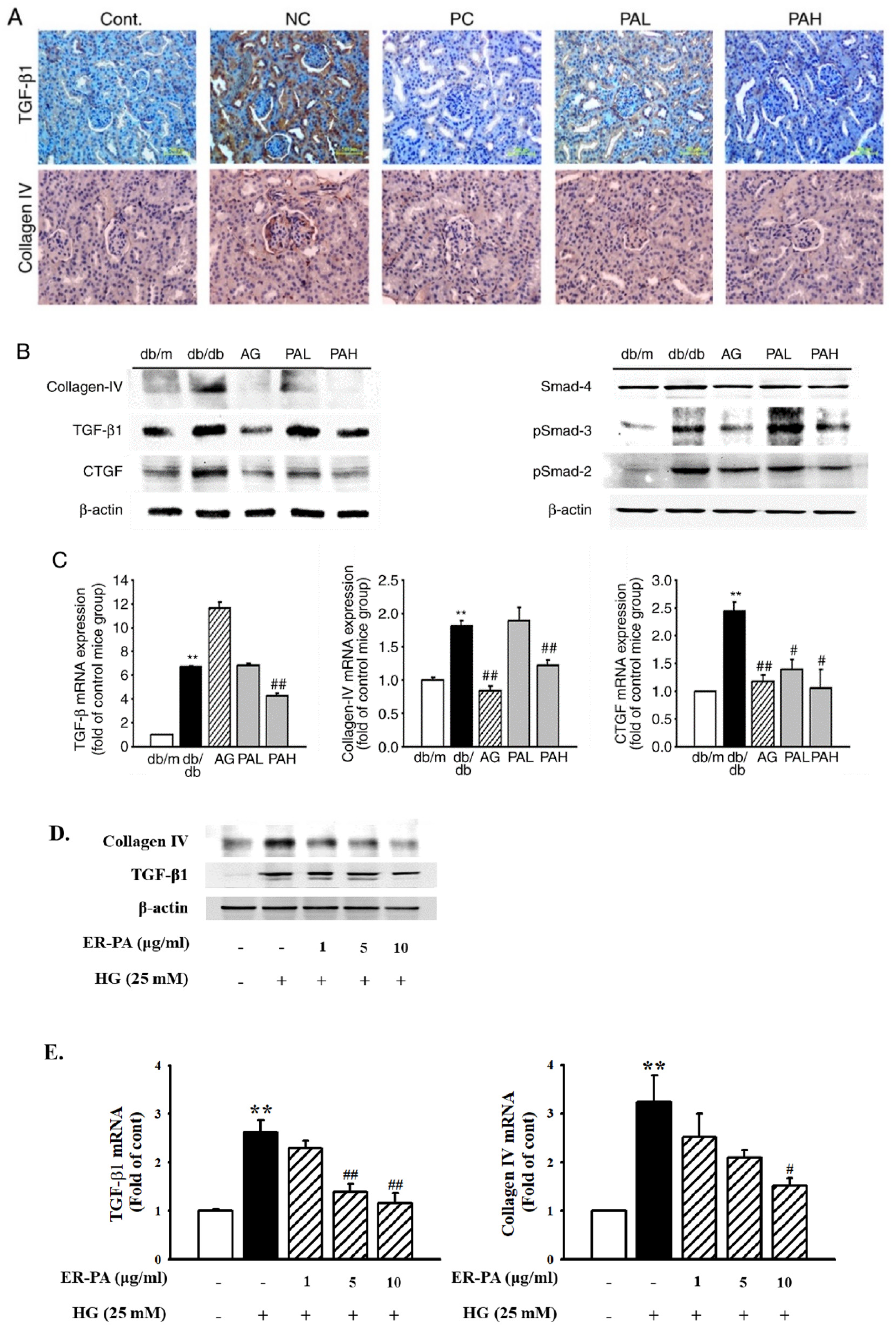
3.5. Effect of the Ethanolic Extract from Rhizome of PA on Renal Fibrosis
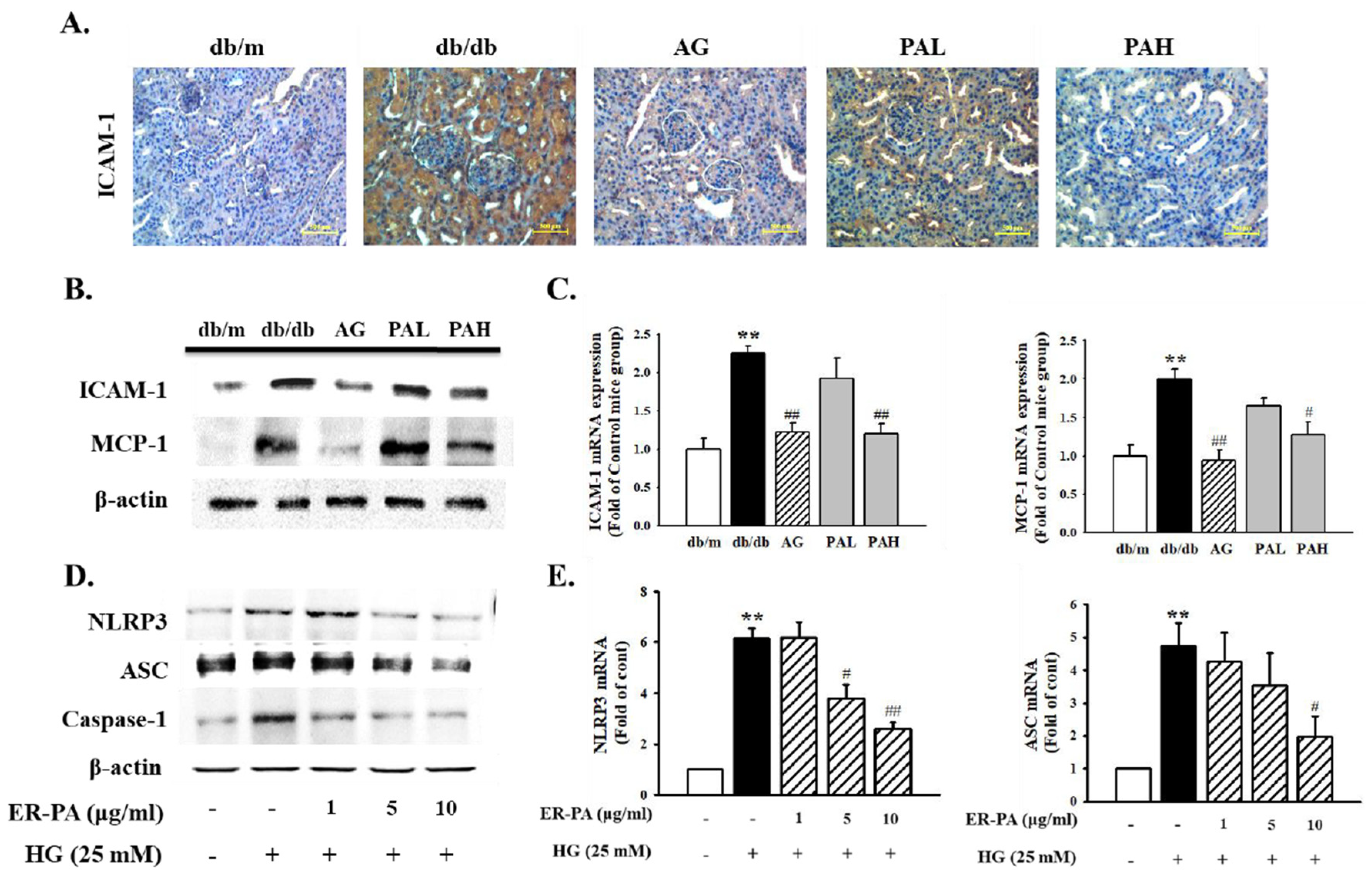
3.6. Effect of the Ethanolic Extract from Rhizome of PA on Renal Inflammation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allen, T.G.; Cooper, M.E.; Lan, H.Y. Use of genetic mouse models in the study of diabetic nephropathy. Curr. Atheroscler. Rep. 2004, 6, 207–214. [Google Scholar] [CrossRef] [PubMed]
- De Zeeuw, D.; Remuzzi, G.; Parving, H.H.; Keane, W.F.; Zhang, Z.; Shahinfar, S.; Snapinn, S.; Cooper, M.E. Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: Lessons from RENAAL. Kidney Int. 2004, 65, 2309–2320. [Google Scholar] [CrossRef] [Green Version]
- Eddy, A.A. Expresssion of genes that promote renal interstitial fibrosis in rats with proteinuria. Kidney Int. 1996, 49, 49–54. [Google Scholar]
- Lee, A.S.; Lee, Y.J.; Lee, S.M.; Yoon, J.J.; Kim, J.S.; Kang, D.G.; Lee, H.S. An aqueous extract of portulaca oleracea ameliorates diabetic nephropathy through suppression of renal fibrosis and inflammation in diabetic db/db mice. Am. J. Chin. Med. 2012, 40, 495–510. [Google Scholar] [CrossRef] [PubMed]
- Riser, B.L.; Denichilo, M.; Cortes, P.; Baker, C.; Grondin, J.M.; Yee, J.; Narins, R.G. Regulation of connective tissue growth factor activity in cultured rat mesangial cells and its expression in experimental diabetic glomerulosclerosis. J. Am. Soc. Nephrol. 2000, 11, 25–38. [Google Scholar] [CrossRef]
- Pozzi, A.; Voziyan, P.A.; Hudson, B.G.; Zent, R. Regulation of matrix synthesis, remodeling and accumulation in glomerulo-sclerosis. Curr. Pharm. Des. 2005, 15, 1318–1333. [Google Scholar] [CrossRef]
- Leask, A.; Abraham, D.J. TGF-beta signaling and the fibrotic response. FASEB J. 2004, 18, 816–817. [Google Scholar] [CrossRef]
- Huang, P.; Zhang, Y.; Jiang, T.; Zeng, W.; Zhang, N. Aldose reductase is a potent regulator of TGF-1 induced expression of fibronectin in human mesangial cells. Mol. Biol. Rep. 2010, 37, 3097–3103. [Google Scholar] [CrossRef]
- Ruotasalainen, V.; Ljungberg, P.; Wartiavaara, J.; Lenkkeri, U.; Kestilä, M.; Jalanko, H.; Holmberg, C.; Tryggvason, K. Nephrine is specifically located at the slit diaphragm of glomerular podocytes. Proc. Natl. Acad. Sci. USA 1999, 96, 7962–7967. [Google Scholar] [CrossRef] [Green Version]
- Fornoni, A.; Ijaz, A.; Tejada, T.; Lenz, O. Role of inflammation in diabetic nephropaty. Curr. Diabetes Rev. 1995, 9, 252–254. [Google Scholar]
- Miyatake, N.; Shikata, K.; Sugimoto, H.; Kushiro, M.; Shikata, Y.; Ogawa, S.; Hayashi, Y.; Miyasaka, M.; Makino, H. Intracellular adhesion molecule 1 mediates mononuclear cell infiltration into rat glomeruli after renal ablation. Nephron 1998, 79, 91–98. [Google Scholar] [CrossRef]
- Zhou, T.; Li, H.Y.; Zhong, H.; Zhong, Z. Relationship between transforming growth factor-β1 and type 2 diabetic nephropathy risk in Chinese population. BMC Med. Genet. 2018, 19, 201. [Google Scholar] [CrossRef] [Green Version]
- Du, N.; Xu, X.; Gao, M.; Liu, P.; Sun, B.; Cao, X. Combination of Ginsenoside Rg1 and Astragaloside IV reduces oxidative stress and inhibits TGF-β1/Smads signaling cascade on renal fibrosis in rats with diabetic nephropathy. Drug Des. Dev. Ther. 2018, 12, 3517–3524. [Google Scholar] [CrossRef] [Green Version]
- Robers, A.B.; Piek, E.; Bottinger, E.P.; Ashcroft, G.; Mitchell, J.B.; Flanders, K.C. Is Smad3 a major player in signal transduction pathways leading to fibrogenesis. Chest 2001, 16, 43–47. [Google Scholar] [CrossRef]
- Jiang, W.; Zhang, Y.; Wu, H.; Zhang, X.; Gan, H.; Sun, J.; Chen, Q.; Guo, M.; Zhang, Z. Role of cross-talk between the Samd2 and MAPK pathways in TGF-beta1-induced collagen IV expression in mesangial cells. Int. J. Mol. Med. 2010, 26, 571–576. [Google Scholar]
- Dai, H.Y.; Ma, L.N.; Cao, Y.; Chen, X.L.; Shi, H.; Fan, Y.P.; Yang, B. Protection of CTGF antibody against diabetic nephropathy in mice via reducing glomerular β-catenin expression and podocyte epithelial-mesenchymal transition. J. Cell Biochem. 2017, 118, 3706–3712. [Google Scholar] [CrossRef]
- Vilaysane, A.; Chun, J.; Seamone, M.E.; Wang, W.; Chin, R.; Hirota, S.; Li, Y.; Clark, S.A. The NLRP3 inflammasome promotes renal inflammation and contributes to CKD. J. Am. Soc. Nephrol. 2010, 21, 1732–1744. [Google Scholar] [CrossRef] [Green Version]
- Sen, Z.; Weida, W.; Jie, M.; Li, S.; Dongming, Z.; Xiaoguang, C. Coumarin glycosides from Hydrangea paniculata slow down the progression of diabetic nephropathy by targeting Nrf2 anti-oxidation and smad2/3-mediated profibrosis. Phytomedicine 2019, 57, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Lunyera, J.; Wang, D.; Maro, V. Traditional medicine practices among community members with diabetes mellitus in Northern Tanzania: An ethnomedical survey. BMC Complement. Altern. Med. 2016, 16, 282. [Google Scholar] [CrossRef] [Green Version]
- Woo, C.S.J.; Lau, J.S.H.; El-Nezami, H. Herbal Medicine: Toxicity and Recent Trends in Assessing Their Potential Toxic Effects. Adv. Bot. Res. 2012, 62, 365–384. [Google Scholar]
- Granica, S.; Piwowarski, J.P.; Poplawska, M.; Jakubowska, M.; Borzym, J.; Kiss, A.K. Novel insight into qualitative standardization of Polygoni avicularis herba (PH.Eur.). J. Pharm. Biomed. Anal. 2013, 72, 216–222. [Google Scholar] [CrossRef]
- Hsu, C.Y. Antioxidant activity of extract from Polygonum aviculare L. Biol. Res. 2006, 39, 281–288. [Google Scholar] [CrossRef] [Green Version]
- Roudkenar, M.H.; Roushandeh, A.M.; Delazar, A.; Halabian, R.; Rad, J.S.; Mehdipour, A.; Bagheri, M.; Jahanian-Najafabadi, A. Effects of polygonum aviculare herbal extract on proliferation and apoptotic gene expression of MCF-7. Daru 2011, 19, 326–331. [Google Scholar]
- Granica, S.; Czerwinska, M.E.; Zyzynska-Granica, B.; Kiss, A.K. Antioxidant and anti-inflammatory flavonol glucuronides from Polygonum aviculare L. Fitoterapia 2013, 91, 180–188. [Google Scholar] [CrossRef]
- Smolarz, H.D.; Budzianowski, J.; Bogucka-Kocka, A.; Kocki, J.; Mendyk, E. Flavonoid glucuronides with anti-leukaemic activity from Polygonum amphibium L. Phytochem. Anal. 2008, 19, 506–513. [Google Scholar] [CrossRef]
- Martín, T.; Rubio, B.; Villaescusa, L.; Fernández, L.; Díaz, A.M. Polyphenolic Compounds from Pericarps of Myrtus communis. Pharm. Biol. 1999, 37, 28–31. [Google Scholar] [CrossRef]
- Jung, H.A.; Kim, A.R.; Chung, H.Y.; Chop, J.S. In Vitro Antioxidant Activity of Some Selected Prunus Species in Korea. Arch. Pharm. Res. 2002, 25, 865–872. [Google Scholar] [CrossRef]
- da Silva Sa, F.; de Paula, J.; dos Santos, P.; de Almeida Ribeiro Oliveira, L. Phytochemical Analysis and Antimicrobial Activity of Myrcia tomentosa (Aubl.) DC. Leaves. Molecules 2017, 22, 1100. [Google Scholar] [CrossRef] [Green Version]
- Kruthiventi, A.K.; Krishnaswamy, N.R. Constituents of the flowers of Persea gratissima. Fitoterapia 2000, 71, 94–96. [Google Scholar] [CrossRef]
- Zhang, H.L.; Nagatsu, A.; Okuyama, H.; Mizukami, H.; Sakakibara, J. Sesquiterpene glycosides from cotton oil cake. Phytochemistry 1998, 48, 665–668. [Google Scholar] [CrossRef]
- Kumari, G.N.K.; Rao, L.J.M.; Rao, N.S.P. Myricetin methyl ethers from Solanum pubescens. Phytochemistry 1984, 23, 2701–2702. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid sensitive method for the quantification of microgram quantities of protein utilising the principle of protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Adams, L.A.; Angulo, P. Role of liver biopsy and serum markers of liver fibrosis in non-alcoholic fatty liver disease. Clin. Liver Dis. 2007, 11, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Adler, S. Structure-function relationships associated with extracellular matrix alternationsin diabetic glomerulopathy. J. Am. Soc. Nephrol. 1994, 5, 1165–1172. [Google Scholar] [CrossRef]
- Chao, C.Y.; Mong, M.C.; Chan, K.C.; Yin, M.C. Anti-glycative and antiinflammatory effects of caffeic acid and ellagic acid in kidney of diabetic mice. Mol. Nutr. Food Res. 2010, 54, 388–395. [Google Scholar] [CrossRef]
- Chen, H.; Charlat, O.; Tartaglia, L.A.; Woolf, E.A.; Weng, X.; Ellis, S.J.; Lakey, N.D. Evidence that the diabetes gene encodes the leptin receptor: Identification of a mutation in the leptin receptor gene in db/db mice. Cell 1996, 84, 491–495. [Google Scholar] [CrossRef] [Green Version]
- de Fronzo, R.A.; Alvestrand, A. Glucose intolerance in uremia: Site and mechanism. Am. J. Clin. Nutr. 1980, 33, 1438–1445. [Google Scholar] [CrossRef] [Green Version]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and abuse of HOMA modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef] [Green Version]
- van Det, N.F.; van den Born, J.; Tamsma, J.T.; Verhagen, N.A.; Berden, J.H.; Bruijn, J.A.; Daha, M.R.; van der Woude, F.J. Effects of high glucose on the production of heparan sulfate proteoglycan by mesangial and epithelial cells. Kidney Int. 1996, 49, 1079–1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, R.; Zhai, R.; Xie, L.; Zheng, Z.; Jian, G.; Chen, T.; Su, J.; Gao, C.; Wang, N.; Yang, X.; et al. Xuesaitong Protects Podocytes from Apoptosis in Diabetic Rats through Modulating PTEN-PDK1-Akt-mTOR Pathway. J. Diabetes Res. 2020, 2020, 9309768. [Google Scholar] [CrossRef]
- Sohn, E.J.; Kim, J.H.; Kim, C.S.; Lee, Y.M.; Jo, K.H.; Shin, S.D.; Kim, J.H.; Kim, J.S. The Extract of Litsea japonica Reduced the Development of Diabetic Nephropathy via the Inhibition of Advanced Glycation End Products Accumulation in db/db Mice. Evid.-Based Complement. Alternat Med. 2013, 2013, 769416. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, R.E.; Cox, A.; Dziadek, M.; Cooper, M.E.; Jerums, G. Extracellular matrix and its interactions in the diabetic kidney: A molecular biological approach. J. Diabetes Complicat. 1995, 9, 252–254. [Google Scholar] [CrossRef]
- Gilbert, R.E.; Cox, A.; Wu, L.L.; Allen, T.J.; Hulthen, U.L.; Jerums, G.; Cooper, M.E. Expression of transforming growth factor-beta1 and type IV collagen in the renal tubulointerstitium in experimental diabetes: Effects of ACE inhibition. Diabetes 1998, 47, 414–422. [Google Scholar] [CrossRef]
- Hattori, T.; Fujitsuka, N.; Kurogi, A.; Shindo, S. Sairei-to may inhibit the synthesis of endothelin-1 in nephritic glomeruli. Nippon Jinzo Gakkai Shi 1997, 39, 121–128. [Google Scholar] [PubMed]
- Collagen-Twigg, S.M.; Joly, A.H.; Chen, M.M.; Tsubaki, J.; Kim, H.S.; Hwa, V.; Oh, Y.; Rosenfeld, R.G. Connective tissue growth factor/IGF-binding protein-related protein-2 is a mediator in the induction of fibronectin by advanced glycosylation end-products in human dermal fibroblasts. Endocrinology 2002, 143, 1260–1269. [Google Scholar] [CrossRef]
- Hiragushi, K.; Sugimoto, H.; Shikata, K. Nitric oxide system is involved in glomerular hyperfiltration in Japanese normo- and micro-albuminutric patients with type2 diabetes. Diabetes Res. Clin. Pract. 2001, 53, 149–159. [Google Scholar] [CrossRef]
- Dong, F.Q.; Li, H.; Cai, W.M.; Tao, J.; Li, Q.; Ruan, Y.; Zheng, F.P.; Zhang, Z. Effects of pioglitazone on expressions of matrix metalloproteinases 2 and 9 in kidneys of diabetic rats. Chin. Med. J. 2004, 117, 1040–1044. [Google Scholar] [PubMed]
- Kang, E.S.; Kim, H.J.; Ahn, C.W.; Park, C.W.; Cha, B.S.; Lim, S.K.; Kim, K.R.; Lee, H.C. Relationship of serum high sensitivity C-reactive protein to metabolic syndrome and microvascular complications in type 2 diabetes. Diabetes Res. Clin. Pract. 2005, 69, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Sohn, E.; Kim, J.; Kim, C.S.; Jo, K.; Kim, J.S. Osteomeles schwerinae Extract Prevents Diabetes-Induced Renal Injury in Spontaneously Diabetic Torii Rats. Evid.-Based Complement. Alternat. Med. 2018, 2018, 6824215. [Google Scholar] [CrossRef]
- Cove-Smith, A.; Hendry, B.M. The Regulation of Mesangial Cell Proliferation. Nephron Exp. Nephrol. 2008, 108, e74–e79. [Google Scholar] [CrossRef]
- Nagai, K.; Arai, H.; Yanagita, M.; Matsubara, T.; Kanamori, H.; Nakano, T.; Iehara, N.; Fukatsu, A.; Kita, T.; Doi, T. Growth arrest-specific gene 6 is involved in glomerular hypertrophy in the early stage of diabetic nephropathy. J. Biol. Chem. 2003, 278, 18229–18234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.L.; Hsu, Y.C.; Lee, P.H.; Lei, C.C.; Wang, J.Y.; Huang, Y.T.; Wang, S.Y.; Wang, F.S. Cannabinoid receptor 1 disturbance of PPAR2 augments hyperglycemia induction of mesangial inflammation and fibrosis in renal glomeruli. J. Mol. Med. 2014, 92, 779–792. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, J.-J.; Park, J.-H.; Lee, Y.-J.; Kim, H.-Y.; Han, B.-H.; Jin, H.-G.; Kang, D.-G.; Lee, H.-S. Protective Effects of Ethanolic Extract from Rhizome of Polygoni avicularis against Renal Fibrosis and Inflammation in a Diabetic Nephropathy Model. Int. J. Mol. Sci. 2021, 22, 7230. https://doi.org/10.3390/ijms22137230
Yoon J-J, Park J-H, Lee Y-J, Kim H-Y, Han B-H, Jin H-G, Kang D-G, Lee H-S. Protective Effects of Ethanolic Extract from Rhizome of Polygoni avicularis against Renal Fibrosis and Inflammation in a Diabetic Nephropathy Model. International Journal of Molecular Sciences. 2021; 22(13):7230. https://doi.org/10.3390/ijms22137230
Chicago/Turabian StyleYoon, Jung-Joo, Ji-Hun Park, Yun-Jung Lee, Hye-Yoom Kim, Byung-Hyuk Han, Hong-Guang Jin, Dae-Gill Kang, and Ho-Sub Lee. 2021. "Protective Effects of Ethanolic Extract from Rhizome of Polygoni avicularis against Renal Fibrosis and Inflammation in a Diabetic Nephropathy Model" International Journal of Molecular Sciences 22, no. 13: 7230. https://doi.org/10.3390/ijms22137230
APA StyleYoon, J.-J., Park, J.-H., Lee, Y.-J., Kim, H.-Y., Han, B.-H., Jin, H.-G., Kang, D.-G., & Lee, H.-S. (2021). Protective Effects of Ethanolic Extract from Rhizome of Polygoni avicularis against Renal Fibrosis and Inflammation in a Diabetic Nephropathy Model. International Journal of Molecular Sciences, 22(13), 7230. https://doi.org/10.3390/ijms22137230





