From Cosmetics to Innovative Cosmeceuticals—Non-Woven Tissues as New Biodegradable Carriers
Abstract
:1. Introduction
2. Why the Use of Polysaccharides
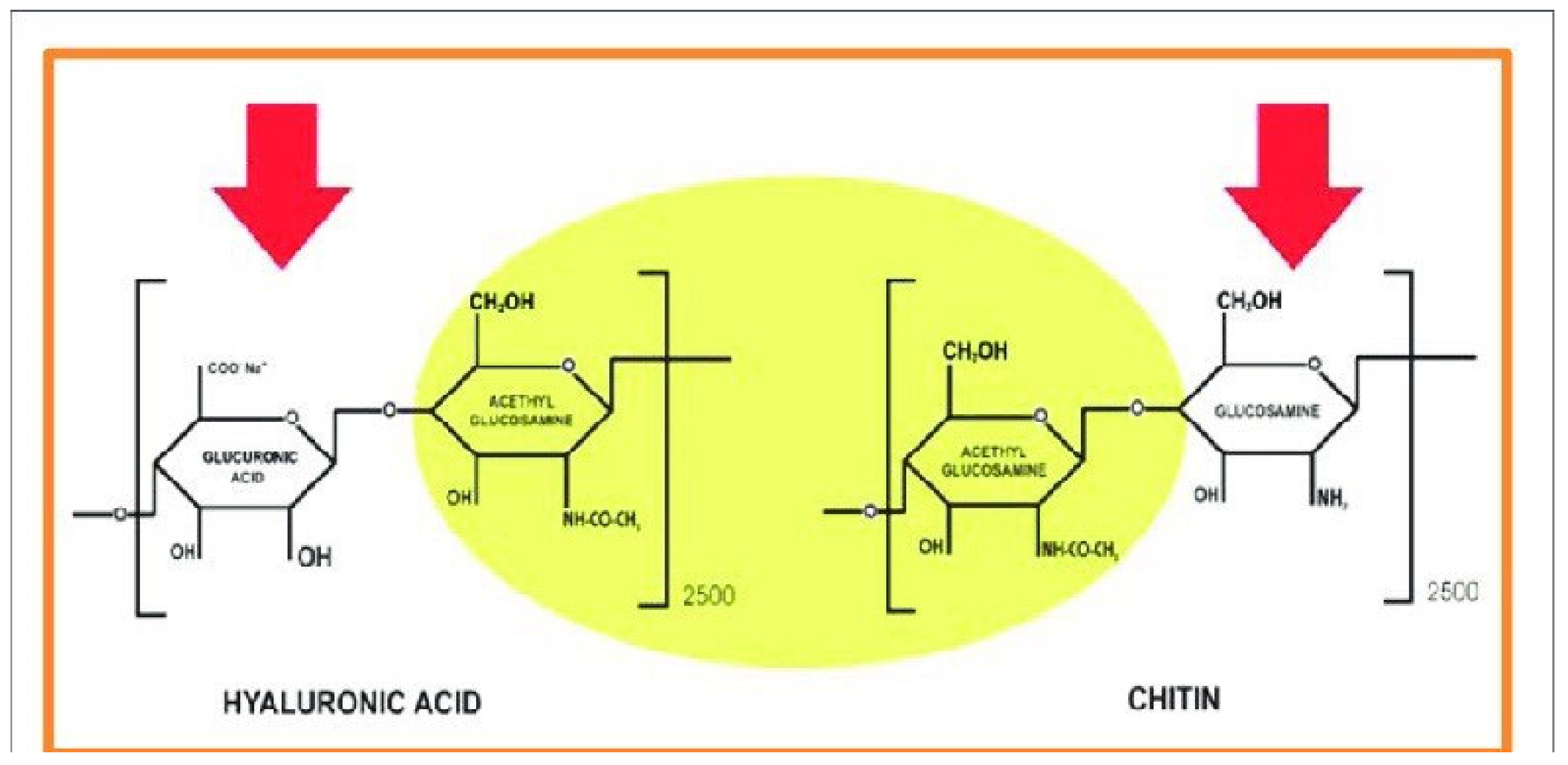
3. Chitin and Its Complexes
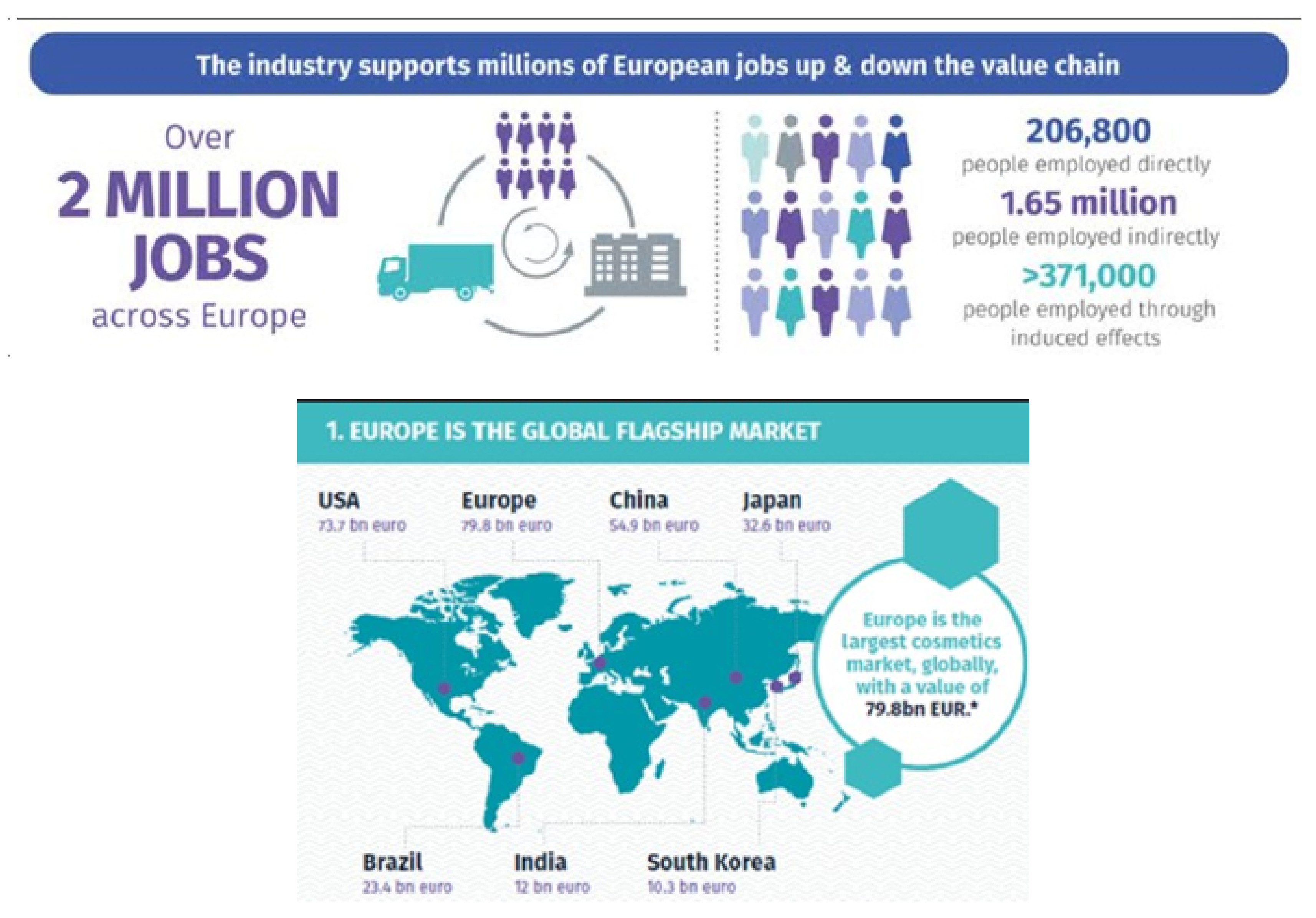
4. Cosmetics, Beauty/Health and Plastic Pollution
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Kligman, A.M.; Graham, J.A. Cosmetic Make-Over in Elderly Women. In A New Look at Old Skin: A Challenge to Cosmetology; Morganti, P., Montagna, W., Eds.; International Ediemme: Rome, Italy, 1986; pp. 197–201. [Google Scholar]
- Dion, D.; Berscheild, E.; Walster, E. What is beautiful is good. J. Pers. Soc. Psychol. 1972, 24, 285–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, D. Appearance and the elderly. In The Psychology of Cosmetic treatment; Graham, J.A., Kligman, A.M., Eds.; Praeger: New York, NY, USA, 1985; pp. 152–158. [Google Scholar]
- Reed, R.E. The Definition of Cosmeceuticals. J. Soc. Cosmet. Chem. 1962, 13, 103–106. [Google Scholar]
- Kligman, A.M. Why Cosmeceuticals? Cosmet. Toil. 1993, 108, 37–38. [Google Scholar]
- Kligman, A.M. Cosmeceuticals as a Third Category. Cosmet. Toit. 1998, 113, 33–39. [Google Scholar]
- Vermeer, B.J.; Gilchrest, B.A. Cosmeceuticals: A proposal for Rational definition, evaluation, and regulation. Arch Dermatol. 1996, 132, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Umbach, W. Cosmeceuticals—The future of Cosmetics? In Cosmeceuticals-Active Skin Treatment; Cosmetic & Toilettres Magazine, Ed.; Aluted Publishing Co.: Carol Stream, IL, USA, 2002; pp. 10–18. [Google Scholar]
- Giacomoni, P.U. Advancement in Skin Aging: The Future Cosmeceuticals. Clin. Dermatol. 2008, 26, 364–366. [Google Scholar] [CrossRef]
- Gao, X.-H.; Zhang, L.; Wei, H.; Chen, H.-D. Efficacy and Safety of Innovative Cosmeceuticals. Clin. Dermatol. 2008, 26, 367–374. [Google Scholar] [CrossRef]
- Elsner, P.; Maibach, H.I. (Eds.) Cosmeceuticals: Drugs vs. Cosmetics; CRC Press: New York, NY, USA, 2000. [Google Scholar]
- Aranaz, I.; Acosta, N.; Civera, C.; Elorza, B.; Mingo, J.; Castro, C.; De los Llanos Gandía, M.; Caballero, A.H. Cosmetic and Cosmeceuticals Aplications of Chitin, Chitosan and Their Derivatives. Polymers 2018, 10, 213. [Google Scholar] [CrossRef] [Green Version]
- Morganti, P.; Paglialunga, S. EU borderline: Cosmetic products review of current regulatory status. Clin. Dermatol. 2008, 26, 392–397. [Google Scholar] [CrossRef]
- McNamara, S.H. FDA Regulation of Cosmeceuticals. In Cosmeceuticals. Active Skin Treatment; C & T, Ed.; Allured Publishing Co.: Carol Stream, IL, USA, 2002; pp. 19–25. [Google Scholar]
- EU Regulation (EC) No1223/2009 of the European Parliament and the Council of 30 November 2009 on Cosmetic Product; European Commission: Bruxelles, Belgium, 2009.
- EC. Comparative Study on Cosmetic Legislation with EU and Other Principal Markets with Special Attention to So-Called Borderline Products; Final Report No: FIF. 20030624. Prepared for EC DG Enterprice; European Commission: Bruxelles, Belgium, 2004. [Google Scholar]
- Morganti, P. Cosmeceuticals. Clin. Dermatol. 2008, 26, 317. [Google Scholar] [CrossRef]
- Morganti, P. Reflections on cosmetics, Cosmceuticals, and nutraceuticals. Clin. Dermatol. 2008, 26, 318–320. [Google Scholar] [CrossRef]
- Wiechers, J.W. Skin Delivery: What it is and Why We Need It. In Skin Delivery Systems; Wiechers, J.W., Ed.; Allured Publishing Co.: Carol Stream, FL, USA, 2008; pp. 1–21. [Google Scholar]
- Fisher, A. Sustainable Skincare in 2021 and Beyond. 2020, Mintel Report. Available online: www.mintel.com (accessed on 20 May 2021).
- Mellow, F.; Varvaresoo, A.; Papageorgiou, S. Renewable Sources: Application on Personal Care Formulations. Int. J. Cosmet. Sci. 2019, 41, 517–525. [Google Scholar] [CrossRef] [Green Version]
- Henningan, C. Covid-19 Increases Demand for Safe and Reliable Beauty and Personal Care Product. 2020. Mintel Reoport. Available online: www.mintel.com (accessed on 20 May 2021).
- Bom, S.; Sorge, J.; Ribeiro, H.M.; Marto, J. A Step Forward on Sustainability in the Cosmetic Industry: A Review. J. Clean Product. 2019, 225, 270–290. [Google Scholar] [CrossRef]
- Amberg, N.; Fogarassy, C. Green Consumer Behavior in the Cosmetic Market. Resources 2019, 8, 137. [Google Scholar] [CrossRef] [Green Version]
- Matic, M.; Puh, B. Consumers’ Purchase Intentions Towards Natural Cosmetic. Ekon. Vjesn. Econviews-Rev. Contemp. Bus. Entrep. Econ. Issues 2016, 29, 53–64. [Google Scholar]
- Almeida, T.; Silvestri, A.J.D.; Vilela, C.; Freire, C.S.R. Bacterial Nanocellulose toward Green Cosmetics: Recent Progresses and Challenges. Int. J. Mol. Sci. 2021, 22, 2836. [Google Scholar] [CrossRef] [PubMed]
- Reis, R.L.; Neves, N.M. (Eds.) Natural-Based Polymers for Biomedical Applications; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological activities of polysaccharide from natural resources: A review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Kumbar, S.; Laurencin, C.; Deng, M. (Eds.) Natural and Synthetic Biomedical Polymers; Elsevier Science: Amsterdam, The Netherlandds, 2014. [Google Scholar]
- Baldwin, A.D.; Kilck, K.L. Polysaccharide-Modified Synthetic Polymeric Biomaterials. Biopolymers 2010, 94, 128–140. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Z.; Zhaki, A.A.; Hui, J.Z.; Muzykantov, V.R.; Tsourkas, H. Multifunctional Nanoparticles: Cost versus benefit of adding targeting and imaging capabilities. Science 2012, 338, 903–910. [Google Scholar] [CrossRef] [Green Version]
- Tirbitt, M.W.; Dahlma, J.E.; Langer, R. Emerging frontiers in drug delivery. J. Am. Chem. Soc. 2016, 138, 704–717. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [Green Version]
- Langer, R.; Tirrek, D.A. Designing Materials for Biology and Medicine. Nature 2004, 428, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasvum, K.K. A Comparative Review of Natural and Synthetic Biopolimer composite Scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef] [PubMed]
- Tziabos, A.O. Polisaccharide Immunomodulators as Therapeutic Agents: Structural Aspects and Biological Functions. Clin. Microbiol. Rev. 2000, 13, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Y.J.; Abatangelo, G. Function of Hyaluronan in Wound Repair. Wound Repair Regen. 1999, 7, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Biagini, G.; Zizzi, A.; Giantomassi, F.; Orlando, F.; Lucarini, G.; Mattioli-Belmonte, M.; Tucci, M.; Morganti, P. Cutaneous Absorption of Nanooctructured Chitin Associated with Natural Synergystic Molecules(Lutein). J. Appl. Cosmetol. 2008, 26, 69–80. [Google Scholar]
- Await, K.G.; Merillon, S.M. (Eds.) Polysaccharides. Bioactivity and Biotechnology; Springer Nature: Basel, Switzerland, 2020. [Google Scholar]
- Kim, S.-K. (Ed.) Chitin and Chitosan Derivatives; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Lee, C.G.; Da Silva, C.A.; Lee, J.Y.; Hartl, D.; Elias, J.A. Chitin Regulation of Immune Responses: An Old Molecule with New Roles. Curr. Opin. Immunol. 2008, 20, 684–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komi, D.E.A.; Sharma, L.; Delacruz, C. Chitin and its effects on inflammatory and immunoresponses. Clin. Rev. Allergy Immunol. 2018, 54, 213–223. [Google Scholar] [CrossRef] [Green Version]
- Epstein, H. Factors in formulating Cosmeceutical Vehicles. In Cosmeceuticals. Active Skin Treatment; Cosmetic & Toilettres, Ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2002; pp. 37–49. [Google Scholar]
- Morganti, P.; Danti, S.; Coltelli, M.B. Chitin and Lignin to Produce Biocompatible Tissues. Res. Clin. Dermatol. 2018, 1, 5–11. [Google Scholar] [CrossRef]
- Morganti, P.; Morganti, G.; Colao, C. Biofunctional Textiles for Skin Aging. Biomedicines 2019, 7, 51. [Google Scholar] [CrossRef] [Green Version]
- Morganti, P.; Del Ciotto, P.; Stoller, M.; Chianese, A. Antibacterial and Antiinlfammatory Green Nanocomposites. Chem. Eng. Trans. 2016, 47, 61–64. [Google Scholar] [CrossRef]
- Morganti, P.; Fusco, A.; Paoletti, I.; Perfetto, B.; Del Ciotto, P.; Palombo, M.; Chianese, A.; Baroni, A.; Donnarumma, G. Antiinflammatory, Immunomodulatory, and Tissue Repair on Human Keratinocytes by Green Innovative Nanocomposites. Materials 2017, 10, 843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donnarumma, G.; Fusco, A.; Morganti, P.; Palombo, M.; Anniboletti, T.; Del Ciotto, P.; Baroni, A.; Chianese, A. Advanced Medications Made by Green Nanocomposites. Int. J. Res. Nano Sci. 2018, 5, 261–270. [Google Scholar]
- Miletic, A.; Ristic, C.; Coltelli, M.B.; Pilic, B. Modification of PLA-based Films by Grafting or Coating. J. Func. Biomater. 2020, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Obisesan, K.A.; Neri, S.; Bugnicourt, E.; Campos, I.; Rodriguez-Turienzo, L. Determination and Quantification of the Distribution of CN-NL Nanopartickes Encapsulating Glycyrrhetic Acid on Novel Textile Surfaces with Hyperspectran Imaging. J. Funct. Biomater. 2020, 11, 327. [Google Scholar] [CrossRef]
- Morganti, P.; Palombo, P.; Palombo, M.; Fabrizi, G.; Cardillo, A.; Slovacchia, F.; Guevara, L.; Mezzana, P. A phosphatidylcholine Hyaluronic acid chitin-nanofibrils complex for a fast skin remodeling and rejuvenating look. J. Clin. Cosmet. Investig. Dermatol. 2012, 5, 213–220. [Google Scholar] [CrossRef] [Green Version]
- Morganti, P.; Morganti, G.; Coltelli, M.B. Chitin Nanomaterials and Nanocomposites for Tissue Repair. In Marine-Derived Biomaterials for Tissue Engineeing Applications; Choi, A.H., Ben-Nissan, B., Eds.; Springer: Singapore, 2019; pp. 523–544. ISBN 978-981-13-8855-2. [Google Scholar]
- Danti, S.; Trombi, L.; Fusco, A.; Azimi, B.; Lazzeri, A.; Morganti, P.; Coltelli, M.-B.; Donnarumma, G. Chitin Nanofibrils and Nanolinin as Functional Agent in Skin Regeneration. Int. J. Mol. Sci. 2019, 20, 2669. [Google Scholar] [CrossRef] [Green Version]
- Morganti, P.; Palombo, M.; Fabrizi, G.; Guarneri, F.; Slovacchia, F.; Cardillo, A.; Del Ciotto, P.; Carezzi, F.; Morganti, G. New Insight on Antiaging activity of Chitin Nanofibril-Hyaluronan block copolymers entrapping active ingredients: In vitro and in vivo study. J. Appl. Cosmetol. 2013, 41, 1–29. [Google Scholar]
- Morganti, P.; Morganti, G. Surgical & Beauty Facial Masks: The New Waste Orobkem of Post-COVID-19. Biomed. Sci. Tech. Res. 2020. [Google Scholar] [CrossRef]
- Morganti, P.; Morganti, G. Post-COVID-19: Opportunity to Produce Biodegradable Goods & Surgical Masks to Save the Environment. J. Health Care Res. 2020, 1, 157–165. [Google Scholar] [CrossRef]
- Morganti, P.; Morganti, G. Textile&Waste: Will be Covid-19 a Cafalist for Changing? J. Textile Eng. Fash. Tech. 2020, 2, 26–31. [Google Scholar]
- Anniboletti, T.; Palombo, M.; Moroni, S.; Bruno, S.; Palombo, P.; Morganti, P. Innovative non-woven tissues. In Green Bioeconomy and Bionanotechnology for a More Sustainable Environment; Morganti, P., Ed.; MDPI Edition: Basel, Switzerland, 2019; pp. 340–360. [Google Scholar]
- Morganti, P.; Coltelli, M.B. A new Carrier for Advanced Cosmeceuticals. Cosmetics 2019, 6, 10. [Google Scholar] [CrossRef] [Green Version]
- Westbrook, G.; Angus, A. Top 10 Global Consumer Trends 2021. Euromonitor Report. 2021. Available online: www.euromonitorinternational.com (accessed on 20 May 2021).
- GWI. Defining the Mental Wellness Economy. Global Wellness Institute Report. 2020. Available online: www.globalwellnessinstitute.com (accessed on 20 May 2021).
- Lobstein, T. COVID-19 and Obesity: The 2021 Atlas. World Obesity Organization Report. 2021. Available online: www.worldobesity.org (accessed on 20 May 2021).
- Gilsenan, K. Available online: https://www.gwi.com/ (accessed on 21 May 2021).
- AMR. Global Cosmetic Market by 2027. Allied Market Research. Available online: www.globenewswire.com (accessed on 20 May 2021).
- CE. Cosmetic and Personal Care Industry. In Cosmetic Europe a Report; Publisher Cosmetic Europe, Cosmetic and Personal Industry Association: Bruxelles, Belgium, 2020. [Google Scholar]
- Geyser, R.; Jambeck, J.R.; Law, K.L. Production, Use and Fate of All Plastics ever Made. Sci. Adv. 2017, 3, e1700782. [Google Scholar]
- Adyel, T.M. Accumulation of Plastic Waste During COVID-19. Science 2020, 369, 1314–1315. [Google Scholar] [CrossRef] [PubMed]
- Benson, N.V.; Bassey, D.E.; Palavisami, T. Covid pollution of COVID-19 pandemic on global waste footprint. Heliyon 2021, 7, e06343. [Google Scholar] [CrossRef]
- Sarkodie, S.A.; Owusu, P.A. Impact of COVID-19 pandemic on waste management. Environ. Dev. Sustain. 2021, 23, 7951–7960. [Google Scholar] [CrossRef]
- Condor. Plastic in the Ocean. Statistics 2020–2021, Condor Ferries. 2021. UK. Available online: www.condorferries.co.uk (accessed on 24 May 2021).
- Hernandez, L.M.; Xu, E.G.; Larsson, H.C.E.; Tahara, R.; Maisura, U.B.; Tufenskji, N. Plastic Teabags Release Billion of Microparticles and Nanoparticles into tea. Environ. Sci. Technol. 2019, 53, 12300–12310. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First Evidence of Microplastics in Human Placenta. Environ. Int. 2021, 146. [Google Scholar] [CrossRef]
- Grinder, N.M.; Vanderlinden, L.; Karthiktraj, R.; Kannan, K.; Teal, S.; Polotsky, A.J.; Powell, T.L.; Yang, I.V.; Jansson, T. Exposure to Phthalate, Alters the Furst Tramester Placental Methylome and Trascriptome in Women. Sci. Rep. 2018, 8, 6086. [Google Scholar] [CrossRef]
- Whitt, M.; Brown, W.; Danes, J.E.; Vorst, K.L. Migration of heavy metals from recycled polyethylene terephthalate during storage and microwave heating. J. Plast. Film Sheeting 2016, 32, 189–207. [Google Scholar] [CrossRef]
- Mao, J.; Jain, A.; Denslow, N.D.; Nouri, M.-Z.; Chen, S.; Wang, T.; Zhu, N.; Koh, J.; Sarma, S.J.; Sumner, B.W.; et al. Bisphenol A and bisphenol S disruptions of the mouse placenta and potential effects on the placenta–brain axis. Proc. Natl. Acad. Sci. USA 2020, 117, 4642–4652. [Google Scholar] [CrossRef]
- Bick, R.; Hasley, E.; Ekenga, C.C. The Global Injustice of Fast Fashion. Environ. Health 2018, 17, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, K.I.; Glaser, D.A. Cosmeceuticals: The New Medicine of Beauty. Mo Med. 2011, 108, 60–63. [Google Scholar] [PubMed]
- Brandt, F.S.; Cazzaniga, A.; Hann, M. Cosmeceuticals: Current Trends and Market Analysis. Semin. Cutan. Surg. 2011, 30, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Bellad, K.A.; Nanjwade, B.K.; Kamble, M.S.; Sricham, T.; Idris, N.F. Development of Cosmeceuticals. World J. Pharm. Pharm. Sci. 2017, 6, 643–691. [Google Scholar]
- BusinessWire. Global Cosmeceuticals Report 2018–-2022: Current State of Industry; BusinessWire: Dublin, Ireland, 2019; Available online: www.businesswire.com (accessed on 22 May 2021).
- Otto, A.; Du Plessis, J.; Wiechers, J.W. Formulation effects of topical emulsions on transdermal and dermal delivery. Int. J. Cosmet. Sci. 2009, 31, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Tampucci, S.; Burgalassi, S.; Chetoni, P.; Monti, D. Cutaneous Permeation and Penetration of Sunscreens: Formulation Strategies and In Vitro Methods. Cosmetsis 2017, 5, 1. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; He, H.A. A review of Cosmetic as skin Delivery. J. Cosmet. Dermatol. 2021. [Google Scholar] [CrossRef]
- Naser, W. Recently Emerged Bioactive Cosmeceuticals for Skin Rejuvenation: A Review. PharmacologyOnline 2020, 2, 243–250. [Google Scholar]
- Morganti, P.; Febo, P.; Cardillo, A.; Donnarumma, G.; Baroni, A. Chitin Nanofibril and Nanolignin: Natural Polymers of Biomedical Interest. J. Clin. Cosmet. Dermatol. 2017, 1. [Google Scholar] [CrossRef]
- Morganti, P.; Morganti, G.; Palombo, M. Resarch and Innovation for Sustainable Products: Polysaccharides for a Smart Circular Economy at Zero Waste. Clin. Res. Clin. Trials 2021, 3. [Google Scholar] [CrossRef]
- Cinelli, P.; Coltelli, M.B.; Signori, F.; Morganti, P.; Lazzeri, A. Cosmetic Packaging to Save the Environment. Cosmetics 2019, 6, 26. [Google Scholar] [CrossRef] [Green Version]
- Morganti, P.; Chen, H.-D.; Morganti, G. Nanocosmetics: Future Perspective. In Nanocosmetics: Fundamentals, Applications and Toxicity; Nanda, A., Nguyen, T.A., Raiendran, S., Slimani, Y., Eds.; Elsevier: Amsterdam, The Netherland, 2020; pp. 455–481. [Google Scholar]
- Hahn, T.; Tafi, E.; Pavl, A.; Salvia, R.; Falabella, P.; Zibek, S. Current State of Chitin Purification on Chitosan Production from Insects. J. Chem. Technol. Biotechnol. 2020, 95, 2775–2795. [Google Scholar] [CrossRef]
- Triunfo, M.; Tafi, E.; Guarnieri, A.; Scieuzo, C.; Hahn, T.; Zibek, S.; Salvia, R.; Falabella, P. Insect Chitin-based Nanomaterials for Innovative Cosmetics and Cosmeceuticals. Cosmetics 2021, 8, 40. [Google Scholar] [CrossRef]
- Chui, M.; Bernasek, A.; Kelly, J.; McMurry, M.; Heath, M. Foreward Thinking on the Bio-Revolution. 2021. MacKinsey & Company Podcast. Available online: www.mackinseyandcompany.com (accessed on 23 May 2021).















Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morganti, P.; Morganti, G.; Gagliardini, A.; Lohani, A. From Cosmetics to Innovative Cosmeceuticals—Non-Woven Tissues as New Biodegradable Carriers. Cosmetics 2021, 8, 65. https://doi.org/10.3390/cosmetics8030065
Morganti P, Morganti G, Gagliardini A, Lohani A. From Cosmetics to Innovative Cosmeceuticals—Non-Woven Tissues as New Biodegradable Carriers. Cosmetics. 2021; 8(3):65. https://doi.org/10.3390/cosmetics8030065
Chicago/Turabian StyleMorganti, Pierfrancesco, Gianluca Morganti, Alessandro Gagliardini, and Alka Lohani. 2021. "From Cosmetics to Innovative Cosmeceuticals—Non-Woven Tissues as New Biodegradable Carriers" Cosmetics 8, no. 3: 65. https://doi.org/10.3390/cosmetics8030065
APA StyleMorganti, P., Morganti, G., Gagliardini, A., & Lohani, A. (2021). From Cosmetics to Innovative Cosmeceuticals—Non-Woven Tissues as New Biodegradable Carriers. Cosmetics, 8(3), 65. https://doi.org/10.3390/cosmetics8030065






